Abstract
Hydrocarbon-decomposing microorganisms identified as representatives of the genera Pseudomonas, Rhodococcus, Acinetobacter, Kocuria, Raoultella, and Candida have been isolated from the oil-contaminated soil samples of the Middle Ob region. They have been screened for the ability to decompose various classes of hydrocarbons in a wide temperature range (6–37°C), in acid media (up to pH 4), and at increased salinity (up to 3%), for the ability to produce biosurfactants, and for the presence of genes encoding enzymes responsible for hydrocarbon decomposition. A microbial consortium has been suggested as the basis of a biological preparation for bioremediation of oil-contaminated soils in the Middle Ob region, including strains of Candida fluviatilis 24p-51, Rhodococcus erythropolis 24-44, Acinetobacter calcoaceticus 7-43, and Pseudomonas extremaustralis 7-31. The modes of cultivation and lyophilization of biomass have been determined for these microorganisms. The efficiency of degradation of oil hydrocarbons by the developed microbial consortium has been evaluated in laboratory model systems. The degree of oil degradation by the microbial consortium in the liquid mineral medium was 56%; in the model soil, 22% in 10 days at 24°C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
In the course of intense development and exploitation of oil fields, natural environment is subjected to significant technogenic load, which often results in contamination and degradation of ecosystems [17]. Most oil fields of West Siberia are situated in the middle reaches of the Ob River in Khanty-Mansi autonomous okrug, Yugra. This is the northern taiga zone of West Siberia with a high percent of waterlogged areas (swamps) [2, 13]. These territories are under permanent negative influence of the processes of oil contamination and technogenic soil salinization. Available physical, chemical, and thermal methods of soil purification do not allow performing the complete remediation of oil-contaminated territories. Bioremediation is an environmentally safe and economically advantageous technology for remediation of oil spills [35]. The decomposition of hydrocarbons in soil due to metabolic activity of microorganisms is determined by some environmental and biological factors, which vary from place to place, such as soil pH, temperature, oxygen availability and concentrations of nutrients, growth and survival of hydrocarbon-decomposing microorganisms, and bioavailability of hydrocarbons [32]. Up to date, there are a lot of data on the capacity of bacteria and fungi isolated from different biotopes for oxidation of oil hydrocarbons [19, 23, 29, 39, 54]. The main criteria for selection of promising hydrocarbon-decomposing microorganisms for remediation of natural environment from oil and petroleum products are: the capacity to decompose different classes of hydrocarbons, the presence of biodegradation plasmids, tolerance towards low pH values and the presence of salt (sodium chloride), and the capacity to synthesize biological surfactants (biosurfactants) and to oxidize hydrocarbons within the wide temperature range [3, 25, 36, 43, 49]. This work was aimed at developing the microbial consortium for remediation of oil-contaminated territories in the Middle Ob region.
OBJECTS AND METHODS
Sampling and sample characteristics. Sampling was carried out in oligotrophic, mesotrophic, and floodplain peatlands and in swampy forest from the peaty gleyzem. In the WRB system, soils of oligotrophic and mesotrophic bogs were classified as Hyperdystric Histosols; soils of the floodplain peatland, as Histic Fluvisols; and soils under swampy forest, as Histic Gleysols. Soil samples for every type of biogeocenoses were taken in several oil-contaminated sites of not reclaimed soils differing in the degree and time of contamination and in the presence/absence of salinization and in their background analogues (detailed description of the samples is presented in [14]).
Concentrations of oil products were determined with IR spectrometry method [8]. The intensity of СО2 emission was evaluated by the quantity of СО2 emitted from moist samples under room temperature relative to the mass of dry soil [10]. Soil acidity was determined according GOST 26423-85.
