Abstract
Mercury is a heavy metal associated with cardiovascular diseases. Studies have reported increased vascular reactivity without changes in systolic blood pressure (SBP) after chronic mercury chloride (HgCl2) exposure, an inorganic form of the metal, in normotensive rats. However, we do not know whether individuals in the prehypertensive phase, such as young spontaneously hypertensive rats (SHRs), are susceptible to increased arterial blood pressure. We investigated whether chronic HgCl2 exposure in young SHRs accelerates hypertension development by studying the vascular function of mesenteric resistance arteries (MRAs) and SBP in young SHRs during the prehypertensive phase. Four-week-old male SHRs were divided into two groups: the SHR control group (vehicle) and the SHR HgCl2 group (4 weeks of exposure). The results showed that HgCl2 treatment accelerated the development of hypertension; reduced vascular reactivity to phenylephrine in MRAs; increased nitric oxide (NO) generation; promoted vascular dysfunction by increasing the production of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2); increased Gp91Phox protein levels and in situ levels of superoxide anion (O ·−2 ); and reduced vasoconstrictor prostanoid production compared to vehicle treatment. Although HgCl2 accelerated the development of hypertension, the HgCl2-exposed animals also exhibited a vasoprotective mechanism to counterbalance the rapid increase in SBP by decreasing vascular reactivity through H2O2 and NO overproduction. Our results suggest that HgCl2 exposure potentiates this vasoprotective mechanism against the early establishment of hypertension. Therefore, we are concluding that chronic exposure to HgCl2 in prehypertensive animals could enhance the risk for cardiovascular diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mercury is a heavy metal used in human activities and is associated with health risks [1,2,3,4,5]. The increasing contamination of the environment with this toxic metal has been attributed to the processes of industrialization and urbanization [6]. Today, the main sources of human mercury exposure are exposure to mercury vapor from dental amalgams; consumption of contaminated fish; vaccination with vaccines containing thimerosal (used as a preservative); contact with insecticides, paints, and cosmetics; and gold digging in mining camps [3, 7,8,9]. The extent of mercury toxicity depends on its chemical form, dose and exposure duration [10, 11]. Once in the human body, mercury can cause damage to different organs and systems, such as the kidneys, liver, central nervous system, and respiratory and cardiovascular systems [2, 9, 12,13,14,15,16,17,18]. In the cardiovascular system, chronic or acute exposure to different forms of mercury has been associated with the development of hypertension, atherosclerosis, acute myocardial infarction and stroke [2, 7, 19].
Some studies have explored the effects of mercury chloride (HgCl2), an inorganic mercury form, on vascular beds. Chronic HgCl2 exposure of normotensive rats increases vascular reactivity and reduces nitric oxide (NO) bioavailability due to increased reactive oxygen species (ROS) production in both conductance and resistance arteries [20,21,22]. It is well known that imbalances between ROS formation and antioxidant capacity due to ROS overproduction cause endothelial dysfunction [13, 14, 23]. It has also been observed that chronic HgCl2 exposure of normotensive rats to doses similar to those in exposed humans increases contractile responses by increasing the production of cyclooxygenase 2 (COX-2)-derived prostanoids [24, 25]. The activation of this inflammatory and oxidative pathway induces structural alterations, as confirmed by the proliferation of vascular smooth muscle cells (VSMCs) in mesenteric resistance arteries (MRAs) [26]. Such effects increase vascular resistance and consequently increase blood pressure. However, chronic exposure of normotensive rats to HgCl2 for 30 days did not change systolic blood pressure (SBP) [20, 22, 27], but 60 days and 180 days of exposure promoted a significant increase in SBP [12, 27].
Despite these results, the effects of chronic exposure to HgCl2 at similar concentrations to those found in exposed humans on the development of hypertension in spontaneously hypertensive rats (SHRs) are still unknown. It is not known if chronic HgCl2 exposure in prehypertensive animals could alter the time course of arterial hypertension, accelerating its development, and which vascular changes would be associated with such effect. Thus, the aim of this study was to investigate the effects of HgCl2 on the vascular reactivity of MRAs and on SBP in young SHRs, especially during the prehypertensive phase.
Materials and Methods
Animals
1-month-old male SHRs that had recently been weaned were obtained from the Animal Quarters of the Health Center of the Federal University of Espírito Santo (CCS-UFES). All experiments were conducted according to the research guidelines established by the Brazilian Societies of Experimental Biology and were approved by the institutional Ethics Committee in Animal Research (CEUA 09/2018). The rats were kept in cages at a controlled temperature under a light–dark cycle of 12–12 h with free access to water and feed. The animals were randomly allocated into two groups: the SHR control group (vehicle) and the SHR HgCl2 group (HgCl2). The SHR control group received intramuscular injections of 0.9% NaCl for 30 days, while the SHR HgCl2 group received intramuscular injections of HgCl2 for the same period to achieve a final plasma concentration of approximately 29 nM, with an initial dose of 4.6 μg/kg and subsequent doses of 0.07 μg/kg/day according to the model of Wiggers et al. [28]. Intramuscular administration was used to better control final plasma concentration reached. The doses were adjusted weekly based on the weights of the rats.
Indirect Measurement of SBP and Body Weight
Noninvasive measurement of SBP was performed on the first day of treatment (onset) and then weekly until the end of the 30-day treatment (weeks 1, 2, 3 and 4) according to the methods of Grizzo and Cordellini [29]. Measurements were performed with a tail plethysmograph (IITC Life Science—23924 Victory Blvd, Woodland Hills, CA). Before the beginning of pressure measurement, the animals were subjected to a 3-day acclimation period. Three measurements were taken from each rat, and the mean was used. We also investigated whether treatment with HgCl2 interfered with normal weight gain. For this, the rats were weighed weekly.
Evaluation of Cardiac Hypertrophy
At the end of 30 days, the animals were anesthetized with intraperitoneal doses of ketamine (50 mg/kg) and xylazine (10 mg/kg). After thoracotomy, the left ventricle (LV) was carefully removed from the heart, dried in an oven at 37 °C for 24 h and weighed. The tibia was removed with the purpose of estimating the bone growth and was measured with a pachymeter. The ratio of LV dry weight (g) to tibial length (mm) was calculated. This ratio was used to normalize the weight of the LV and to determine whether cardiac hypertrophy was present.
