Abstract
Close to spherical nanosized niobium diboride particles with an average diameter of 65 nm were synthesized by the reaction of amorphous boron with niobium powder at 1073 K in an argon atmosphere in KCl and Na2B4O7 ionic melts (autoclave, argon pressure of 4 MPa, 32 h).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Niobium diboride NbB2 has a high melting point, thermodynamic stability, high values of hardness, strength, wear resistance, thermal and electrical conductivity, chemical and corrosion inertia, and hence it is used in various areas of modern industry [1, 2].
To synthesize NbB2 nanoparticles, methods developed for the production of transition metal diborides of groups IV‒VI are usually applied: high-temperature solid-phase synthesis from elements, borothermal reduction of various niobium oxides and salts, carbothermal reduction of niobium and boron oxides, or reduction of niobium and boron oxides with magnesium, reactions of niobium chlorides with alkali metal borohydrides at elevated temperatures and pressures, mechanochemical synthesis, and chemical vapor-phase deposition [3–10].
As an alternative method for the production of NbB2 nanoparticles we consider in this work the so-called current-free method [11], which is based on the current-free transfer of boron to a metal in ionic melts of various chemical compositions and structures according to the procedures previously developed for the production of TiB2 and VB2 nanoparticles [12, 13]. The use of ionic melts as a reaction medium, due to the special features of their structure and properties, makes it possible to obtain metal borides in the form of highly dispersed powders.
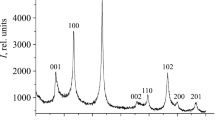
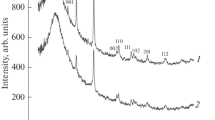
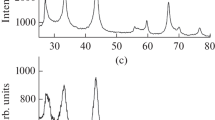
We synthesized NbB2 nanoparticles by the reaction of amorphous boron with niobium powder in ionic KCl and Na2B4O7 melts in atmosphere of argon with a pressure of 4 MPa at 1073 K and the reaction duration of 32 h. The resulting niobium diboride, according to the results of chemical and energy dispersion analyses, has the composition NbB1.97–2.01O0.01–0.03 without traces of hydrogen and chloride ions. According to the XRD data, niobium diboride crystallizes in the hexagonal syngony (space group P6/mmm, structural type AlB2, a = 0.3100‒0.3104, с = 0.3278‒0.3280 nm), the lattice parameters are consistent with published data.
The average diameters (d, nm) obtained for melts of NbB2 particles (according to electron microscopic and X-ray studies, and also by measuring specific surface area) and areas of coherent scattering (Dhkl, nm) are shown below:
NbB2 from Na2B4O7 d ~65 (electron microscopy), ~67 (Ssp = 13.0 m2/g), Dhkl ~60;
NbB2 from KСl d ~64 (electron microscopy), ~67 (Ssp = 13.0 m2/g), Dhkl ~59.
Regardless of the chemical composition and the nature of the ionic melt, the average diameter of the NbB2 powder particles is close to 65 nm.
The transfer of B to Nb observed in a Na2B4O7 or KCl ionic melt, according to [11], can be explained by the formation of lower valence B2+ ions by reaction (1) and their interaction with niobium to form NbB2 by reaction (2).
To refine the qualitative composition of the surface of NbB2 nanoparticles, their X‒ray photoelectron spectra were recorded, according to which the main component of the powders is NbB2, the electron binding energy at the Nb 3d5/2 level is 203.4 eV and at the B 1s level is 188.4 eV, which is consistent with the published data. Along with the lines characteristic of niobium diboride, weak lines corresponding to boron oxide and niobium oxide Nb2O5 were observed.
Prior to the reaction of amorphous boron with niobium powder in an ionic melt, a niobium powder with a particle size of 10–15 µm was prepared by heating a commercial niobium powder with a particle size of ~45 µm at 1173 K in a vacuum of 1.3×10–1 Pa and subjected to five hydrogenation‒dehydrogenation cycles [14]. Then the resulting niobium powder and amorphous boron in stoichiometric amounts (1 : 2, g-at) were mixed and activated in a Pulverisette 6 ball planetary mill (niobium balls, ball loading 1 : 10, rotation speed 400 rpm, processing time 40 min) in an argon atmosphere at room temperature.
The activated mixture of powdered Nb (9.21 g) and B (2.16 g) together with Na2B4O7 or KCl (14.0 g each) in a quartz ampoule was placed in a stainless steel autoclave reactor in a high‒purity argon atmosphere. The reactor was evacuated to a residual pressure of 1.3×10–1 Pa, filled with argon at a pressure of 4 MPa, and heated for 32 h at 1073 K. The reactor was then cooled to room temperature, and the reaction mixture was discharged. The speck was crushed and successively treated with distilled water, ethyl alcohol, and acetone, and evacuated to a residual pressure of 1.3×10–1 Pa. The resulting powder was again placed in the reactor, treated with hydrogen in the flow mode at a pressure of 5 MPa at 373 K, evacuated at room temperature up to a residual vacuum of 1.3×10–1 Pa, and discharged from the reactor in an argon atmosphere. All subsequent work with the niobium diboride obtained in this way, including sampling for analysis, was carried out in an argon atmosphere.
The X-ray phase analysis (XRD) of the obtained NbB2 nanoparticles was performed on an ADP-2 diffractometer (monochromatic CuKα-radiation). The error in determining the periods of the NbB2 crystal lattice did not exceed 0.0003 nm. The areas of coherent scattering of Dhkl in the direction perpendicular to the hkl plane were estimated from powder diffractograms using Scherer formula (3).