Media and conditions for cultivation of microorganisms. The complete Luria-Bertani medium [46] and mineral Evans’ medium [26] were used in the work. To obtain agar media, 2% (weight/volume) agar (Difco, USA) was added. To obtain media with decreased acidity, pH was brought to 5 and 4 with concentrated hydrochloric acid, and 3% (weight/volume) NaCl was added to obtain media with increased salinity. Microorganisms were cultivated in liquid media in Erlenmeyer flasks of 750 mL in volume or in test tubes of 50 mL in volume with their mixing on rotation shaker at 120 rpm. The temperature and time of cultivation are given in descriptions of particular experiments.
One of the following substrates was added to liquid mineral volume in amount of 2% (weight/volume for solid and volume/volume for liquid substances) as the only source of carbon and energy: crude oil, diesel fuel, naphthalene, β-methylnaphthalene, phenanthrene, anthracene, pyrene, fluorene, camphor, benzene, ethylbenzene, phenol, sodium protocatechoate, sodium salicylate, 4-methylcatechol, nonane, decane, undecane, dodecane or hexadecane, and hexane and octane. They were introduced into the flask arm in order to grow in vapor. Diesel fuel for Evans’ agar was introduced in disposable plastic pipette end on the foil of turned over Petri dish.
Determination of the number of microorganisms. For soil samples, 1-g weighed portion was taken and introduced into the flask with 100 mL of physiological solution; for dry preparations, 1-g weighed portion was introduced into 9 mL of physiological solution. The samples were agitated during 30 min. Next, we used standard serial dilution method with inoculation to separate colonies on agar media (Luria-Bertani medium) to determine the total number of microorganisms; Evans’ medium with diesel fuel was used to determine the number of decomposers of crude oil. Petri dishes were incubated at 24°С during 2 days (Luria-Bertani medium) and 5 days (Evans’ medium). Inoculations were carried out in triplicate.
Isolation and characterization of hydrocarbon-oxidizing microorganisms. Weighed portion (5 g) of the mixture of collected soil samples was placed into Erlenmeyer flask with 100 mL of Evans’ medium (рН 5, 3% sodium chloride, 2% volume/volume crude oil). Cultivation was carried out during 10 days under 24°C and 20 days under 6°C. Then Luria-Bertani agar was inoculated. Morphologically distinguishable colonies of cells were inoculated up to separate colonies to obtain pore cultures. Isolated pure strains were repeatedly checked out on hydrocarbon oxidizing activity by the growth in mineral Evans’ medium with oil under 24°С during 10 days.
Taxonomic identification of most active hydrocarbon-oxidizing strains and search the genes responsible for hydrocarbon decomposition. Genomic DNA of bacteria was isolated using the diaGene Kit (Russia). Plasmid DNA was isolated by alkaline lysis [7].
Identification of isolated hydrocarbon-decomposing microorganisms was carried out via determination of primary nucleotide sequences in the fragment of 16S rRNA gene (for bacteria) and ITS-region (for yeast fungi). Polymerase chain reaction (PCR) was carried out in a thermocycler GeneAmp PCR System 9700 (Applied Biosystems, USA). Nucleotide sequence, temperature of primer annealing, and the size of PCR products are given in [11, 12]. The obtained PCR products were purified using GeneJET PCR Purification Kit (Thermo Scientific, USA) according the protocol of producing company [28].
The nucleotide sequence of amplicons was determined in a sequenator Applied Biosystems 3130 × 1 using the sequencing kit BigDye v.3.1. The identity of nucleotide sequences was analyzed using the BLASTN program [18].
Electrophoresis was carried out in 0.8–1% horizontal agarose gel and 0.5 × Tris/Borate/EDTA [46]. Visualization of DNA was carried out by adding ethidium bromide to the agarose in final concentration 0.5 µg/mL.
Pulsed-field gel electrophoresis was carried out according to the protocol of producer (Bio-Rad, USA). Preparation of inserted blocks was carried out according to [15].
Determination of substrate specificity of microorganisms−decomposers. Microorganisms were cultivated in the tubes with 10 mL of Evans’ medium with substrate added under 24°С during 10 days.