In Vitro Analysis of Vascular Reactivity
After euthanasia, the third branch of the MRA was selected and immersed in Krebs–Henseleit solution (KHS, in mM: 115 NaCl, 25 NaHCO3, 4.7 KCl, 1.2 MgSO4 7H2O, 2.5 CaCl2, 1.2 KH2PO4, 11.1 glucose and 0.01 Na2 EDTA) at 4 °C. The branch of the MRA was segmented into rings of 2 mm width, and the segments were mounted in a wire myograph for force measurements (Model Myo Tech Danish, Model 410A and 610M, JP-Trading I/S, Aarhus, Denmark). The rings were stretched until a tension was achieved that was considered optimal with respect to their internal diameter. For this, in each artery, the internal stress-to-diameter relationship was calculated, and the internal circumference was determined corresponding to a transmural pressure of 100 mmHg for a relaxed vessel (Mulvany and Halpern 1977). After 30 min of rest in oxygenated KHS solution heated to 37 °C with a pH 7.4, the rings were exposed to 120 mM potassium chloride (KCl) to evaluate the functionality of the vascular smooth muscle. Then, the presence of intact endothelium was confirmed by 80% acetylcholine-induced relaxation of the vessels after precontraction with phenylephrine. After 30 min of stabilization, phenylephrine (10−8 to 10−3 M) dose–response curves were created, and the effects of L-NAME (100 μM; a nonspecific nitric oxide synthase inhibitor), indomethacin (5 μM; a nonspecific COX inhibitor), CAY 10441 (1 mM; a prostacyclin receptor antagonist), SQ 29.548 (1 μM; a thromboxane A2 receptor antagonist), SC 19220 (10 μM; a prostaglandin E2 receptor 1 antagonist), apocynin (30 μM; an NADPH oxidase inhibitor), catalase (1000 U/mL; a hydrogen peroxide scavenger) and tetraethylammonium (2 mM; a nonspecific potassium channel blocker) were evaluated. Acetylcholine (10−11 to 10−5 M) and sodium nitroprusside (10−11 to 10−5 M) concentration–response curves were also analyzed in control groups to evaluate the endothelium-dependent and endothelium-independent relaxation, respectively.
In Situ Detection of Superoxide Anion Production
The oxidative fluorescent dye dihydroethidium (DHE, Sigma-Aldrich, St. Louis, MO, USA) was used to evaluate the vascular production of superoxide anion (O ·−2 ) in situ as previously described [22, 30]. The MRA rings were frozen, cut crosswise into section 10 μm in diameter and mounted on glass slides for 30 min at 37 °C in Krebs-HEPES buffer (in mM: 130 NaCl, 5.6 KCl, 2 CaCl2, 0.24 MgCl2, 8.3 HEPES and 11 glucose, pH 7.4). Then, they were exposed to a buffer with DHE (2 μM) and incubated for 30 min in a wet chamber at 37 °C. Digital images were acquired with a magnification of × 400 by optical fluorescence microscopy (Nikon Eclipse Ti, Melville, NY, USA). Images of the SHR control and HgCl2 groups were obtained with the same adjustments and analyzed using MetaMorph software (Molecular Devices LLC, San Jose, Califórnia, USA).
Nitric Oxide Production
The in situ production of NO was determined using 4,5-diaminofluorescein (DAF-2). The dissected MRAs were frozen and sectioned in a cryostat as described by Ribeiro Júnior et al. [31]. Next, these sections were incubated at 37 °C with 8 mM DAF-2 in phosphate buffer (0.1 M) containing CaCl2 (0.45 mM). After 30 min, digital images were acquired with a magnification of × 400 by optical fluorescence microscopy (Nikon Eclipse Ti, Melville, NY, USA). The images were analyzed using MetaMorph software.
Western Blot Analysis
Frozen samples of MRAs were sonicated with ice-cold RIPA buffer (Sigma-Aldrich, St Louis, USA). The lysate was centrifuged at 6000 rpm, the supernatant was collected, and the protein concentration was determined by Bradford assay (Bio-Rad). Laemmli solution was added to aliquots containing 40 µg of protein from each animal, and the samples were loaded into 7.5, 10 or 12% acrylamide TGX Stain-Free Gels (Bio-Rad). The proteins were separated by electrophoresis in a Mini-PROTEAN Tetra cell system (Bio-Rad) for 2 h at 120 V in a running buffer containing 140 mM glycine, 37 mM Tris base and 1% sodium dodecyl sulfate (Sigma-Aldrich, St Louis, USA). The gels were then activated by UV irradiation (ChemiDoc XRS+ Imaging System, Bio-Rad) for 1 min, which produced a fluorescent signal from the tryptophan residues present on the proteins. The separated proteins were transferred to nitrocellulose membranes (Bio-Rad) for 18 h at 0.25 A in an ice-cold transfer solution containing 140 mM glycine, 37 mM Tris base and 20% methanol. The total protein content for each sample was determined by imaging the fluorescence emission (ChemiDoc XRS+ , Bio-Rad). The membranes were blocked with 5% skimmed milk (Molico, Nestlé) in Tris-buffered solution with Tween 20 (TBST) for 1 h under agitation at room temperature and incubated with primary antibodies diluted in TBST, including anti-Gp91Phox (1:700, BD Transduction Laboratories, San Jose, USA), anti-COX 2 (1:800, Cayman Chemical, Ann Arbor, MI, USA), catalase (1:14000, Sigma-Aldrich, St Louis, USA) and anti-peNOS Ser1177 (1:500, BD Transduction Laboratories, San Jose, USA), under agitation overnight at 4 °C. After being thoroughly washed, the membranes were incubated with StrepTactin-HRP conjugate (1:5000) and with anti-mouse (1:5000, Sigma-Aldrich, St Louis, USA) or anti-rabbit (1:5000, Sigma-Aldrich, St Louis, EUA) secondary antibodies in 3% bovine serum albumin (Sigma-Aldrich, St Louis, USA) in TBST for 1 h with agitation before again being washed with TBST. A chemiluminescent substrate (0.2 mM coumaric acid, 1.25 mM luminol, 0.1 M Tris–HCl and 0.06% hydrogen peroxide) was added to the membranes, and images were obtained with a ChemiDoc XRS+ system. The intensity of luminescence was quantified and normalized by the total protein content of the sample using Image Lab 6.0.1 Software (Bio-Rad).
Drugs and Reagents
Mercury chloride, apocynin, sodium nitroprusside, phenylephrine hydrochloride, acetylcholine chloride, L-NAME, catalase, tetraethylammonium (TEA), indomethacin and DHE were purchased from Sigma-Aldrich (St Louis, MO, USA). CAY 10441, SC 19220 and SQ 29.548 were purchased from Cayman Chemical. Potassium chloride was obtained from Merck. Xylazine was obtained from Ceva. Ketamine was obtained from Vetnil. The primary antibodies anti-Gp91Phox and anti-peNOS Ser1177 were obtained from BD Biosciences, anti-COX-2 was obtained from Cayman Chemical, and anti-catalase was obtained from Sigma-Aldrich. Salts and reagents, when not specified, were of analytical grade and were obtained from Sigma-Aldrich and Merck (Darmstadt, Germany).
