Abstract
Humans are adversely affected by exposure to cadmium (Cd) as it induces oxidative stress which damages kidneys, bones pulmonary tissues, liver, cardiovascular, immune, reproductive systems and influences endocrine secretions. In the present study tubotaiwine treatment regulated systolic, diastolic and mean arterial blood pressure of the Cd exposed rats. Tubotaiwine significantly promoted vascular responsiveness to Phe, ACh and SNP and reversed Cd mediated decrease in eNOS and increase in iNOS expression. Treatment of the Cd exposed rats with tubotaiwine reduced number of smooth muscle cells, decreased collagen content and promoted content of elastin in aortic artery walls. Tubotaiwine treatment significantly suppressed Cd-induced increase in MMP-2 and MMP-9 in rat aortic artery tissues. Increase in O2–, urinary nitrate/nitrite, MDA, carbonyl level and decrease in GSH production in rat blood and thoracic aorta tissues were effectively reversed by tubotaiwine treatment. Tubotaiwine treatment of the rats significantly reduced Cd-induced increase in blood, liver, heart and kidney tissue Cd content in dose dependent manner. Thus, tubotaiwine suppresses Cd induced hypertension in rats by reducing arterial stiffness, inhibition of oxidative stress and increasing vascular remodeling. Therefore, tubotaiwine has beneficial effect on Cd induced hypertension in rats and may be developed as a potential candidate for treatment of hypertension.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
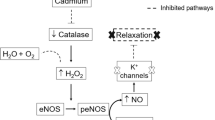
Cadmium (Cd) in ionic form (Cd2+) is one of the highly toxic metals distributed widely in the environment. Exposure of humans to Cd is gradually increasing in multiple ways such as through food sources, intake of tobacco, and via industrial contamination [1]. Animals are adversely affected by exposure to Cd resulting in induction of oxidative stress which damages kidneys, bones pulmonary tissues, liver, cardiovascular, immune, reproductive systems and influences endocrine secretions [2]. The most susceptible target of Cd in humans is vascular system leading to development of various cardiovascular diseases including, hypertension, atherosclerosis and diabetes [3]. It has been demonstrated that increase in Cd2+ blood level is directly associated with the elevation of blood pressure [3]. However, the mechanism by which Cd accumulation raises blood pressure to induce hypertension is yet to be clearly known. Studies have identified activation of oxidative stress by Cd exposure as one of the factors involved in vascular dysfunction and organ damage [4]. Presence of vasodilator (nitric oxide) is reduced while as inflammation, vascular tissue injury, lipid, protein and DNA damage is increased in vasculature by oxidative stress [4]. It has been demonstrated that suppression of ROS, promotion of NO bioavailability and enhancement in arterial remodeling and stiffening play important role in the treatment of hypertension [5].
Antioxidant natural products and metal chelating agents have been described to play important role in the treatment of disorders/diseases caused by metal poisoning. These antioxidants exhibit their effect by scavenging of free radicals, regulation of gene expression and signalling pathways and thereby inhibit cell death [6]. Alstonia scholaris Linn., commonly called as saptaparni or devil’s tree is growing widely in dried forests of Western Himalayas, Ghats and in the Southern region [6]. Traditionally the plant bark has been used for treatment of rheumatism, chronic ulcers, fever caused by malarial infection, abdominal disorders, asthma, bronchitis and pruritis [6]. Milky juice of the plant is mixed with oil and used for relaxing the pain in rheumatic patients. Phytochemical investigation revealed the presence of many alkaloids including, echitamine, tubotaiwine, akaummicine, echitamidine, picrinine, and strictamine in the plant extract [7]. The plant extract has been shown to possess immune-stimulatory, hepatoprotective and anti-hypertensive properties [7]. Moreover, studies have found anti-diabetic, anti-hyperlipidemic, anti-inflammatory, analgesic, antioxidant, immunostimulant, anti-asthmatic, hepatoprotective and anti-anxiety properties of the plant extract [8]. Ethanolic extract of the plant leaves has been shown to possess anti-arthritic activity in vivo in rat model of arthritis [9]. The present study investigated the protective effect of tubotaiwine (Fig. 1) against hypertension in Cd-induced rat model and evaluated the underlying mechanism.
