Abstract—
Reaction between vanadium and boron powders in the stoichiometric ratio 1 : 2 at a temperature of 800°C, argon pressure of 4 MPa, and reaction time of 32 h in Na2B4O7 and KCl ionic melts has been studied by X-ray diffraction, energy dispersive X-ray analysis, scanning electron microscopy, X-ray photoelectron spectroscopy, frustrated total internal reflection IR spectroscopy, thermal analysis, and elemental analysis. The results demonstrate that, independent of the composition and nature of the melts, the reaction yields phase-pure vanadium diboride nanoparticles with an average diameter near 90 nm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Vanadium diboride, VB2, has a high melting point (~2745°C); thermodynamic stability; high hardness, strength, and wear resistance; chemical stability, corrosion resistance, etc. Owing to this, it has found a variety of industrial applications and is used in the fabrication of vanadium boride–air batteries [1–5]. In modern materials research, the interest in Group IV and V metal borides has considerably increased with the advent of nanoparticulate materials based on these borides, because the physicochemical, physicomechanical, and other properties of such materials differ significantly from those of bulk materials [6, 7]. In connection with this, the development of new effective methods for the preparation of vanadium boride nanoparticles is a topical issue.
VB2 can be prepared by the following methods: direct elemental synthesis (high-temperature sintering), borothermal reduction of various vanadium oxides and salts, carbothermal reduction of vanadium and boron oxides, mechanochemical synthesis, and plasma synthesis in a low-temperature plasma flow [8–18].
Sintering is a simple method capable of ensuring vanadium boride synthesis at a high rate [8]. However, VB2 thus prepared has the form of a fused sinter cake containing vanadium oxide and boron oxide impurities. As shown by Wei et al. [9], rather coarse VB2 powder, with a particle size under 300 nm, can be prepared by the borothermal reduction of NH4VO3 in the range 900–1000°C under an argon atmosphere in a NaCl/KCl ionic melt or with no melt according to the reaction schemes

Vanadium diboride powder can also be prepared by the borothermal reduction of vanadium oxide, V2O3, in vacuum above 1500°C by reaction (2) [10] or via a boron carbide route at a temperature of 1500°C in an argon atmosphere by reaction (3) [11].
Kim et al. [12] proposed a mechanochemical process for the synthesis of vanadium diboride in a high-energy ball mill according to the reaction scheme
The VB2 powder obtained after lithium chloride removal ranges in particle size from 15 to 60 nm.
Yeh and Wang [13] studied vanadium borides with the compositions V3B2, VB, V5B6, V3B4, V2B3, and VB2, prepared by self-propagating high-temperature synthesis from vanadium and amorphous boron powders. Vanadium boride nanopowder with a particle size of ~36 nm can be prepared by mechanochemical combustion of a mixture of Mg, V2O5, and B2O3 in a high-energy ball mill [14]. Shi et al. [15] obtained VB2 nanoparticles 50 to 100 nm in size by reacting VCl4 with NaBH4 and Mg at a temperature of 650°C in a steel autoclave reactor. VB2 nanoparticles ~10 nm in size can be prepared by reacting VCl3 with NaBH4 in a eutectic mixture of anhydrous lithium and sodium chlorides in a quartz reactor at a temperature of 900°C [16]. Using high-energy disintegration in modern milling–crushing devices, one can obtain fine powders of various compounds, including VB2 [18].
Each of the above-mentioned methods has its own advantages and disadvantages. Some of them ensure a high production rate, others make it possible to obtain vanadium diboride nanoparticles of stoichiometric composition and particular size at relatively low temperatures, etc.
As an alternative approach to the synthesis of VB2 nanoparticles, we propose a so-called “currentless” process. Basic to this approach is “currentless” vanadium transport to boron in ionic melts with various chemical compositions and structures (anhydrous sodium tetraborate or potassium chloride) using a technique developed previously for the synthesis of TiB2 nanoparticles [19]. Owing to specific features of their structure and properties, the use of ionic melts as reaction media makes it possible to obtain metal borides in the form of fine powders.