Statistical Analyses
The results are expressed as the mean ± SEM for the number of rats in each sample group. The vasoconstriction responses induced by phenylephrine are expressed as the percent contraction at 120 mM KCl. The endothelium-dependent and endothelium-independent vasodilator responses are expressed as a percentage of the precontraction with phenylephrine. The data were analyzed using Student’s t test or two-way ANOVA followed by Bonferroni’s test. To compare the magnitudes of the effects of treatment on the contractile vascular response, the differences in the area under the curve (dAUC) between groups were used. The area under the curve (AUC) was calculated with a computer program (GraphPad Prism 6, GraphPad Software, Inc., San Diego, CA), and the differences are expressed as the percent dAUC between the control and experimental groups. For protein levels, the data are expressed as the ratio between signals on the immunoblot corresponding to the studied protein and the total protein. P < 0.05 was considered to indicate significance.
Results
Body Weight
Body weight values were similar between the groups at beginning and at the end of treatment (control = 178 ± 23.5 g vs. HgCl2 = 184 ± 16.9 g, n = 24, P > 0.05), showing that the 30-day exposure to HgCl2 did not influence body weight gain.
Evaluation of SBP
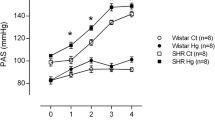
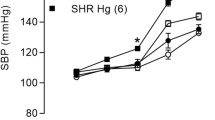
SBP increased from the first week of treatment, and exposure to HgCl2 significantly accelerated the development of hypertension in the exposed group (Table 1).
Cardiac Hypertrophy
The degree of cardiac hypertrophy, if present, was estimated by the ratio of the dry weight of the left ventricle (g) to the tibia length (mm). The LV weight, tibial length and VE/tibia length ratio did not differ between the studied groups (control: 3.8 ± 0.2 mg/mm, n = 10; HgCl2: 3.4 ± 0.09 mg/mm, n = 10; P > 0.05).
Effects of HgCl2 on Vasoconstrictor and Vasodilator Responses
Chronic exposure to HgCl2 did not interfere with the vascular response to KCl (control: 2.28 ± 0.16 mN/mm, n = 9; HgCl2: 2.26 ± 0.27 mN/mm, n = 9; P > 0.05). In contrast, significantly lower reactivity to phenylephrine was observed in the HgCl2-exposed group than in the control group (Fig. 1a). There were no differences in the vasodilator responses induced by acetylcholine or sodium nitroprusside (Fig. 1b, c respectively) in MRA segments in any of the studied groups.
Effects of chronic mercury exposure on the vascular reactivity of MRAs. Concentration–response curves for phenylephrine (a), acetylcholine (b) and sodium nitroprusside (c) in MRA segments from the control and HgCl2 groups. The number of animals used is indicated in parentheses. The results (mean ± SEM) are expressed as the percent contraction induced by 120 mM KCl. Two-way ANOVA followed by Bonferroni posttest: *P < 0.05 (control group vs. HgCl2 group)
Influence of NO on the Vasoconstrictor Response Induced by Phenylephrine
To investigate whether chronic exposure to HgCl2 altered the role of NO in the vasoconstrictor response to phenylephrine in MRAs, segments with intact endothelium were preincubated with L-NAME (100 μM). Incubation with L-NAME increased vascular reactivity only in the SHR HgCl2 group (Fig. 2a, b). Adding to these findings, in situ quantification of NO showed an increase in the production of this vasodilator agent in MRA rings exposed to HgCl2, although no changes in endothelial nitric oxide synthase (eNOS) levels were observed (Fig. 2c, d).
Effects of chronic mercury exposure on NO-mediated vasodilator response in MRA rings. Effect of blockade of nitric oxide synthesis with L-NAME (100 μM) on the concentration–response curve for phenylephrine in MRA segments from the control (a) and HgCl2 (b) groups in the absence and presence of L-NAME. c Vascular NO production and densitometric analysis of the Western blot results for phosphorylated eNOS (peNOS) protein levels (d) in MRAs from the control and HgCl2 groups. Representative blots are also shown. The number of animals used is indicated in parentheses. The results (mean ± SEM) for the phenylephrine response are expressed as a percentage of the contraction induced by 120 mM KCl. Two-way ANOVA followed by Bonferroni posttest: *P < 0.05 (control group vs. HgCl2 group). Unpaired t test: *P < 0.05 (control group vs. HgCl2 group)
Effect of ROS on the Vasoconstrictor Response to Phenylephrine
To analyze whether chronic exposure to HgCl2 altered the production of ROS in MRA rings, contraction due to phenylephrine was evaluated in the presence of apocynin (30 μM) and catalase (1000 U/mL). Incubation with apocynin and catalase significantly increased the vasoconstrictor response to phenylephrine in the SHR HgCl2 group, suggesting a major participation of O ·−2 and H2O2 (Fig. 3a–d). In addition, DHE analysis confirmed the increase in O ·−2 in MRAs of the SHR HgCl2 group (Fig. 3e). Western blot analysis indicated an increase in Gp91Phox levels (Fig. 3f) in the SHR HgCl2 group with no increase in catalase levels (Fig. 3g).
Mercury chloride increases vascular oxidative stress. Effect of the NADPH oxidase inhibitor apocynin (30 μM) on the concentration–response curve for phenylephrine in MRA segments from the control (a) and HgCl2 (b) groups. Effect of the H2O2 scavenger catalase (1000 U/mL) on the concentration–response curve for phenylephrine in rat MRA segments from the control (c) and HgCl2 (d) groups. e Vascular superoxide anion production in segments of MRAs and densitometric analysis of the Western blot results for Gp91Phox (f) and catalase (g) protein levels in MRAs from the control and HgCl2 groups. Representative blots are also shown. The number of animals used is indicated in parentheses. The results (mean ± SEM) for the phenylephrine response are expressed as a percentage of the contraction induced by 120 mM KCl. Two-way ANOVA followed by Bonferroni posttest: *P < 0.05 (control group vs. HgCl2 group). Unpaired t test: *P < 0.05 (control group vs. HgCl2 group)
Participation of Potassium Channels After Exposure to HgCl2 on the Vasoconstrictor Response
To better investigate the effect of chronic exposure to HgCl2 on the vascular response to phenylephrine in MRAs, we also evaluated the modulatory role of EDHF. For this, phenylephrine concentration–response curves were obtained in the presence of TEA (2 mM). However, there were no changes in contractile response in either group (Fig. 4a, b). To evaluate which vasodilatory agent, NO or H2O2, had a stronger effect, we coincubated MRAs with TEA + L-NAME or TEA + catalase and observed increases in the contractile response only in the group exposed to HgCl2 for both coincubations. A major increase after coincubation with TEA + L-NAME (Fig. 4c–f) was observed.