MATERIALS AND METHODS
Animals and Experimental Protocols
Total sixty male neonatal Sprague–Dawley rats (body weight 40–45 g; age 18 days) were provided by the Beijing Vital River Laboratory Animal Technology (Beijing, China). The rats were kept under environmentally regulated conditions (temperature 23 ± 2°C, humidity 40–50%, and exposed to 12 h light/dark cycles). All rats were given free access to rodent diet and water. The study protocols were conducted strictly in accordance with the guidelines issued by the National Institutes of Health, USA. Acclimatization for 1-week was followed by separation of the rats randomly into 6-groups of 10 each: normal, tubotaiwine (10 mg/kg), model, and three tubotaiwine treatment groups (at 2.5, 5, and 10 mg/kg doses). Tubotaiwine was given to rats alternately for 8-weeks through intragastrical route in physiological saline. Rats in the model and three treatment groups were given deionized water containing CdCl2 (100 mg/L) to induce hypertension.
Blood Pressure Measurement and Vascular Reactivity Determination
After 8-weeks of treatment rats were sacrificed using anesthetization with ketamine (100 mg/kg) and xylazine (2.5 mg/kg) doses. Previously reported protocol was used for measurement of blood pressure and assessment of vascular reactivity of the rats. Spontaneous breathing was maintained by tracheotomy and body temperature of the rats was kept constant using heating pads. Carotid artery of the rats was exposed carefully and then cannulated using polyethylene tubing connected with pressure transducer for recording the blood pressure. Left jugular vein of the rats was also exposed and then cannulated using polyethylene tubing to infuse the vasoactive agents. Baseline measurements were made prior to infusion of ach (10 nmol/kg), SNP (10 nmol/kg), and Phe (0.03 mmol/kg) through intravenous route. Infusion of drugs was followed by allowing the rats to stabilize for 5 min so that blood pressure reaches the baseline level. Percentage of the blood pressure measured immediately prior to drug infusion (baseline) was taken to represent the change in blood pressure. Completion of the experiment was followed by sacrificing the rats using overdose of anaesthesia to collect the blood from abdominal aorta for determination of GSH level and oxidative stress makers. Then, aorta of the rats was excised for assessment of the level of superoxide ions.
Determination of Nitrate/Nitrite, Oxidative Stress Markers, and Glutathione Level
Nitrate and nitrite level present in the rat urine was measured using reported methodology. The established lucigenin-enhanced chemiluminescence protocol was used for determination of superoxide level in rat aorta tissues. Previously reported TBA assay was used to measure the levels of malondialdehyde (MDA) in plasma samples, liver, heart and kidney tissues. Marker of protein oxidation, protein carbonyl in plasma and tissues was determined by assessment of carbonyl groups based on DNPH reaction protocol. The GSH level in blood samples of rats was measured spectrophotometrically by established methodology.
Western Blot Assay
Following lysis of aorta, protein concentration in the lysate was quantified using bicinchoninic acid assay. The protein samples (50 μg protein/lane) were separated by electrophoresis on SDS–PAGE (10% gel) and subsequently transferred to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA) which were blocked by incubation with 5% non-fat milk powder for 1 h at room temperature. Afterwards membranes were incubated for overnight with primary antibodies against eNOS, iNOS, and β-actin (all Abcam, Cambridge, MA, USA) at 4°C. Membranes were then washed with Tris-buffered saline with Tween® 20 (TBST; Sigma-Aldrich; Merck KGaA) followed by incubation for 1 h with fluorescently labeled goat anti-rabbit IgG secondary antibodies (Abcam, Cambridge, MA, USA) at 37°C. The ECL reagent (Beyotime Institute of Biotechnology) and Odyssey far-infrared fluorescence scanning imaging system (LI-COR Biosciences, Lincoln, NE, USA) were used for visualization of the proteins bands.