Production of biological surface-active substances. Microorganisms were grown in Evans’ medium with diesel fuel under 24°C during 5 days. Culture liquid was settled during 30 min in separating funnels, getting rid of the film of residual substrate. The measurements were carried out with du Nouy (anchor-ring) method in tensiometer К6 (Kruss, Germany) under room temperature. Surface tension of reference solution (mineral Evans’ medium) was 72 mN/m.
Model systems with crude oil. The experiments with liquid medium were carried out in Erlenmeyer flasks containing 100 mL of Evans’ medium and 2, 10, or 20% (volume/volume) of crude oil. The experiments with sand were carried out in the containers with 1 kg of washed and sterilized river sand with 10% (weight/weight) of crude oil. The suspension of microorganisms grown on Luria-Bertani medium or the sample of dry preparation diluted in physiological solution was taken for inoculum. Inoculation rate was calculated in order to reach final concentration of microorganisms 1 × 107 CFU per one mL or g of the medium. Culturing was carried out during 10 days under 24°C. The flasks were placed on rotation shaker (120 rpm), the sand was carefully loosened at the moment of inoculation and after 5 days. Every system was prepared in triplicate.
Evaluation of degree of oil degradation. The residual content of oil hydrocarbons in liquid medium and sand was determined by gas chromatography after extraction by methylene chloride [4]. The measurements were carried out using gas chromatograph with flame ionization detector Agilent 6890N (Agilent, USA) and capillary column DB-1 (30 m × 0.25 mm × 0.50 µm). The degree of hydrocarbon degradation was evaluated in comparison with the control (model system with oil and without microorganisms). Every measurement was carried out in triplicate.
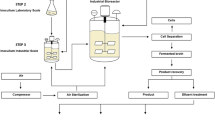
Culturing of the strains of microbial consortium. The work was carried out in the unique scientific unit Experimental Processing Unit Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, registration number 279918 on the website www.ckp-rf.ru. The inoculum was grown on Luria-Bertani medium, and the volume of introduced inoculum was 100 mL. The culturing was carried out in a 10-L fermenter (ANKUM), containing 7.5 L of the medium of following composition (g/L): peptone 7.0, yeast extract 5.0, sodium chloride 1.0, manganese sulfate heptahydrate 0.1, magnesium sulfate heptahydrate 0.3, ammonium sulfate 5.0, dipotassium hydrogen phosphate dodecahydrate 2.0, potassium dihydrogen orthophosphate dodecahydrate 0.5, and glucose 25.0. The following parameters of the cultivation process were maintained: temperature 28°C, рН 7.0, rate of agitation 450 rpm, aeration 0.5–1.0 L/min. Duration of the process of culturing microorganisms was r 16–22 h (up to finishing of the growth of optical density during 2–4 h).
Microbial biomass was separated in centrifugal apparatus К-70 (Janezki, Germany) at 4500 rpm and temperature 5°C during 30 min.
Dry preparation obtaining. The obtained biomass was mixed at the rate 1 : 1 (weight/volume) with 5% solution of sodium glutamate. The suspension was frozen at –40°C. Lyophilization was carried out in vacuum freeze dryers КС-30. Dry preparation was packed into sterilized polyethylene bags.
Statistical treatment the results. The results were treated using the firmware statistical package Excel (MS Office 2020).
RESULTS AND DISCUSSION
Detailed description of collected samples of the upper horizons of the main soil types in dominating types of biogeocenoses of the Middle Ob region is presented in the work [14]. Soils were classified as oligotrophic peat, mesotrophic peat, peat bog alluvial soil, and peaty gleyzem. Though alluvial and mesotrophic soils are less abundant in the area in comparison with oligotrophic peat soils, they play a big role in supporting the bio- and pedodiversity [1]. The characteristics of collected soil samples are presented in Table 1. The рН value averaged 5.2 for all biotopes. Maximum carbon dioxide emission was found in all clean background samples, and this apparently attested to the active metabolism of microbial community. High concentration of oil products and low pH values in contaminated samples of oligotrophic peat soil had a negative effect on the amount of oil decomposers and the intensity of CO2 emission. However, this effect was not observed in contaminated samples from the other biotopes. Positive correlation was found between the number of oil decomposers and the intensity of carbon dioxide emission.