Influence of potassium channels on the vascular reactivity of exposed and non-mercury exposed rats. Effect of a nonspecific channel blocker for potassium, TEA (2 mM), on the concentration–response curve for phenylephrine in MRA segments from the control (a) and HgCl2 (b) groups. Effect of double blockade with TEA (2 mM) plus L-NAME (100 μM) on the concentration–response curve for phenylephrine in MRA segments from the control (c) and HgCl2 (d) groups. Effect of double blockade with TEA (2 mM) plus catalase (1000 u/mL) on the concentration–response curve for phenylephrine in MRA segments from the control (e) and HgCl2 (f) groups. The number of animals used is indicated in parentheses. The results (mean ± SEM) for the phenylephrine response are expressed as a percentage of the contraction induced by 120 mM KCl. Two-way ANOVA followed by Bonferroni posttest: *P < 0.05 (control group vs. HgCl2 group)
Influence of Prostanoids Derived from the Arachidonic Acid-Cyclooxygenase Pathway on the Vasoconstrictor Response to Phenylephrine
To investigate whether chronic HgCl2 exposure alters the participation of prostanoids derived from the arachidonic acid-cyclooxygenase pathway in the contractile response to phenylephrine, the SHR control and HgCl2 groups were incubated with indomethacin (5 μM), CAY 10441 (1 mM), SQ 29.548 (1 μM; a thromboxane A2 receptor antagonist) and SC 19220, a prostaglandin E2 receptor 1 (EP1) antagonist (10 μM). With indomethacin, there was a greater reduction in the SHR HgCl2-treated group than in the SHR control group (Fig. 5a–c), suggesting a greater participation of COX in this group with the production of a vasoconstrictor agent. This finding was reinforced by the Western blot analysis results, which indicated an increase in COX-2 levels (Fig. 5d) in the HgCl2-exposed group. CAY 10441 reduced the reactivity in both groups with equal magnitude, as shown in Fig. 6a–c, but the presence of SQ 29.548 did not alter the contractile response in the SHR HgCl2 group (Fig. 6d, e). Such results suggest that thromboxane A2 (TXA2) was not involved. In contrast, SC 19220 promoted a greater reduction in the SHR control group than in the SHR HgCl2 group (Fig. 6f–h).
The role of cyclooxygenase on vascular reactivity in exposed and non-mercury-exposed rats. Effect of the nonspecific COX inhibitor indomethacin (10 μM) on the concentration–response curve for phenylephrine in MRA segments from the control (a) and HgCl2 (b) groups. Differences in the area under the concentration–response curves (dAUCs) in the presence and absence of indomethacin (c). Densitometric analysis of the Western blot results for COX-2 protein levels (d) in MRAs from the control and HgCl2 groups. Representative blots are also shown. The number of animals used is indicated in parentheses. The results (mean ± SEM) for the phenylephrine response are expressed as a percentage of the contraction induced by 120 mM KCl. Two-way ANOVA followed by Bonferroni posttest: *P < 0.05 (control group vs. HgCl2 group). Unpaired t test: *P < 0.05 (control group vs. HgCl2 group)
Effects of chronic mercury exposure on the role of prostanoids derived from the COX pathway in MRA segments. Effect of the prostacyclin receptor antagonist CAY 10441 (100 nM) on the concentration–response curve for phenylephrine in MRA segments from the control (a) and HgCl2 (b) groups. Effect of SQ 29.548 (1 μM), a TXA2 receptor antagonist, on the concentration–response curve for phenylephrine in MRA segments from the control (d) and HgCl2 (e) groups. Effect of the EP1 receptor antagonist SC 19.220 (10 μM) on the concentration–response curve for phenylephrine in MRA segments from the control (f) and HgCl2 (g) groups. Differences in the area under the concentration–response curves (dAUCs) in the presence and absence of CAY 10441 (c) and SC 19.220 (h). The number of animals used is indicated in parentheses. The results (mean ± SEM) for the phenylephrine response are expressed as a percentage of the contraction induced by 120 mM KCl. Two-way ANOVA followed by Bonferroni posttest: *P < 0.05 (control group vs. HgCl2 group). Unpaired t test: *P < 0.05 (control group vs. HgCl2 group)
Discussion
Our findings showed, for the first time, that chronic intramuscular administration of HgCl2 increases SBP, accelerates the development of hypertension in young SHRs and induces a vasoprotective adaptation mechanism to support increased blood pressure. H2O2 and NO overproduction occurred to counterregulate the early augmentation of blood pressure, reducing vascular reactivity. HgCl2 also appears to reduce the participation of TXA2 and prostaglandin receptors in the contractile response to phenylephrine.
In our study, we used an experimental model developed by Wiggers et al. [22] of rats chronically exposed to controlled intramuscular doses of HgCl2 in which the final plasma HgCl2 concentration reached 8 ng/mL (29 nM), similar to concentration found in plasma of exposed humans [3]. Studies using the same model of treatment have demonstrated the occurrence of endothelial dysfunction in resistance and conductance arteries in Wistar rats [20, 22, 24, 25, 32]. Considering that chronic exposure to HgCl2 generates endothelial dysfunction in adult normotensive rats, which is a risk factor for hypertension, we analyzed the impact of this same model of treatment on young SHRs before the establishment of hypertension. Although the studies above demonstrated the presence of endothelial dysfunction, there was no change in SBP after 30 days of exposure in adult normotensive rats. In contrast, increased SBP was observed after treatment regimens of 180 and 60 days [12, 27]. In our study, a rapid increase in SBP was observed as early as the first week of treatment, indicating that chronic exposure to HgCl2 increased SBP and accelerated the development of hypertension in young SHRs, which has not been observed in adult SHRs [33].
Arterial hypertension imposes an increased afterload on the myocardium due to the elevation of peripheral vascular resistance [34] and a relationship between cardiac hypertrophy and ventricular dysfunction resulting from increased SBP levels in adult SHRs has been reported [35]. However, in our study, despite the increase in SBP in exposed young SHRs, the animals did not present cardiac hypertrophy at this stage of development.