Determination of MMP-2 and MMP-9 Level
Localization of MMP-2 and MMP-9 in rat thoracic aorta was assessed by incubation of the dewaxed tissues with specific antibodies (ab37150; Abcam and ab19016; Millipore, respectively). The previously reported immunohistochemical technique employing R.T.U. Vectastain ABC kit (Vector Laboratories, Inc., CA, USA) was used for analysis of MMP-2 and MMP-9 levels. Experimental setup was established so that a strong and specific signal for the desired antigen is generated.
Cadmium Assay
Content of Cd in blood samples and tissues of the rats was measured using the previously known protocol [24]. The inductively coupled plasma mass spectrometer (Agilent 7500 ICP-MS model, Santa Clara, CA, USA) was used for determination of Cdcontent as per the manufacturer’s protocol. The Cd content in tissues and blood samples were expressed as mg/g and mg/L, respectively.
Statistical Analysis
The data presented are the mean ± SEM. The data were analyzed using SPSS19.0 statistical software package (IBM Corp., Armonk, NY, USA). Comparisons between various groups was made using one-way analysis of variance (ANOVA) followed by a Newman–Keuls post-hoc test; P < 0.05 was taken to represent the statistically significant differences.
RESULTS
Tubotaiwine Suppresses Hypertension and Improves Vascular Dysfunction in Cd Exposed Rats
Exposure to Cd caused a significant (P < 0.05) increase in systolic as well as diastolic blood pressure and raised the mean arterial pressure in rats compared to the normal group (Table 1). Treatment with tubotaiwine at 2.5, 5, and 10 mg/kg doses significantly (P < 0.05) reduced Cd induced increase in systolic as well as diastolic blood pressure in rats. Moreover, Cd induced elevation in mean arterial pressure was also significantly (P < 0.05) lowered in rats on treatment with 2.5, 5, and 10 mg/kg doses of tubotaiwine after 8 weeks.
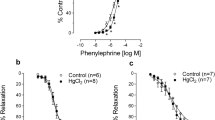
In Cd exposed rats, a significant (P < 0.05) impairment in vasodilating and vasoconstricting activity was observed as evident by vascular hypo-responsiveness to the Phe, ACh and SNP (Fig. 2). However, tubotaiwine treatment of the rats at 2.5, 5, and 10 mg/kg doses significantly (P < 0.05) promoted vascular responsiveness to Phe, ACh, and SNP. Increase in vascular responsiveness in Cd exposed rats was comparable to normal group on treatment with 10 mg/kg dose of tubotaiwine.
The data presented are the mean ± SEM; MAP, mean arterial pressure.
Tubotaiwine Inhibits Cd-Induced Increase in iNOS Level
In Cd exposed rats, the eNOS level was significantly (P < 0.05) reduced while as iNOS expression was elevated in aortic tissues compared to the normal group (Fig. 3). Treatment of the rats with tubotaiwine at 2.5, 5, and 10 mg/kg doses significantly (P < 0.05) reversed Cd mediated decrease in eNOS level. Additionally, tubotaiwine treatment also caused a significant (P < 0.05) reduction in iNOS expression in Cd exposed rats.
Tubotaiwine Regulates Vascular Remodeling in Cd Exposed Rats
Exposure to Cd caused a significant (P < 0.05) elevation in number of smooth muscle cells and increase in content of collagen in the aortic wall compared to the normal rats (Fig. 4).
Effect of tubotaiwine on vascular remodeling in Cd exposed rats. Number of smooth muscle cells, collagen and elastin protein content in the Cd exposed and tubotaiwine treated rats was determined by H and E, picrosirius red, and Miller’s elastic staining, respectively after 8 weeks; * P < 0.05, ** P < 0.01 vs. normal rats.
Moreover, Cd exposure led to a significant (P < 0.05) reduction in content of elastin in aortic artery of the rats compared to the normal group. Treatment of the Cd exposed rats with tubotaiwine at 2.5, 5, and 10 mg/kg doses significantly (P < 0.05) suppressed number of smooth muscle cells and reduced content of collagen in the aortic artery. Additionally, tubotaiwine treatment at 2.5, 5, and 10 mg/kg doses effectively promoted content of elastin in aortic artery walls of the rats.