Trofimov et al. demonstrated that major contribution to carbon dioxide production in studied oil-contaminated samples was apparently made by decomposing microorganisms, which use oil compounds in their metabolism [14]. Detailed study of the main physicochemical properties of biotopes and characteristics of hydrocarbon soil status, and evaluation of the quantity of oil decomposers allowed selecting the following samples from three biotopes: R4.1, RHB2.2.2, and М2. The following properties were taken as the criteria of selection: low pH value, concentration of oil hydrocarbons >25%, high value of the intensity of carbon dioxide emission, and the fraction of oil decomposers of more than 20%. As oil-contaminated samples of the peat bog alluvial soil and peat gleyzem were single, they were also taken for further experiments.
Twenty-four strains were isolated during enrichment cultivation of the mixture of soil samples at 24°C and 20 strains, at 6°C. The obtained pure cultures of microorganisms were repeatedly tested for their capacity for growth on oil, and this allowed selecting 14 oil-decomposing microorganisms: 24р-61, 24-21, 7р-81, 24р-83, 24-41, 7р-72, 24-44, 7р-51, 7р-62, 7-43, 7-31, 24р-71, 24р-51, and 7-41.
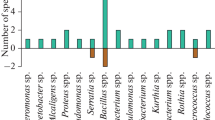
According to the data of some researchers, domination of the following bacteria genera was observed in the samples of contaminated soils around oil-processing plants, agricultural lands, or industrial locations: Megamonas, Paenibacillus, Bacillus, Aquicella, Alicyclobacillus, Anaeromyxobacter, Bdellovibrio, Nitrospiria, Oscillospiria, Mycobacterium, Rhodococcus, Pseudomonas, Burkholderia, Chromobacterium, Xanthomonas, and Acinetobacter [21, 42, 53]. In our study, representatives of bacteria from genera Pseudomonas (5 strains), Bacillus (1 strain), Raoutella (1 strain), Acinetobacter (1 strain), and Kocuria (1 strain) were selected (Table 2). The species of genus Pseudomonas are often the most widespread microorganisms in oil-contaminated soils, and this fact attests to significant contribution of strains of this genus to the process of decomposition of hydrocarbons [20]. It was found that five representatives of yeasts Candida fluviatilis were present in isolated strains additionally to bacteria. According to Polyakova [44], higher frequency and diversity of yeasts of ascomycete affinity of genera Pichia, Debaryomyces, Metschnikowia, and Candida was a characteristic feature of the taxonomic structure of microbial community of peat in comparison with other soils. We can make an assumption that bacteria of genus Pseudomonas and yeasts Candida fluviatilis are the specific microorganisms of soil biotopes in the Middle Ob region and can be considered important participants of biodegradation processes.
To compose the most efficient consortium, it is necessary to take into consideration the features of climate and soil of the region, where the remediation measures are planned, as well as general principles of biodegradation providing the possibility for microbial destruction of contaminating substrates. The particular criteria of selecting the strains were determined on the basis of this reason.
According to [14], saline soils with low pH values are typical for different biotopes in the Middle Ob region. Mean summer temperatures in this region range from 7 to 35°C. The analysis of experimental results on the capacity of microorganisms to decompose oil degradation in the temperature range from 6 to 37°С under low рН values and in presence of sodium chloride allowed revealing the strains with the most active growth under these conditions: Candida fluviatilis 24-21, 24р-51, 24р-71, 24р-83; Acinetobacter calcoaceticus 7-43; Rhodococcus erythropolis 24-44; and Pseudomonas extremaustralis 7-31 (Tables S1, S2). It should be noted that yeasts Candida fluviatilis predominated under these conditions. As is commonly known, yeast fungi often predominate in the media with high salinity and increased acidity.