EXPERIMENTAL
Starting chemicals. Vanadium powder 10 to 15 μm in particle size was prepared as follows: off-the-shelf vanadium powder was activated by heating at 900°C in a vacuum of 1.3 × 10–1 Pa and then subjected to five hydrogenation–dehydrogenation cycles as described elsewhere [20, 21]. The residual hydrogen content of the powder was within 1.0 × 10–3% and the residual oxygen content did not exceed 3.0 × 10–3%. As a source of 99.999+%-pure hydrogen, we used a self-contained laboratory-scale hydrogen generator containing hydrided TiFe and LaNi5 intermetallic phases as working materials. Its operating principle was described in detail previously [22, 23]. In our preparations, we also used reagent-grade potassium chloride and high-purity (99.998%) argon (Russian Federation Purity Standard TU 2114-005-0024760-99). Commercially available amorphous boron (B 99A, Russian Federation Purity Standard TU 1-92-154-90) 10 to 20 μm in particle size was held in vacuum at a residual pressure of 1.3 × 10–1 Pa at a temperature of 300°C. Anhydrous sodium tetraborate was obtained by holding commercially available reagent-grade Na2B4O7 · 5H2O in a vacuum of 1.3 × 10–1 Pa at a temperature of 350°C.
Characterization techniques. The phase composition of powder samples was determined by X-ray diffraction on an ADP-2 diffractometer (monochromatized CuKα radiation). The lattice parameters of VB2 were determined with an accuracy of 0.0003 nm or better. Using X-ray powder diffraction data, we estimated the crystallite size along the normal to an hkl plane, Dhkl, by the Scherrer formula:
where k is the anisotropy coefficient (which was taken to be 0.9 in this study), λ is the X-ray wavelength (\({{\lambda }_{{{\text{Cu}}{{K}_{{\alpha }}}}}}\) = 1.54178 Å), θ is the diffraction angle, and β is the full width at half maximum of the diffraction peak (in radians).
IR frustrated total internal reflection (FTIR) spectra were measured in the range from 500 to 4000 cm–1 using a PerkinElmer Spectrum 100 Fourier transform spectrometer and a Vertex 70V spectrometer, both equipped with accessories for taking reflection spectra.
The powders were characterized by simultaneous thermal analysis in a Netzsch STA 409 PC Luxx thermoanalytical system in combination with a QMS 403 C Aëolos mass spectrometer at a constant heating rate of 10°C/min under flowing argon in the temperature range from 20 to 1000°C.
The samples were also examined by electron microscopy and energy dispersive X-ray (EDX) analysis on a Zeiss Supra 25 field emission scanning electron microscope equipped with an INCA x-sight X‑ray spectrometer system. Electron-microscopic images were obtained at low electron beam accelerating voltages (~4 kV). At such accelerating voltages, the contribution of the substrate to the measured signal was negligible, if any. EDX analysis data were collected at an accelerating voltage of ~8 kV. Electron micrographs of the VB2 powder samples with various particle sizes were processed as an array of particles to give the particle size distribution using the Image-ProExpress 4.0 program.
X-ray photoelectron spectra were measured using a PHOIBOS 150 MCD electron spectrometer system. The specific surface area (S) of the samples was determined at liquid nitrogen temperature using a Quadrasorb SI analyzer. Hydrogen, oxygen, and chlorine were determined using a CHNS/O Vario Micro cube element analyzer. Boron and vanadium were determined by standard analytical techniques and EDX analysis.
Experimental procedure. Stoichiometric amounts of the V and B powders were mixed with sodium tetraborate and potassium chloride in a vibratory mill (50-cm3 grinding vessel, vanadium balls, ball-to-powder weight ratio of 1 : 1, 10-mm vibration amplitude, frequency of 28 Hz) under an argon atmosphere at room temperature until complete homogenization of the mixture (8 h). Next, a weighed amount of the resultant mixture was loaded into a corundum crucible, which was then placed in a steel autoclave reactor. The reactor was pumped down to a residual pressure of 1.3 × 10–1 Pa, filled with argon at a pressure of 4 MPa, and maintained at a temperature of 800°C for a preset time. Next, the reactor was cooled to room temperature and the reaction mixture was withdrawn. The sinter cake was comminuted, and the resultant powder was sequentially washed with distilled water, ethanol, and acetone. Next, the ampule was pumped down to a residual pressure of 1.3 × 10–1 Pa and filled with argon. The synthesized powder was again placed in the reactor, exposed to flowing hydrogen under a pressure of 5 MPa at 100°C, evacuated at room temperature to a residual vacuum of 1.33 × 10–1 Pa, and withdrawn from the reactor in an argon atmosphere. All subsequent manipulations with the vanadium diboride thus prepared, including sampling for analyses, were carried out under an argon atmosphere.