In rat aortic walls, Cd exposure led to a significant (P < 0.05) increase in level of MMP-2 and MMP-9 in comparison to the normal group (Fig. 5). However, tubotaiwine treatment at 2.5, 5, and 10 mg/kg doses significantly (P < 0.05) suppressed Cd-induced increase in MMP-2 and MMP-9 in rat aortic artery tissues. The MMP-2 and MMP-9 levels were reduced close to that of the normal groups in Cd exposed rats on treatment with 10 mg/kg dose of tubotaiwine.
Tubotaiwine Suppresses Oxidative Stress Induction by Cd-Exposure
Exposure to Cd significantly elevated O2– level and reduced GSH in blood and thoracic aorta tissues of the rats compared to the normal group (Table 2).
Treatment with tubotaiwine at 2.5, 5, and 10 mg/kg doses significantly (P < 0.05) reversed Cd-induced increase in O2– level and reduction on GSH production in rat blood and thoracic aorta tissues. Tubotaiwine treatment at 10 mg/kg dose was found to be most effective in reducing O2– level and promoting GSH production in Cd-exposed rats. The urinary nitrate/nitrite level showed a significant (P < 0.05) increase in Cd exposed rat aortic tissues compared to the normal rats. However, on treatment with 2.5, 5, and 10 mg/kg doses of tubotaiwine, the Cd-induced urinary nitrate/nitrite level was significantly (P < 0.05) reduced in rat aortic tissues. Plasma samples, liver, heart and kidney tissues of the Cd exposed rats showed significantly (P < 0.05) elevated level of MDA compared to the normal rats. Moreover, carbonyl level was also found to be significantly (P < 0.05) higher in Cd exposed rat plasma samples, liver, heart and kidney tissues in comparison to the normal group. Tubotaiwine treatment at 2.5, 5, and 10 mg/kg doses significantly (P < 0.05) reversed Cd-induced changes in MDA production and carbonyl level in the rat plasma samples, liver, heart and kidney tissues. Tubotaiwine was found to be most effective in alleviating Cd induced changes in MDA production and carbonyl level at 10 mg/kg dose.
Accumulation of Cd is Reduced by Tubotaiwinein Blood and Tissues
Blood samples, liver, heart and kidney tissues of the rats showed significantly (P < 0.05) raised levels of Cd content following 8 weeks of Cd exposure compared to the control group (Fig. 6).
However, tubotaiwine treatment of the rats at 2.5, 5, and 10 mg/kg doses significantly (P < 0.05) reduced Cd-induced increase in blood, liver, heart and kidney tissue Cd content in dose dependent manner. Tubotaiwine treatment of the rats at 10 mg/kg dose for 8 weeks, reduced Cd-induced increase in blood, liver, heart and kidney tissue Cd content close to the normal group.
DISCUSSION
The present study demonstrated that tubotaiwine alleviated hypertension and improved vascular dysfunction induced by Cd exposure in rat model. Tubotaiwine treatment inhibited generation of oxidants, promoted GSH expression, reduced vascular remodeling induction by MMP-upregulation and regulated bioavailability of NO in Cd exposed rats.
In consistence with the previous literature Cd exposure of rats elevated blood pressure indicating its nature as risk factors for the animal health [10]. Vascular function in Cd exposed rats is impaired due to induction of oxidative stress leading to endothelium damage and increase in count of VSMCs [11]. Exposure of animals to Cd has been reported to attenuate the VSMC response to several vasoactive agents [12]. It is believed that Cd promotes Ca2+-channel antagonism to reduce the Phe-induced contracting potential of the arteries [12]. Suppression eNOS expression is associated with reduction in vaso-relaxation ability of the arterial endothelium by Cd exposure which accounts for decrease in response to ACh [12]. In the present study Cd exposure led a significant (P < 0.05) increase in systolic as well as diastolic blood pressure and raised the mean arterial pressure in rats. However, fortunately tubotaiwine treatment significantly (P < 0.05) reduced Cd induced increase in systolic as well as diastolic blood pressure in rats in dose-dependent manner. Additionally, Cd induced elevation in mean arterial pressure was also significantly (P < 0.05) lowered in rats on treatment with tubotaiwine. Vasodilating and vasoconstricting activity of the Cd exposed rats showed a significant impairment that was evident by vascular hypo-responsiveness to the Phe, ACh, and SNP. However, tubotaiwine treatment of the rats significantly (P < 0.05) promoted vascular responsiveness to Phe, ACh, and SNP. These findings indicated that tubotaiwine decreases hypertension in rats and increased responsiveness to vasoactive agents such as Phe, ACh, and SNP.
