Abstract
Near-spherical niobium diboride nanoparticles with an average diameter of ~17 nm, crystallizing in the hexagonal system and belonging to the space group P6/mmm, were obtained by reacting NbCl5 with NaBH4 in ionic melts of alkali metal halides at 1073 K under an argon pressure of 4 MPa for 15 h in an autoclave.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Niobium diboride has a high melting point, as well as high hardness, strength, wear resistance, and thermal and electrical conductivities combined with chemical and corrosion inertness, which makes it useful in various industrial applications [1–3]. Creation of nanostructured boride materials significantly expands the scope of their application and provides motivation to design new methods for the synthesis of nanoscale high-melting borides [4, 5].
The NbB2 nanoparticles are commonly prepared by the synthesis techniques developed for Group IV and VI transition metal diborides: high-temperature solid-state synthesis from elements or “currentless” synthesis method involving the reaction of boron and niobium in ionic melts; borothermal reduction of various oxides and salts of niobium; carbothermic reduction of niobium and boron oxides or reduction of niobium and boron oxides by magnesium; thermolysis of the corresponding metal borohydrides or their complex derivatives; reaction of transition metal chlorides with alkali metal borohydrides without isolation of transition metal borohydride derivatives at elevated temperatures and pressures; mechanochemical synthesis; chemical vapor deposition (CVD) [6–19].
For this study, we considered an alternative method for obtaining NbB2 nanoparticles by reaction (1).
In the temperature range 673–1173 K reaction (1) leading to formation of niobium diboride is characterized by high thermodynamic probability (Table 1). Reaction (1) is exothermic. Calculations of the Gibbs free energy change showed that, in the temperature range indicated, reaction (1) is energetically favorable and is promoted by rising temperature. The thermodynamic data for NbB2 were taken from [20], and those for other substances, from NIST Chemistry WebBook [21].
We obtained niobium diboride nanoparticles from niobium pentachloride and sodium borohydride according to reaction (1) in ionic melts of alkali metal halides under an argon pressure of 4 MPa at a temperature of 1073 K and at a reaction time of 15 h. Reaction (1) should be run at a temperature (1073 K) that slightly exceeds the melting points of KCl (1049 K) and KBr (1007 K). The argon pressure in the reactor over the reactants melt (4 MPa) should ensure the lack of contact of the melt with traces of air oxygen and nitrogen. In view of the above, these parameters were chosen by analogy with the synthesis of NbB2 in ionic melts according to reaction (2) [16, 17].
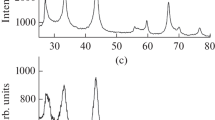
As shown by chemical and energy-dispersive analyses, the resulting niobium diboride nanoparticles have the composition NbB1.99–2.02O0.01–0.02 and are free from traces of hydrogen and halide ions. The XPA data indicate that the obtained samples do not contain noticeable quantities of impurity phases (Fig. 1). Niobium diboride crystallizes in the hexagonal system (space group P6/mmm) with lattice constants a 0.3107–0.3115 nm and c 0.3277–0.3290 nm, which agrees with the published data [22].
Table 2 compares the average diameters of the NbB2 particles as estimated using the electron microscopic and X-ray diffraction analysis data and the results of the specific surface measurements. It is seen that, irrespective of the chemical composition of the ionic melt, the average diameter of the NbB2 powder particles is close to ~17 nm, with the niobium diboride particles being noticeably agglomerated.
To refine the qualitative composition of the surface of the NbB2 nanoparticles, their X-ray photoelectron spectra (XPS) were recorded. The XPS data show that the main component of the powders is NbB2; specifically, the electron binding energy of the Nb 3d5/2 level is 203.6 eV, and that of the B1s level, 188.1 eV, which is consistent with the published data [9, 16, 17]. Along with the lines characteristic of niobium diboride, the XPS spectra contain weak lines corresponding to boron oxide B2O3 and niobium oxide Nb2O5.
Thus, compared to the known procedures, the method involving the use of ionic melts in the reaction of NbCl5 with NaBH4 allows synthesizing smaller nearly spherical NbB2 nanoparticles under milder conditions.
EXPERIMENTAL
Sodium borohydride of >99.5% purity was obtained by recrystallization of technical-grade NaBH4 from 1 N NaOH solution and dried at 1.33×10–1 Pa and 373 K. We used chemically pure grade NbCl5 and high-purity argon [99.998%, TU (Technical Specifications) 2114-005-0024760-99]. The source of hydrogen with a purity of at least 99.999% was an autonomous laboratory hydrogen generator, containing the hydride phases based on LaNi5 and TiFe intermetallic compounds as the working material, whose operation principle was described in detail in [23].