RESULTS AND DISCUSSION
Table 1 summarizes the present results on reaction between vanadium and boron powders in the molar ratio 1 : 2 at a temperature of 800°C, argon pressure of 4 MPa, and reaction time of 32 h in Na2B4O7 and KCl ionic melts.
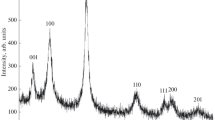
According to the chemical and EDX analysis data, the vanadium diboride prepared under the described conditions had the composition VB2.01–2.02. X-ray diffraction characterization showed that the synthesized vanadium diboride powders (hexagonal symmetry, sp. gr. P6/mmm) were single-phase: we did not detect any significant amounts of impurity phases (Fig. 1). The lattice parameters of the samples (Table 1) agree rather well with those reported for VB2 in the literature: a = 0.2994–2998 nm and c = 0.3048–3056 nm [24]. Scherrer analysis of nine reflections in the range 2θ = 10°–110° showed that the crystallite size Dhkl in the VB2 particles obtained in Na2B4O7 and KCl ionic melts was ~80 and ~85 nm, respectively. The amorphous component of VB2 shows up as a halo centered at 20° (the halo may also be due in part to the substrate on which the sample was produced).
According to the scanning electron microscopy data, the vanadium diboride powders prepared in the ionic melts indicated above consist of particles differing in shape, some of which are nearly spherical, with an average diameter of ~90 nm in both ionic melts (Fig. 2, Table 2). The particle diameter evaluated from the measured specific surface area of the VB2 powders prepared in Na2B4O7 and KCl ionic melts (S = 12.5 and 13.1 m2/g, respectively), under the assumption that the particles are spherical in shape (theoretical density of VB2, 5.066 g/cm3) is ~95 and ~90 nm, respectively.
Table 2 compares the average diameters of the VB2 particles evaluated from the X-ray diffraction, specific surface area, and electron microscopy data. It follows from these data that, independent of the chemical composition and nature of the ionic melts, the average diameter of the VB2 powder particles is near 90 nm.
During heating of the vanadium diboride samples from 20 to 1000°C in an argon atmosphere, we did not detect any noticeable changes attributable to heat release or absorption or weight loss. To more accurately determine the qualitative surface composition of the vanadium diboride powders, we measured their X‑ray photoelectron spectra. The results demonstrate that the major component of the powders is VB2: the V 2p3/2 binding energy is 512.6 eV and the B 1s binding energy is 188.6 eV, which agrees with previously reported data [25, 26]. In addition to the lines characteristic of vanadium diboride, there are weak lines corresponding to boron oxides (boric acid), vanadium oxides, and elemental boron (191.5, 516.8, 532.8, 529.7, and 186.9 eV); that is, the surface layer of the vanadium diboride particles up to ~40 Å in thickness contains small amounts of vanadium oxides, boron oxide or boric acid, and elemental boron inclusions.
The X-ray photoelectron spectroscopy and IR spectroscopy results lend support to the former assumption: according to previous work [27, 28], the position of the B 1s peak at 188.6 eV corresponds to B2O3. The FTIR IR spectra of the powders are essentially identical to the spectrum of phase-pure boric anhydride [29], without features characteristic of H3BO3 (3200, 1450, or 1196 cm–1) [30].
Thus, according to ideas formulated by Ilyushchenko et al. [31], the observed B transport to V in the Na2B4O7 or KCl ionic melt can be accounted for by the formation of lower valence ions, B2+, according to the reaction scheme
These ions then react with vanadium to form VB2 according to the scheme
CONCLUSIONS
We have studied reaction between vanadium and boron powders in the stoichiometric ratio 1 : 2 at a temperature of 800°C, argon pressure of 4 MPa, and reaction time of 32 h in Na2B4O7 and KCl ionic melts. The results demonstrate that, independent of the composition and nature of the melts, the reaction yields phase-pure vanadium diboride nanoparticles with an average diameter near 90 nm.
REFERENCES
Serebryakova, T.I., Neronov, V.A., and Peshev, P.D., Vysokotemperaturnye boridy (High-Temperature Borides), Chelyabinsk: Metallurgiya, 1991.