Production of NO from endothelial cells is associated with disaggregation of platelets, vasodilation, and relaxation of smooth muscles, plats anti-inflammatory and anti-thrombogenic role [13]. Suppression of eNOS expression and loss of eNOS substrate or cofactors are involved in reduction of NO bioavailability. It has been demonstrated that eNOS level is reduced in the blood samples of hypertensive rat model established by Cd exposure [12]. Vascular dysfunction induction by Cd exposure leads to loss of endothelial integrity and tissue inflammation [3]. Pro-inflammatory mediators like TNF-α induce iNOS expression and produce excessive NO content leading to many disorders [14]. In the present study Cd exposure of rats significantly (P < 0.05) reduced eNOS level and elevated iNOS expression in aortic tissues. However, treatment of the rats with tubotaiwine significantly (P < 0.05) reversed Cd mediated decrease in eNOS level. Additionally, tubotaiwine treatment also caused a significant (P < 0.05) reduction in iNOS expression in Cd exposed rats.
Vascular remodeling during hypertension is characterized by extracellular matrix reorganization induced by synthesis and degradation of several proteins. It has been found that activation of MMP through NADPH oxidase induced ROS production is associated with increase in arterial mechanical stretch. Previous studies have shown that Cd exposure promotes levels of matrix metalloproteinases—MMP-9 and MMP-2 which play prominent role in tissue inflammation [15]. In the present study exposure of rats to Cd caused a significant (P < 0.05) elevation in number of smooth muscle cells and increased content of collagen in the aortic wall. Exposure to Cd led to a significant (P < 0.05) reduction in content of elastin in aortic artery of the rats. Treatment of the Cd exposed rats with tubotaiwine significantly (P < 0.05) suppressed number of smooth muscle cells and reduced content of collagen in the aortic artery. Additionally, tubotaiwine treatment effectively promoted content of elastin in aortic artery walls of the rats. In rat aortic walls, Cd exposure led to a significant (P < 0.05) increase in level of MMP-2 and MMP-9. However, tubotaiwine treatment significantly (P < 0.05) suppressed Cd-induced increase in MMP-2 and MMP-9 in rat aortic artery tissues.
Exposure to Cd has been demonstrated to raise ROS production, weaken antioxidant defense systems by reducing GSH expression, deplete metals needed for enzyme activity and promote oxidative attack susceptibility of the cells. Generation of ROS like O2– is elevated and iNOS expression is increased by Cd exposure through NF-kB activation [16]. In the present study Cd exposure significantly elevated O2– level and reduced GSH in blood and thoracic aorta tissues of the rats. Treatment with tubotaiwine significantly (P < 0.05) reversed Cd-induced increase in O2– level and reduction on GSH production in rat blood and thoracic aorta tissues. Treatment with tubotaiwine suppressed Cd-induced urinary nitrate/nitrite level in rat aortic tissues. Tubotaiwine treatment significantly (P < 0.05) reversed Cd-induced changes in MDA production and carbonyl level in the rat plasma samples, liver, heart and kidney tissues. Tubotaiwine treatment of the rats significantly (P < 0.05) reduced Cd-induced increase in blood, liver, heart and kidney tissue Cd content in dose dependent manner.
CONCLUSIONS
In summary, tubotaiwine suppresses Cd-induced hypertension in rats by reducing arterial stiffness, inhibition of oxidative stress and increasing vascular remodeling. Treatment with tubotaiwine suppressed matrix metalloproteinase level, reduced MDA level and promoted GSH activity in Cd exposed rats. Therefore, tubotaiwine has beneficial effect on Cd- induced hypertension in rats and may be developed as a potential candidate for treatment of hypertension.