Aliphatic, branched-chain, and cycloaliphatic alkanes and different mono- and polycyclic aromatic hydrocarbons (PAHs) are the most widespread hydrocarbons, which are the constituents of oil and products of oil refining. Despite the fact that soil microorganisms utilize a wide range of substrates, bioremediation of territories contaminated by a complex set of hydrocarbons requires combined participation of microorganisms with different genetic systems of catabolism of hydrocarbons. The results of analysis of the stains for their capacity for growth on different hydrocarbon-containing substrates are presented in Table 2. It was found that pseudomonades 7р-81, 7-41, 7-31, and 7р-62 could decompose both aliphatic and aromatic hydrocarbons. Yeast Candida fluviatilis and bacterium Rhodococcus erythropolis 24-44 grew most actively in mineral medium with n-alkanes.
Knowledge of metabolic ways and genes participating in decomposition of hydrocarbons helps us to enhance the efficiency of bioremediation. Therefore, the presence of genes responsible for decomposition of n-alkanes and aromatic hydrocarbons in the studied strains was determined by the PCR method with primers for the sequences of known catabolic genes.
The studied strains were tested with PCR method for the presence of alkB gene encoding alkane hydroxylase, with primers allowing to amplify the fragment of this gene in such phylogenetically remote genera as Pseudomonas, Rhodococcus, Burkolderia, Bacillus, Mycobacterium, Amycolicicoccus, Nocardioides, Prauserella, Micromonospora, Frankia, Alcanivorax, etc. [50]. The gene alkB can be the marker for determining bioremediation potential of oil-contaminated territories [50]. Specific amplification of gene alkB was observed in four studied strains: P. extremaustralis 7-31, P. fluorescens 7-41, P. veronii 7p-62, and P. fluorescens 7p-81 (Table S3). Rhodococcus erythropolis 24-44 can grow on alkanes with chain length more than С9, but gene alkB encoding alkane hydroxylase was not found in this strain. It should be noted that capacity for alkane degradation in Rhodococcus sp. strains can be determined by the presence of CYP genes, which are also responsible for alkane degradation [40]. It is likely that CYP genes are responsible for alkane oxidation in the strain Rhodococcus erythropolis 24-44.
The inclusion of oxygen atom into aromatic ring with participation of hydroxylated dioxygenase is the initial stage of PAH degradation in most microorganisms. Genes phnAc and nahAc encoding great subunits of phenanthrene 3,4- and naphthalene 1,2-dioxygenases, respectively, are considered to be good markers for evaluation of the biodegradation potential of microflora in soils contaminated with oil and oil products [34, 52]. The gene of specific amplification phnAc was not found in the studied samples. When amplifying the gene of big subunit of naphthalene 1,2-dioxygenase, PCR products of corresponding size were obtained only in the case of pseudomonades: 7-31, 7-41, 7p-51, 7p-62, and 7p-81.
The strains P. putida 7p-51 and P. fluorescens 7p-81 can grow on naphthalene as well as on phenanthrene as the only source of carbon and energy. However, the results of PCR analysis demonstrated that only the sequence of the gene of great subunit of naphthalene 1,2-dioxygenase was present in these strains. It is known that naphthalene 1,2-dioxygenase possesses wide substrate specificity and can participate in oxidation of more high-molecular-weight PAHs such as phenanthrene, anthracene, and biphenyl [31, 47]. It is likely that naphthalene 1,2-dioxygenase also participates in the destruction of phenanthrene ring in these strains.
Salicylate is the key intermediate product of catabolism of many aromatic hydrocarbons [22, 24, 27, 33]. To study genetical control of salicylate degradation in studied strains, the PCR analysis was carried out for the presence of the following catabolic genes: nahG, nahH, and nagG, encoding salicylate 1-hydroxylase, catechol 2,3-dioxygenase, and big subunit of oxygenase component of salicylate 5-hydroxylase, respectively. When amplifying the gene nagG, PCR-products were not obtained from the studied samples. Gene sequences of salicylate 1-hydroxylase (nahG) and catechol 2,3-dioxygenase (nahH) were found only in the strains of genus Pseudomonas: 7-31, 7-41, 7p-51, 7p-62, and 7p-81.