Carenco, S., Portehault, D., Boissiere, C., Mezailles, N., and Sanchez, C., Nanoscaled metal borides and phosphides: recent developments and perspectives, Chem. Rev., 2013, vol. 113, no. 10, pp. 7981–8065. https://doi.org/10.1021/cr400020d
Lefler, M., Stuart, J., Parkey, J., and Licht, S., Higher capacity, improved conductive matrix VB2 air batteries, J. Electrochem. Soc., 2016, vol. 163, no. 5, pp. A781–A784. https://doi.org/10.1149/2.0031606jes
Licht, S., Ghosh, S., Wang, B., Jiang, D., Hettige, C., Lau, J., and Asercion, J., An 11 electron redox couple for anodic charge storage: VB2, ECS Trans., 2011, vol. 35, no. 33, pp. 21–29. https://doi.org/10.1149/1.3655434
Prokhorov, A.M., Lyakishev, N.P., Burkhanov, G.S., and Dementev, V.A., High-purity transition-metal borides: promising materials for present-day technology, Inorg. Mater., 1996, vol. 32, no. 11, pp. 1195–1201.
Andrievskii, R.A., Osnovy nanostrukturnogo materialovedeniya. Vozmozhnosti i problemy (Principles of Nanostructured Materials Research: Potentialities and Critical Issues), Moscow: BINOM. Laboratoriya Znanii, 2012.
Andrievski, R.A. and Khatchoyan, A.V., Nanomaterials in Extreme Environments: Fundamentals and Applications, Berlin: Springer, 2016. https://doi.org/10.1007/978-3-319-2533-2
Levashov, E.A., Rogachev, A.S., Kurbatkina, V.V., Maksimov, Yu.M., and Yukhvid, V.I., Perspektivnye materialy i tekhnologii samorasprostranyayushchegosya vysokotemperaturnogo sinteza (Self-Propagating High-Temperature Synthesis: Promising Materials and Technologies), Moscow: Izdatel’skii Dom Mosk. Inst. Stali i Splavov, 2011.
Wei, Y., Huang, Zh., Zhou, L., and Ran, S., Novel borothermal synthesis of VB2 powders, Int. J. Mater. Res., 2015, vol. 9, pp. 1–3. https://doi.org/10.3139/146.111286
Peshev, P., Leyarovska, L., and Bliznakov, G., On the borothermic preparation of some vanadium, niobium and tantalum borides, J. Less-Common Met., 1968, vol. 15, pp. 259–267. https://doi.org/10.1016/0022-5088(68)90184-7
Krutskii, Yu.L., Maksimovskii, E.A., Krutskaya, T.M., Popov, M.V., Netskina, O.V., Nikulina, A.A., Cherkasova, N.Yu., and Kvashina, T.S., Synthesis of highly dispersed vanadium diboride with the use of nanofibrous carbon, Russ. J. Appl. Chem., 2017, vol. 90, no. 9, pp. 1379–1385.
Kim, J.W., Shim, J.H., Ahn, J.P., Cho, Y.W., Kim, J.H., and Oh, K.H., Mechanochemical synthesis and characterization of TiB2 and VB2, Mater. Lett., 2008, vol. 62, pp. 2461–2464. https://doi.org/10.1016/j.matlet.2007.12.022
Yeh, C.L. and Wang, H.J., Combustion synthesis of vanadium borides, J. Alloys Compd., 2011, vol. 509, pp. 3257–3261. https://doi.org/10.1016/j.jallcom.2010.12.004
Hassanzadeh-Tabrizi, S.A., Davoodi, D., Beykzadeh, A.A., and Salahshour, S., Fast mechanochemical combustion synthesis of nanostructured vanadium boride by a magnesiothermic reaction, Ceram. Int., 2016, vol. 42, pp. 1812–1816. https://doi.org/10.1016/j.ceramint.2015.09.144
Shi, L., Gu, Y., Chen, L., Yang, Z., Ma, J., and Qian, Y., Low-temperature synthesis of nanocrystalline vanadium diboride, Mater. Lett., 2004, vol. 58, pp. 2890–2892. https://doi.org/10.1016/j.matlet.2004.05.013
Portehaut, D., Devis, S., Beaunier, P., Gervais, C., Giordano, C., Sanchez, C., and Antonietti, M., A general solution route toward metal boride nanocrystals, Angew. Chem., 2011, vol. 50, pp. 3262–3265. https://doi.org/10.1002/anie.201006810
Nozdrin, I.V., Galevskii, G.V., Shiryaeva, L.S., and Terent’eva, M.A., Particle size of vanadium and chromium borides and carbides in a plasma flux, Izv. Vyssh. Uchebn. Zaved., Chern. Metall., 2011, no. 10, pp. 12–17.
Avvakumov, E.G, Mekhanicheskie metody aktivatsii khimicheskikh protsessov (Mechanical Techniques for the Activation of Chemical Processes), Novosibirsk: Nauka, 1989.