Given that HgCl2 exposure altered the natural course of development of hypertension, we investigated the effects of this metal on the resistance of the vascular bed. According to our findings, chronic exposure to HgCl2 reduced the vasoconstrictor response to phenylephrine, and although it has been shown that young SHRs may exhibit reduced vascular reactivity compared to age-matched control rats, HgCl2 exposure appears to favor this occurrence [36,37,38,39,40,41]. However, HgCl2 exposure did not alter the vasodilatory responses to acetylcholine and sodium nitroprusside. These findings suggest that prehypertensive SHRs may have both intact endothelium-dependent and intact endothelium-independent relaxation mechanisms [39, 42, 43]. Similarly, in adult Wistar rats, alterations caused by HgCl2 were not observed in the independent relaxation of the endothelium; however, acetylcholine relaxation was impaired in the groups treated with the metal [22, 24].
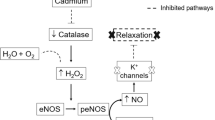
To understand the reduction in the vasoconstrictor response to phenylephrine, we investigated the effects of HgCl2 on NO modulation. SHRs present greater activation of endothelium-dependent vasodilatory mechanisms, such as increased NO production and increased levels of eNOS and inducible nitric oxide synthase (iNOS), in conductance and resistance vessels than do other types of rats. These changes can also be found in 10-weeks-old SHRs as part of an adaptive process against hypertension [44,45,46,47,48]. In our study, the vascular response to phenylephrine did not change in the SHR control group after incubation with L-NAME, suggesting that there was no increase in NO production, possibly because the animals were very young and their SBP was still low. However, after chronic HgCl2 exposure, increased vascular reactivity to phenylephrine was observed upon L-NAME incubation, indicating that, even in very young SHRs, HgCl2 exposure increased NO production in resistance arteries. Supporting our functional findings, in situ-quantified NO levels were higher in the HgCl2-exposed group than in the control group, which is consistent with the fact that the SHR HgCl2 group had an even higher blood pressure than the control group, possibly increasing the vasodilator protection mechanism. This result might be related to the reduction in reactivity observed in the SHR HgCl2 group. Despite the increased NO production in HgCl2-exposed rats, there was no difference in eNOS levels between the groups. Thus, our finding may have been due to the involvement of iNOS, an isoform expressed in inflammatory conditions, such as hypertension [49, 50] and HgCl2 contamination [51].
Given that HgCl2 exposure for 30 days increases ROS production in adult normotensive rats [20,21,22, 24, 28, 52], we investigated the effects of HgCl2 on this pathway in young SHRs. Incubation with apocynin, in situ quantification of O ·−2 and Gp91Phox protein levels suggested that O ·−2 production was increased in the SHR HgCl2 group compared to the SHR control group. In addition, the same group showed increased reactivity when incubated with catalase, suggesting an increase in the production of H2O2, which acts as a vasodilator agent [53,54,55], therefore, HgCl2 exposure augments oxidative stress in prehypertensive SHRs. These results can be explained by the fact that in SHR HgCl2 group, HgCl2 tended to increase ROS, and HgCl2 also appears to increase the levels of superoxide dismutase (SOD) as a compensatory mechanism to enable metabolism of the O ·−2 molecule to H2O2 [56]; this effect can be observed in adult SHRs but not in prehypertensive young SHRs, as we have shown in the control SHR group [57, 58]. As we did not find changes in catalase levels, the increased H2O2 production may have been associated with a decrease in catalase activity.
In addition to NO, hydrogen peroxide, a factor derived from the endothelium, seems to contribute to vasodilation, perhaps by hyperpolarization through the activation of potassium channels [55]. Given that the contribution of EDHF to endothelium-dependent vasodilation is more important in resistance vessels than in conductance vessels [59], we incubated our arteries with TEA to evaluate the role of potassium channels. There was no change in vascular reactivity in either group. To investigate the effect of H2O2 on the potassium channels in the HgCl2-exposed group, we coincubated the samples with catalase and TEA. The results after coincubation suggested that H2O2 was acting by another pathway since, even after blocking of potassium channels, a vasodilatory response remained. Studies have shown that another vasodilator action of H2O2 could take place through activation of soluble guanylate cyclase [53].
Since HgCl2 increased the production of two vasodilatory agents, NO and H2O2, we investigated which was the major determinant of the reduction in vascular reactivity to phenylephrine. For this, we also incubated the arteries with TEA plus L-NAME, which caused a significant increase in reactivity greater than that caused by the incubation with TEA plus catalase, suggesting a higher participation of NO than H2O2 in the vasodilatory response.
Among some specificities of SHR models, the COX pathway is highly activated, since arterial hypertension itself increases the participation of vasoconstrictor prostanoids derived from COX in resistance arteries [60, 61]. Moreover, previous studies have demonstrated increased participation of COX-derived vasoconstrictor prostanoids due to chronic exposure to low doses of HgCl2 in normotensive adult rats [20, 24, 25, 27]. However, the participation of COX in young prehypertensive SHRs has never been described. Upon incubating the MRAs with indomethacin, we observed a reduction in the contractile response to phenylephrine in both groups, but the reduction was greater in the HgCl2 group, suggesting COX participation in arteries from the SHR HgCl2 group. This result was confirmed by the increase in COX-2 levels.
Considering that HgCl2, treatment reduced vascular reactivity to phenylephrine but increased COX-2 levels, we investigated the participation of prostacyclin (PGI2). The rings were incubated with a PGI2 receptor antagonist, and a similar reduction in vascular reactivity to phenylephrine was found in both groups. PGI2 is an antithrombotic and antiplatelet agent and is usually a vasodilator [62,63,64,65]; however, in some cardiovascular diseases, such as hypertension, PGI2 can also act as an endothelial-derived contraction factor by activating TXA2 and prostaglandin receptors, contributing to endothelial dysfunction [66,67,68,69]. This fact, coupled with the fact that young SHRs present PGI2 receptor dysfunction due to impairment of adenylate cyclase stimulation [67], may help to explain our results. According to our findings, PGI2 did not participate in the reduction in reactivity observed in the SHR HgCl2 group. We then investigated TXA2 and prostaglandin receptors.