The reaction between NbCl5 and NaBH4 in the corresponding ionic melt was carried out as follows. First, the reaction mixture was activated in a Pulverisette 6 planetary ball mill (niobium balls, feed to grinding balls ratio 1 : 10, rotation speed 400 rpm, treatment time 2 min) in an argon atmosphere at room temperature. Then, the activated mixture of powdered NbCl5 (3.3 g) with NaBH4 (1.1 g) together with KCl, KBr, or eutectic mixture 50 mol % NaCl–50 mol % KCl (15 g each) in a quartz ampule was placed in a stainless steel autoclave reactor under high-purity argon. The reactor was evacuated to a residual pressure of 1.3×10–1 Pa, filled with argon at a pressure of 4 MPa, and heated at 1073 K for 15 h. Next, the reactor was cooled to room temperature, and the reaction mixture was unloaded. The cake was crushed and sequentially treated with distilled water, ethanol, and acetone and then evacuated to a residual pressure of 1.3×10–1 Pa. Next, the resulting powder was again placed in the reactor and treated with hydrogen in a continuous-flow mode under a pressure of 5 MPa at 373 K, evacuated at room temperature to a residual pressure of 1.3× 10–1 Pa, filled with argon, and unloaded from the reactor in an argon atmosphere.
X-ray powder diffraction analysis of the obtained NbB2 nanoparticles was carried out on an ADP-2 diffractometer (monochromatic CuKα radiation). The error in determining the NbB2 crystal lattice constants did not exceed 0.0003 nm. From the X-ray powder diffraction patterns the size of the coherent scattering region Dhkl was estimated using the Scherrer formula (3) (in the direction perpendicular to the hkl plane).
where k is the anisotropy coefficient, which in this study was taken to be equal to 0.9; λ, X-ray wavelength (for λCuKα 1.54178 Å); θ, diffraction angle; and β, full width at half maximum of the diffraction peak (rad).
Electron microscopic and energy-dispersive X-ray analyses were carried out using a set of instruments, consisting of a Zeiss Supra 25 field emission scanning electron microscope and an INCA x-sight X-ray energy dispersive spectrometer. X-ray photoelectron spectra (XPS) were recorded on a PHOIBOS 150 MCD electron spectrometer system. The specific surface area of the samples (Ssp) was determined on a Quadrasorb SI analyzer. Using the measured Ssp data the size of the NbB2 particles was estimated under the assumption of their spherical shape according to formula (4).
where dx is the particle size, and γ, theoretical density of NbB2.
The contents of boron, niobium, chloride and boride ions, and oxygen were estimated by standard analytical methods, as well as by energy dispersive X-ray analysis. The hydrogen content was determined on a CHNS/O Vario EL cube Elementar elemental analyzer. The pressure in the system was measured with standard pressure gauges (MO) of accuracy class 0.4.
REFERENCES
Serebryakova, T.I., Neronov, V.A., and Peshev, P.D., Vysokotemperaturnye boridy (High-Temperature Borides), Chelyabinsk: Metallurgiya, 1991.
Carenco, S., Portehault, D., Boissiere, C., Mezailles, N., and Sanchez, C., Chem. Rev., 2013, vol. 113, no. 10, p. 7981. https://doi.org/10.1021/cr400020d
Andrievskii, R.A., Osnovy nanostrukturnogo materialovedeniya. Vozmozhnosti i problemy (Fundamentals of Nanostructured Materials Science: Opportunities and Challenges), Moscow: BINOM. Laboratoriya Znanii, 2012.