Volkova, L.S., Shulga, Yu.M., and Shilkin, S.P., Synthesis of nano-sized titanium diboride in a melt of anhydrous sodium tetraborate, Russ. J. Gen. Chem., 2012, vol. 82, no. 5, pp. 819–821.
Semenenko, K.N., Shilkin, S.P., Burnasheva, V.V., Korobov, I.I., Volkova, L.S., and Govorkova, L.V., Reactions of CeFe2–LaFe2 alloys with nitrogen in the presence of hydrogen, Zh. Obshch. Khim., 1983, vol. 53, no. 5, pp. 961–966.
Fokin, V.N., Fokina, E.E., and Shilkin, S.P., Synthesis of coarsely crystalline metal hydrides, Russ. J. Gen. Chem., 1996, vol. 66, no. 8, pp. 1210–1212.
Semenenko, K.N., Shilkin, S.P., Burnasheva, V.V., Volkova, L.S., and Mozgina, N.G., Reactions of some intermetallic compounds between rare-earth and iron-group metals with nitrogen in the presence of hydrogen, Zh. Obshch. Khim., 1987, vol. 57, no. 4, pp. 729–732.
Fokin, V.N., Troitskaya, S.L., Shilkin, S.P., Fokina, E.E., and Rumynskaya, Z.A., Reaction of titanium hydride with oxygen, Zh. Obshch. Khim., 1992, vol. 62, no. 8, pp. 1719–1725.
Diagrammy sostoyaniya dvoinykh metallicheskikh sistem: Spravochnik (Phase Diagrams of Binary Metallic Systems: A Handbook), Lyakishev, N.P., Ed., Moscow: Mashinostroenie, 1996, vol. 1.
Aleshin, V.G., Kharlamov, A.N., and Chudinov, M.G., Surface condition of refractory compounds studied by X-ray photoelectron spectroscopy, Izv. Akad. Nauk SSSR, Neorg. Mater., 1979, vol. 15, no. 4, pp. 672–676.
Terlan, B., Levin, A.A., Börrnert, F., Simon, F., Oschatz, M., Schmidt, M., Cardoso-Gil, R., Lorenz, T., Baburin, I.A., Joswig, J.-O., and Eychmüller, A., Effect of surface properties on the microstructure, thermal, and colloidal stability of VB2 nanoparticles, Chem. Mater., 2015, vol. 27, no. 14, pp. 5106–5115. https://doi.org/10.1021/acs.chemmater.5b01856
Joyner, D.J. and Hercules, D.M., Chemical bonding and electronic structure of B2O3, H3BO3, and BN: ESCA, Auger, SIMS and SXS study, J. Chem. Phys., 1980, vol. 72, no. 2, pp. 1095–1108. https://doi.org/10.1063/1.439251
Ong, C.W., Huang, H., Zheng, B., Kwok, R.W.M., Hui, Y.Y., and Lau, W.M., X-ray photoemission spectroscopy of nonmetallic materials: electronic structures of boron and BxOy , J. Appl. Phys., 2004, vol. 95, no. 7, pp. 3527–3534. https://doi.org/10.1063/1.1651321
Sidorov, T.A. and Sobolev, N.N., Infrared and Raman spectra of boric anhydride: III. Interpretation of the vibrational spectrum of boric anhydride and calculation of the isotopic effect, Opt. Spektrosk., 1958, vol. 4, no. 1, pp. 9–16.
Bethell, D.E. and Sheppard, N., The infrared spectrum and structure of boric acid, Trans. Faraday Soc., 1955, vol. 51, pp. 9–15.
Ilyushchenko, N.G., Anfinogenov, A.I., and Shurov, N.I., Vzaimodeistvie metallov v ionnykh rasplavakh (Interactions between Metals in Ionic Melts), Moscow: Nauka, 1991.
ACKNOWLEDGMENTS
This work was supported by the Russian Foundation for Basic Research (state research target, theme no. 0089-2019-0007) and carried out using equipment at the Shared Analytical Facilities Center, Institute of Problems of Chemical Physics, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Kravchenko, S.E., Domashnev, I.A., Dremova, N.N. et al. Synthesis of Vanadium Diboride Nanoparticles via Reaction of Amorphous Boron with Vanadium in KCl and Na2B4O7 Ionic Melts. Inorg Mater 55, 443–448 (2019). https://doi.org/10.1134/S002016851905011X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002016851905011X