Pecanha et al. [24] demonstrated that 30-day exposure to HgCl2 increased prostaglandin E2 (PGE2) and TXA2 in the vasoconstrictor response to phenylephrine in the aortas of adult normotensive rats. Interestingly, when we blocked the TXA2 receptor, the SHR control group showed a small reduction in reactivity, while no changes were observed in the HgCl2-exposed group, suggesting that TXA2 was not playing a role in the MRAs of young SHRs. Blockade of EP1 receptors strongly reduced the reactivity in the SHR control group, but in the HgCl2-treated group, the reduction in reactivity was smaller. Taken together, these findings show that the reduction in reactivity in the SHR HgCl2 group was due to increases in NO and H2O2 production.
In conclusion, our study shows, for the first time, that chronic exposure to HgCl2 increases blood pressure and accelerates the development of hypertension in young SHRs. This process seems to potentiate a mechanism of adaptation in these animals to support high blood pressures that involves increased generation of reactive oxygen species, specifically H2O2, and NO. Together, these agents promote reductions in vascular reactivity to counterbalance the increase in blood pressure. In addition, in young SHRs, the COX-2 pathway does not contribute to the hypertension and vascular alteration found after HgCl2 exposure. Finally, these findings show that chronic exposure to HgCl2 could be a risk factor for cardiovascular diseases, as it accelerates hypertension development in presposed individuals.
References
Langworth, S. (1997). Exposure to mercury vapor and impact on health in the dental profession in Sweden. Journal of Dental Research,76(7), 1397–1404.
Salonen, J. T., Seppänen, K., Lakka, T. A., Salonen, R., & Kaplan, G. A. (2000). Mercury accumulation and accelerated progression of carotid atherosclerosis: A population-based prospective 4-year follow-up study in men in eastern Finland. Atherosclerosis,148(2), 265–273.
Clarkson, T. W., Magos, L., & Myers, G. J. (2003). The toxicology of mercury—Current exposures and clinical manifestations. New England Journal of Medicine,349(18), 1731–1737.
McKelvey, W., Gwynn, R. C., Jeffery, N., Kass, D., Garg, R. K., Thorpe, L. E., et al. (2007). A biomonitoring study of lead, cadmium, and mercury in the blood of New York city adults. Environmental Health Perspectives,115(10), 1435–1441.
Virtanen, J. K., Rissanen, T. H., Voutilainen, S., & Tuomainen, T. P. (2007). Mercury as a risk factor for cardiovascular diseases. Journal of Nutritional Biochemistry,18(2), 75–85.
Bastami, K. D., Bagheri, H., Kheirabadi, V., Hamzehpoor, A., Ghorghani, N. F., Harami, S. R. M., et al. (2014). Distribution and ecological risk assessment of heavy metals in surface sediments along southeast coast of the Caspian Sea. Marine Pollution Bulletin,81(1), 262–267. https://doi.org/10.1016/j.marpolbul.2014.01.029.
Virtanen, J. K., Voutilainen, S., Rissanen, T. H., Mursu, J., Tuomainen, T. P., Korhonen, M. J., et al. (2005). Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in Eastern Finland. Arteriosclerosis, Thrombosis, and Vascular Biology,25(1), 228–233.
Clarkson, T. W., Vyas, J. B., & Ballatori, N. (2007). Mechanisms of mercury disposition in body. American Journal of Industrial Medicine,50(10), 757–764.
Salonen, J. T., Seppänen, K., Nyyssönen, K., Korpela, H., Kauhanen, J., Kantola, M., et al. (1995). Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in Eastern Finnish men. Circulation,91(3), 645–655.
Clarkson, T. W. (1972). The biological properties and distribution of mercury. Biochemical Journal,130(2), 61–63.
Zalups, R. K., & Lash, L. H. (1994). Invited review: Advances in understanding the renal transport and toxicity of mercury. Journal of Toxicology and Environment Health,42(1), 01–44.
Carmignani, M., & Boscolo, P. (1984). Cardiovascular homeostasis in rats chronically exposed to mercuric chloride. Archives of Toxicology. Supplement,7, 383–388. https://doi.org/10.1007/978-3-642-69132-4_66.
Kishimoto, T., Oguri, T., & Tada, M. (1995). Effect of methylmercury (CH3HgCl) injury on nitric oxide synthase (NOS) activity in cultured human umbilical vascular endothelial cells. Toxicology,103(1), 1–7.
Kishimoto, T., Oguri, T., Abe, M., Kajitani, H., & Tada, M. (1995). Inhibitory effect of methylmercury on migration and tube formation by cultured human vascular endothelial cells. Archives of Toxicology,69(6), 357–361.
Weinsberg, F., Bickmeyer, U., & Wiegand, H. (1995). Effects of inorganic mercury (Hg2 +) on calcium channel currents and catecholamine release from bovine chromaffin cells. Archives of Toxicology,69(3), 191–196.
Lim, H. E., Shim, J. J., Lee, S. Y., Lee, S. H., Kang, S. Y., Jo, J. Y., et al. (1998). Mercury inhalation poisoning and acute lung injury. Korean Journal of Internal Medicine,13(2), 127–130.
Boffetta, P., Sallsten, G., & Garcia-Gomez, M. (2001). Mortality from cardiovascular diseases and exposure to inorganic mercury. Occupational and Environmental Medicine,58, 461–466.
Omanwar, S., Ravi, K., & Fahim, M. (2011). Persistence of EDHF pathway and impairment of the nitric oxide pathway after chronic mercury chloride exposure in rats: Mechanisms of endothelial dysfunction. Human and Experimental Toxicology,30(11), 1777–1784.
Houston, M. C. (2011). Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. Journal of Clinical Hypertension (Greenwich),13(8), 621–627.
Lemos, N. B., Angeli, J. K., Faria, T. O., Ribeiro Junior, R. F., Vassallo, D. V., Padilha, A. S., et al. (2012). Low mercury concentration produces vasoconstriction, decreases nitric oxide bioavailability and increases oxidative stress in rat conductance artery. PLoS ONE,7(11), e49005.
Furieri, L. B., Galán, M., Avendaño, M. S., García-Redondo, A. B., Aguado, A., Martínez, S., et al. (2011). Endothelial dysfunction of rat coronary arteries after exposure to low concentrations of mercury is dependent on reactive oxygen species. British Journal of Pharmacology,162(8), 1819–1831.
Wiggers, G. A., Peçanha, F. M., Briones, A. M., Pérez-Girón, J. V., Miguel, M., Vassallo, D. V., et al. (2008). Low mercury concentrations cause oxidative stress and endothelial dysfunction in conductance and resistance arteries. American Journal of Physiology-Heart and Circulatory Physiology,295(3), 1033–1043.
Félétou, M., & Vanhoutte, P. M. (2006). Endothelial dysfunction: A multifaceted disorder (The Wiggers Award Lecture). American Journal of Physiology-Heart and Circulatory Physiology,291(3), 985–1002.