REFERENCES
ATSDR, Toxicological Profile for Cadmium, Agency for Toxic Substances and Disease Registry, Department of Health and Humans Services, Public Health Service, Centers for Disease Control, Atlanta, GA, U.S.A., 2012, pp. 1–430.
Cuypers, A., Plusquin, M., Remans, T., Jozefczak, M., Keunen, E., et al., Cadmium stress: an oxidative challenge, Biometals, 2010, vol. 23, pp. 927–940.
Alissa, E.M. and Ferns, G.A., Heavy metal poisoning and cardiovascular disease, J. Toxicol., p. 2011.
Xu, S. and Touyz, R.M., Reactive oxygen species and vascular remodelling in hypertension: still alive, Can. J. Cardiol., 2006, vol. 22, pp. 947–951.
Lee, M.Y. and Griendling, K.K., Redox signaling, vascular function, and hypertension, Antioxid. Redox Signal., 2008, vol. 10, pp. 1045–1059.
Young, I.S. and Woodside, J.V., Antioxidants in health and disease, J. Clin. Pathol., 2001, vol. 54, pp. 176–186.
Arulmozhi, S., Mazumder, P.M., Narayan, L.S., and Thakurdesai, P.A., In vitro antioxidant and free radical scavenging activity of fractions from Alstonia scholaris, Linn. R. Br. Int. J. Pharm. Tech. Res., 2010, vol. 2, pp. 18– 25.
Deepti, B., Archana, J., and Manasi, J., Antidiabetic and antihyperlipidemic effect of Alstonia scholaris Linn. bark in streptozocin induced diabetic rats, Indian J. Pharm. Educ., 2011, vol. 45, pp. 114–120.
Arulmozhi, S., Mazumder, P.M., Sathiyanarayanan, L., and Ashok, P., Anti-arthritic and antioxidant activity of leaves of Alstonia scholaris, Linn. R. Br. Eur. J. Integr. Med., 2011, vol. 3, pp. e83–e90.
Satarug, S., Garrett, S.H., Sens, M.A., and Sens, D.A., Cadmium, environmental exposure, and health outcomes, Environ. Health Perspect., 2010, vol. 118, pp. 182–190.
Wang, Y., Fang, J., Leonard, S.S., and Rao, K.M., Cadmium inhibits the electron transfer chain and induces reactive oxygen species, Free Radic. Biol. Med., 2004, vol. 36, pp. 1434–1443.
Tzotzes, V., Tzilalis, V., Giannakakis, S., Saranteas, T., Papas, A., et al., Effects of acute and chronic cadmium administration on the vascular reactivity of rat aorta, Biometals, 2007, vol. 20, pp. 83–91.
Forstermann, U. and Munzel, T., Endothelial nitric oxide synthase in vascular disease: from marvel to menace, Circulation, 2006, vol. 113, pp. 1708–1714.
Aktan, F., iNOS-mediated nitric oxide production and its regulation, Life Sci., 2004, vol. 75, pp. 639–653.
Kundu, S., Sengupta, S., Chatterjee, S., Mitra, S., and Bhattacharyya, A., Cadmium induces lung inflammation independent of lung cell proliferation: a molecular approach, J. Inflamm. (London), 2009, vol. 6, p. 19.
Lambertucci, R.H., Leandro, C.G., Vinolo, M.A., Nachbar, R.T., Dos Reis Silveira, L., et al., The effects of palmitic acid on nitric oxide production by rat skeletal muscle: mechanism via superoxide and iNOS activation, Cell Physiol. Biochem., 2012, vol. 30, pp. 1169–1180.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests. Statement on the welfare of animals. Approval for the study was obtained from the Animal Ethics Committee, The First People’s Hospital of Jingmen City, Hubei Province.
Rights and permissions
About this article
Cite this article
Gao, L., Li, X. Protective Effect of Tubotaiwine on Cadmium-Induced Hypertension in Rats through Reduction in Arterial Stiffness and Vascular Remodeling. Dokl Biochem Biophys 500, 368–375 (2021). https://doi.org/10.1134/S1607672921050136
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1607672921050136