Here k is the anisotropy coefficient, which was assumed to be 0.9; λ is the X-ray wavelength (λCuKα 1.54178 Å), θ is the diffraction angle, β is the width of the diffraction peak at half its height (in radians).
Electron microscopic studies and X-ray energy-dispersion analysis were carried out on a set of instruments consisting of a Zeiss Supra 25 scanning autoemission electron microscope and an INCA X-sight X-ray spectral installation. The X-ray photoelectron (XPE) spectra were recorded on a PHOIBOS 150 MCD electronic spectrometer for chemical analysis. The specific surface area of the samples (S) was determined using a Quadrasorb SI analyzer. The source of hydrogen with a purity of at least 99.999% was an autonomous laboratory hydrogen generator containing hydride phases based on LaNi5 and TiFe intermetallides as the working material. Its operation principle is described in detail [15].
The chemical composition of NbB2 nanoparticles was determined by standard analytical methods, and also by the results of X-ray energy dispersion analysis.
REFERENCES
Carenco, S., Portehault, D., Boissiere, C., Mezailles, N., and Sanchez, C., Chem. Rev., 2013, vol. 113, no. 10, p. 7981. https://doi.org/10.1021/cr400020d
Andrievski, R.A. and Khatchoyan, A.V., Nanomaterials in Extreme Environments, Fundamentals and Applications, Berlin: Springer Int. Publ., 2016. https://doi.org/10.1007/978-3-319-2533-2
Jha, M., Ramanujachary, K.V., Lofland, S.T., Gupta, G., and Ganguli, A.K., Dalton Trans., 2011, vol. 40, p. 7879. https://doi.org/10.1039/c1dt10468c
Gai, P., Yang, Z., Shi, L., Chen, L., Zhao, A., Gu, Y., and Qian, Y., Mater. Lett., 2005, vol. 59, p. 3550. https://doi.org/10.1016/j.matlet.2005.07.051
Ma, J., Du, Y., Wu, M., Li, G., Feng, Z., Guo, M., Sun, Y., Song, W., Lin, M., and Guo, X., J. Alloys Compds., 2009, vol. 468, p. 473. https://doi.org/10.1016/j.jallcom.2008.01.021
Jothi, P.R., Yubuta, K., and Fokwa, B.P.T., Adv. Mater., 2018, vol. 30, no. 14, p. 1704181-1. https://doi.org/10.1002/adma.201704181
Portehaut, D., Devis, S., Beaunier, P., Gervais, C., Giordano, C., Sanchez, C., and Antonietti, M., Angew. Chem., 2011, vol. 50, p. 3262. https://doi.org/10.1002/ange.201006810
Jafari, M., Tajizadegan, H., Golabgir, M.H., Chami, A., and Torabio, O., Int. J. Refr. Met. Hard Mater., 2015, vol. 50, p. 86. https://doi.org/10.1016/j.ijrmhm.2014.10.017
Balci, Ö., Aĝaoĝullari, D., Övecoĝlu, M.L., and Duman, I., Trans. Nonferrous Met. Soc. China, 2016, vol. 26. P.747. https://doi.org/10.1016/S1003-6326(16)64165-1
Gupta, A., Singhal, V., and Pandey, O.P., J. Alloys Compd., 2018, vol. 736, p. 306. https://doi.org/10.1016/j.jallcom.2017.10.257
Ilyushchenko, N.G., Afinogenov, A.I., and Shurov, N.I., Vzaimodeistvie metallov v ionnykh rasplavakh (Interaction of Metals in Ionic Melts), Moscow: Nauka, 1991. 176 с.
Volkova, LS., Shulga, Yu.M., and Shilkin, S.P., Russ. J. Gen. Chem., 2012, vol. 82, no. 5, p. 819. https://doi.org/10.1134/S1070363212050027
Kravchenko, S.E., Domashnev, I.A., Dremova, N.N., Vinokurov, A.A., and Shilkin, S.P., Inorg. Mater., 2019, vol. 55, no. 5, p. 443. https://doi.org/10.1134/S002016851905011X
Fokin, V.N., Fokina, E.E., Tarasov, B.P., and Shilkin, S.P., Int. J. Hydrogen Energy, 1999, vol. 24, nos. 2–3, p. 111. https://doi.org/10.1016/S0360-3199(98)00070-6
Fokin, V.N., Fokina, E.E., and Shilkin, S.P., Zh. Obshch. Khim., 1996, vol. 66, no. 8, p. 1249.
Funding
The work was fulfilled in the framework of the state task (state registration no. АААА-А19119061890019-5) using equipment of Analytical center of collective use of the Institute of Problems of Chemical Physics of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Additional information
Translated from Zhurnal Obshchei Khimii, 2021, Vol. 91, No. 2, pp. 326–328 https://doi.org/10.31857/S0044460X21020153.
Rights and permissions
About this article
Cite this article
Kravchenko, S.E., Vinokurov, A.A., Dremova, N.N. et al. Synthesis of Niobium Diboride Nanoparticles by the Reaction of Amorphous Boron with Niobium in KCl and Na2B4O7 Ionic Melts. Russ J Gen Chem 91, 302–304 (2021). https://doi.org/10.1134/S1070363221020195
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221020195