Pecanha, F. M., Wiggers, G. A., Briones, A. M., Perez-Giron, J. V., Miguel, M., Garcia-Redondo, A. B., et al. (2010). The role of cyclooxygenase (COX)-2 derived prostanoids on vasoconstrictor responses to phenylephrine is increased by exposure to low mercury concentration. Journal of Physiology and Pharmacology,61(1), 29–36.
Rizzetti, D. A., Torres, J. G., Escobar, A. G., Peçanha, F. M., Santos, F. W., Puntel, R. L., et al. (2013). Apocynin prevents vascular effects caused by chronic exposure to low concentrations of mercury. PLoS ONE,8(2), e55806.
Aguado, A., Galán, M., Zhenyukh, O., Wiggers, G. A., Roque, F. R., Redondo, S., et al. (2013). Mercury induces proliferation and reduces cell size in vascular smooth muscle cells through MAPK, oxidative stress and cyclooxygenase-2 pathways. Toxicology and Applied Pharmacology,268(2), 188–200. https://doi.org/10.1016/j.taap.2013.01.030.
Rizzetti, D. A., Torres, J. G., Escobar, A. G., da Silva, T. M., Moraes, P. Z., Hernanz, R., et al. (2017). The cessation of the long-term exposure to low doses of mercury ameliorates the increase in systolic blood pressure and vascular damage in rats. Environmental Research,155, 182–192. https://doi.org/10.1016/j.envres.2017.02.022.
Wiggers, G. A., Stefanon, I., Padilha, A. S., Peçanha, F. M., Vassallo, D. V., & Oliveira, E. M. (2008). Low nanomolar concentration of mercury chloride increases vascular reactivity to phenylephrine and local angiotensin production in rats. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology,147(2), 252–260.
Grizzo, L. T., & Cordellini, S. (2008). Perinatal lead exposure affects nitric oxide and cyclooxygenase pathways in aorta of weaned rats. Toxicological Sciences,103(1), 207–214.
Ribeiro Júnior, R. F., Pavan, B. M., Fiorim, J., Simoes, M. R., Dias, F. M., Lima, F. L., et al. (2012). Myocardial contractile dysfunction induced by ovariectomy requires AT 1 receptor activation in female rats. Cellular Physiology and Biochemistry,30(1), 1–12.
Ribeiro Júnior, R. F., Marques, V. B., Nunes, D. O., de Ronconi, K. S., de Araújo, J. F. P., Rodrigues, P. L., et al. (2016). Tributyltin chloride increases phenylephrine-induced contraction and vascular stiffness in mesenteric resistance arteries from female rats. Toxicology and Applied Pharmacology,295, 26–36. https://doi.org/10.1016/j.taap.2016.02.005.
Vassallo, D. V., Simões, M. R., Furieri, L. B., Fioresi, M., Fiorim, J., & Almeida, E. A. S. (2011). Toxic effects of mercury, lead and gadolinium on vascular reactivity. Brazilian Journal of Medical and Biological Research,44(9), 939–946.
Vassallo, D. V., Azevedo, B. F., Giuberti, K., Simões, M. R., Ribeiro Junior, R. F., & Salaices, M. (2018). Effects of chronic exposure to mercury on angiotensin-converting enzyme activity and oxidative stress in normotensive and hypertensive rats. Arquivos Brasileiros de Cardiologia,112, 1–7.
Bristow, M. R. (1999). Mechanisms of development of heart failuve in the hypertensive patient. Cardiology,92, 3–6.
Bing, O. H. L., Brooks, W. W., Robinson, K. G., Slawsky, M. T., Hayes, J. A., Litwin, S. E., et al. (1995). The spontaneously hypertensive rat as a model of the transition from compensated left ventricular hypertrophy to failure. Journal of Molecular and Cellular Cardiology,27, 383–396. https://doi.org/10.1016/S0022-2828(08)80035-1.
Arribas, S., Gordon, J., Daly, C. J., Dominiczak, A. F., & McGrath, J. C. (1996). Confocal microscopic characterization of a lesion in cerebral vessel of the stroke-prone spontaneously hypertensive rat. Stroke,27, 1118–1123.
Mizutani, K., Ikeda, K., Kawai, Y., & Yamori, Y. (1999). Biomechanical properties and chemical composition of the aorta in genetic hypertensive rats. Journal of Hypertension,17, 481–487.
Török, J., Koprdová, R., Cebová, M., Kuneš, J., & Kristek, F. (2006). Functional and structural pattern of arterial responses in hereditary hypertriglyceridemic and spontaneously hypertensive rats in early stage of experimental hypertension. Physiological Research,55, 65–71.
Tsuda, K., Kuchii, M., Nishio, I., & Masuyama, Y. (1987). Presynaptic α2-adrenoceptor mediated regulation of norepinephrine release in perfused mesenteric vasculatures in young and adult spontaneously hypertensive rats. Japanese Circulation Journal,51, 25–32.
Szemeredi, K., Bagdy, G., Stull, R., Keiser, H. R., Kopin, I. J., & Goldstein, D. S. (1988). Sympathoadrenomedullary hyper-responsiveness to yohimbine in juvenile spontaneously hypertensive rats. Life Sciences,43(13), 1063–1068.
Zhao, Y., Vanhoutte, P. M., & Leung, S. W. S. (2012). Endothelial nitric oxide synthase-independent release of nitric oxide in the aorta of the spontaneously hypertensive rat. Journal of Pharmacology and Experimental Therapeutics,344(1), 15–22.
Ibarra, M., López-Guerrero, J. J., Mejía-Zepeda, R., & Villalobos-Molina, R. (2006). Endothelium-dependent inhibition of the contractile response is decreased in aorta from aged and spontaneously hypertensive rats. Archives of Medical Research,37(3), 334–341.
Cacanyiova, S., Berenyiova, A., Kristek, F., Drobna, M., Ondrias, K., & Grman, M. (2016). The adaptive role of nitric oxide and hydrogen sulphide in vasoactive responses of thoracic aorta is triggered already in young spontaneously hypertensive rats. Journal of Physiology and Pharmacology,67, 501–512.
Wu, C. C., Hong, H. J., Chou, T. C., Ding, Y. A., & Yen, M. H. (1996). Evidence for inducible nitric oxide synthase in spontaneously hypertensive rats. Biochemical and Biophysical Research Communications,466, 459–466.
Chou, T. C., Yen, M. H., Li, C. Y., & Ding, Y. A. (1998). Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension,31(2), 643–648.