Albert, B. and Hillebrecht, H., Angew. Chem., Int. Ed., 2009, vol. 48, no. 46, p. 8640. https://doi.org/10.1002/anie.200903246
Andrievski, R.A. and Khatchoyan, A.V., Nanomaterials in Extreme Environments, Fundamentals and Applications, Berlin: Springer Int. Publ., 2016. https://doi.org/10.1007/978-3-319-25331-2
Matsudaira, T., Itoh, H., and Naka, S., J. Less-Common Met., 1989, vol. 155, no. 2, p. 207. https://doi.org/10.1016/0022-5088(89)90229-4
Jha, M., Ramanujachary, K.V., Lofland, S.T., Gupta, G., and Ganguli, A.K., J. Dalton Trans., 2011, vol. 40, p. 7879. https://doi.org/10.1039/c1dt10468c
Maeda, H., Yoshikawa, T., Kusakabe, K., and Morooka, S., J. Alloys Compd., 1994, vol. 215, p. 127. https://doi.org/10.1016/0925-8388(94)90829-X
Gai, P., Yang, Z., Shi, L., Chen, L., Zhao, A., Gu, Y., and Qian, Y., Mater. Lett., 2005, vol. 59, p. 3550. https://doi.org/10.1016/j.matlet.2005.07.051
Ma, J., Du, Y., Wu, M., Li, G., Feng, Z., Guo, M., Sun, Y., Song, W., Lin, M., and Guo, X., J. Alloys Compd., 2009, vol. 468, p. 473. https://doi.org/10.1016/j.jallcom.2008.01.021
Jothi, P.R., Yubuta, K., and Fokwa, B.P.T., Adv. Mater., 2018, vol. 30, no. 14, p. 1704181. https://doi.org/10.1002/adma.201704181
Portehaut, D., Devis, S., Beaunier, P., Gervais, C., Giordano, C., Sanchez, C., and Antonietti, M., Angew. Chem., 2011, vol. 50, p. 3262. https://doi.org/10.1002/ange.201006810
Jafari, M., Tajizadegan, H., Golabgir, M.H., Chami, A., and Torabio, O., Int. J. Refr. Met. Hard Mater., 2015, vol. 50, p. 86. https://doi.org/10.1016/j.ijrmhm.2014.10.017
Balci, Ö., Aĝaoĝullari, D., Övecoĝlu, M.L., and Duman, I., Trans. Nonferrous Met. Soc. China, 2016, vol. 26, p.747. https://doi.org/10.1016/S1003-6326(16)64165-1
Gupta, A., Singhal, V., and Pandey, O.P., J. Alloys Compd., 2018, vol. 736, p. 306. https://doi.org/10.1016/j.jallcom.2017.10.257
Kravchenko, S.E., Vinokurov, A.A., Dremova, N.N., Nadkhina, S.E., and Shilkin, S.P., Russ. J. Gen. Chem., 2021, vol. 91, no. 2, p. 302. https://doi.org/10.1134/S1070363221020195
Kravchenko, S.E., Kovalev, D.Yu., Vinokurov, A.A., Dremova, N.N., Ivanov, A.V., and Shilkin, S.P., Inorg. Mater., 2021, vol. 57, no.10, p. 1005. https://doi.org/10.1134/S002016852110006X
Kravchenko, S.E., Torbov, V.I., and Shilkin, S.P., Russ. J. Inorg. Chem., 2011, vol. 56, no. 4, p. 506. https://doi.org/10.1134/S0036023611040164
Andrievski, R.A., Kravchenko, S.E., and Shilkin, S.P., Jpn. J. Appl. Phys., 1994, vol. 10, p. 198.
Bolgar, A.S., Serbova, M.I., Fesenko, V.V., Serebryakova, T.I., and Isaeva, L.P., Teplofiz. Vys. Temp., 1980, vol. 18, no. 6, p. 1180.
Burgess, D.R. Jr., Thermochemical Data, in NIST Chemistry WebBook, NIST Standard Reference Database no. 69, Linstrom, P.J. and Mallard, W.G., Eds., Gaithersburg, MD: National Institute of Standards and Technology, 20899. https://doi.org/10.18434/T4D303
Diagrammy sostoyaniya dvoinykh metallicheskikh sistem: Spravochnik (Phase Diagrams of Binary Metallic Systems: Handbook), Lyakishev, N.P., Ed., Moscow: Mashinostroenie, 1996, vol. 1.
Fokin, V.N., Fokina, E.E., and Shilkin, S.P., Zh. Obshch. Khim., 1996, vol. 66, no. 8, p. 1249.
Funding
This study was financially supported by the Ministry of Science and Higher Education of the Russian Federation within the framework of the State Assignment (state registration no. АААА-А19-119061890019-5) using the equipment of the Analytical Center for Collective Use, Institute of Problems of Chemical Physics, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Vinokurov, A.A., Dremova, N.N., Nadkhina, S.E. et al. Formation of Niobium Diboride Nanoparticles by the Reaction of Niobium Pentachloride with Sodium Borohydride in Ionic Melts of Alkali Metal Halides. Russ J Gen Chem 92, 272–275 (2022). https://doi.org/10.1134/S1070363222020189
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222020189