Vaziri, N. D., Ni, Z., & Oveisi, F. (1998). Upregulation of renal and vascular nitric oxide synthase in young spontaneously hypertensive rats. Hypertension,31(6), 1248–1254.
Briones, A. M., Alonso, M. J., Marin, J., & Salaices, M. (1999). Role of iNOS in the vasodilator responses induced by l-arginine in the middle cerebral artery from normotensive and hypertensive rats. British Journal of Pharmacology,126(1), 111–120.
Briones, A. M., Balfagón, G., Marín, J., Alonso, M. J., & Salaices, M. (2000). Influence of hypertension on nitric oxide synthase expression and vascular effects of lipopolysaccharide in rat mesenteric arteries. British Journal of Pharmacology,131(2), 185–194.
Förstermann, U., Closs, E. I., Pollock, J. S., Nakane, M., Schwarz, P., Gath, I., et al. (1994). Nitric oxide synthase isozymes. Hypertension,23, 1121–1131.
Förstemann, U., Nakane, M., Tracey, W. R., & Pollock, J. (1993). Isorfoms of nitric oxide syntase: Functions in the cardiovascular system. European Heart Journal,14, 10–15.
Faria, T. O., Simões, M. R., Vassallo, D. V., Forechi, L., Almenara, C. C. P., Marchezini, B. A., et al. (2018). Xanthine oxidase activation modulates the endothelial (vascular) dysfunction related to HgCl2 exposure plus myocardial infarction in rats. Cardiovascular Toxicology,18(2), 161–174.
Azevedo, B. F., Simões, M. R., Fiorim, J., Botelho, T., Angeli, J. K., Vieira, J., et al. (2016). Chronic mercury exposure at different concentrations produces opposed vascular responses in rat aorta. Clinical and Experimental Pharmacology and Physiology,43(7), 712–719.
Hayabuchi, Y., Mori, K., Nakaya, Y., Sakamoto, S., Matsuoka, S., & Kuroda, Y. (1998). Lactate-induced vascular relaxation in porcine coronary arteries is mediated by Ca2 + -activated K + channels. Journal of Molecular and Cellular Cardiology,30(2), 349–356.
Gil-Longo, J., & González-Vázquez, C. (2005). Characterization of four different effects elicited by H2O2 in rat aorta. Vascular Pharmacology,43(2), 128–138.
Félétou, M. (2009). Calcium-activated potassium channels and endothelial dysfunction: Therapeutic options? British Journal of Pharmacology,156(4), 545–562.
Ferreira, A. L. A., & Matsubara, L. S. (1997). Radicais livres: Conceitos E Mecanismo De Lesão. Revista da Associacao Medica Brasileira Impact,43(1), 61–69.
Zalba, G., Beaumont, F. J., José, G. S., Fortuño, M. A., Fortuño, A., Etayo, J. C., et al. (2012). Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension,35(5), 1055–1061.
Gongora, M. C., Laude, K., Folz, J. R., Kim, H. W., McCann, L., Qin, Z., et al. (2006). Role of extracellular superoxide dismutase in hypertension. Hypertension,48(3), 473–481.
Urakami-Harasawa, L., Shimokawa, H., Nakashima, M., Egashira, K., & Takeshita, A. (1997). Importance of endothelium-derived hyperpolarizing factor in human arteries. Journal of Clinical Investigation,100(11), 2793–2799.
Alvarez, Y., Briones, A. M., Balfagón, G., Alonso, M. J., & Salaices, M. (2005). Hypertension increases the participation of vasoconstrictor prostanoids from cyclooxygenase-2 in phenylephrine responses. Journal of Hypertension,23(4), 767–777.
Virdis, A., Colucci, R., Fornai, M., Duranti, E., Giannarelli, C., Bernardini, N., et al. (2007). Cyclooxygenase-1 is involved in endothelial dysfunction of mesenteric small arteries from angiotensin II-infused mice. Hypertension,49, 679–686.
Moncada, S., & Vane, J. R. (1978). Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2 and prostacyclin. Pharmacological Reviews,30(3), 293–331.
Corriu, C., Félétou, M., Edwards, G., Weston, A. H., & Vanhoutte, P. M. (2001). Differential effects of prostacyclin and iloprost in the isolated carotid artery of the guinea-pig. European Journal of Pharmacology,426(1–2), 89–94.
Parkington, H. C., Coleman, H. A., & Tare, M. (2004). Prostacyclin and endothelium-dependent hyperpolarization. Pharmacological Research,49(6), 509–514.
Félétou, M., & Vanhoutte, P. (2007). Endothelium dependent hyperpolarizations: Past beliefs and presente facts. Annals of Medicine,39(7), 495–516.
Gluais, P., Lonchampt, M., Morrow, J. D., Vanhoutte, P. M., & Feletou, M. (2005). Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: The Janus face of prostacyclin. British Journal of Pharmacology,146(6), 834–845.
Gomez, E., Schwendemann, C., Roger, S., Simonet, S., Courchay, C., Paysant, J., et al. (2008). Aging and prostacyclin responses in aorta and platelets from WKY and SHR rats. American Journal of Physiology-Heart and Circulatory Physiology,295(5), 2198–2211.
Vanhoutte, P. M., & Tang, E. H. C. (2008). Endothelium-dependent contractions: When a good guy turns bad! Journal of Physiology,586(22), 5295–5304.
Liu, D., Li, H., Zhou, Y., Zhang, Y., Luo, W., & Liu, B. (2015). A vasoconstrictor response to COX-1-mediated prostacyclin synthesis in young rat renal arteries that increases in pre-hypertensive conditions. American Journal of Physiology-Heart and Circulatory Physiology,309, 804–811.
Acknowledgements
This study was supported by Grants from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) 302968/2016-4 and PRONEX-CNPq/FAPES (Fundação de Amparo a Pesquisa do Espírito Santo) 80598773. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest with the contents of this article.
Ethical Approval
All experimental procedures were performed in accordance with the guidelines for the care and handling of laboratory animals as recommended by the Brazilian Societies of Experimental Biology, and the study protocols were previously approved by the Ethics Committee in Animal Research of the Federal University of Espirito Santo (09/2018 CEUA-UFES).
Additional information
Handling Editor: Lorraine Chalifour.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fardin, P.B.A., Simões, R.P., Schereider, I.R.G. et al. Chronic Mercury Exposure in Prehypertensive SHRs Accelerates Hypertension Development and Activates Vasoprotective Mechanisms by Increasing NO and H2O2 Production. Cardiovasc Toxicol 20, 197–210 (2020). https://doi.org/10.1007/s12012-019-09545-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-019-09545-6