Five strains of pseudomonads—P. extremaustralis 7-31, P. fluorescens 7-41, P. putida 7p-51, P. veronii 7p-62 and P. fluorescens 7p-81—simultaneously contained the sequences of genes nahAc, nahG, and nahH, and this fact attested to the presence of classical operons of naphthalene catabolism in these strains [5].
It is known that the genes responsible for catabolism of different xenobiotics can have chromosomal location as well as to be situated on conjugated plasmids of big size [30, 45]. Two approaches were used to detect plasmid DNA in the studied strains: alkaline lysis and pulsed-field gel electrophoresis in agar inserting blocks. The combination of these two methods allowed determination of the presence in a strain of plasmids of relatively big size (more than 200 kilobase pairs). Plasmid DNAs of more than 180 kbp in size were found in five strains of genus Pseudomonas (P. extremaustralis 7‑31, P. fluorescens 7-41, P. putida 7p-51, P. veronii 7p-62, and P. fluorescens 7p-81). Because we failed to find plasmid DNA in remaining nine strains with any of the above-listed approaches, it is safe to say about the absence of plasmids in the following strains: Candida fluviatilis 24-21, Raoutella planticola 24-41, Candida fluviatilis 24p-51, Kocuria rosea 24p-61, Candida fluviatilis 24p-71, Candida fluviatilis 24p-83, Acinetobacter calcoaceticus 7-43, Candida fluviatilis 7p-72, and Rhodococcus erythropolis 24-44. The presence of the gene nahH is an indirect evidence of plasmid localization of catabolic operons [48], and there it is possible that the genes of naphthalene biodegradation in the studied strains of pseudomonads are also situated in plasmids.
Microorganisms have different mechanisms to provide efficient mechanisms for consuming hydrocarbon substrates, for example, such as production of surface-active substances [35]. Biosurfactants are the amphiphilous compounds, which can decrease significantly surface and interphase tension. The capacity for producing such compounds is widely distributed among oil-oxidizing microorganisms [16, 38]. The presence in growth medium of biosurfactants provides for emulsioning of water-insoluble substrates, including hydrocarbons [37]. It is expected that such mechanism promotes the increase of substrate bioavailability for cells, and this makes easier substrate use as the source of carbon and energy and, consequently, allows increasing the efficiency of biodegradation [41]. The decrease in surface tension of culture medium by no less than to 40 mN/m is considered as the most important criterion of selecting the producers of biosurfactants [51]. Among the studied microorganisms, significant decrease of surface tension (to 30 ± 1 mN/m) was recorded for only one strain, Rhodococus erythropolis 24-44 (Table S4), and this suggested the capacity y of this strain to produce the efficient biosurfactants.
Thus, the following criteria of the the most efficient strains to be selected into the resultant consortium were defined: the presence of a wide range of oxidizable substrates of different classes; the capacity y for growing on hydrocarbons in a wide temperature range; the capacity y for growing on hydrocarbons in acid media; the capacity for growing on hydrocarbons under increased salt content; and the presence of plasmids capable of producing biosurfactants.
The range of oxidizable substrates was evaluated separately for three classes of compounds: alkanes, MAH (monocyclic aromatic hydrocarbons), and PAH. The strains that obtained high ranks “3” and “4”,offer the greatest promise for including into the the consortium: Candida fluviatilis 24р-51, Rhodococcus erythropolis 24-44, Acinetobacter calcoaceticus 7-43, Pseudomonas fluorescens 7-41, Pseudomonas extremaustralis 7-31, and Pseudomonas fluorescens 7р-81. Poor settling of cells of the strain 7р-81 was found in the experiment, and this aggravated very much the obtaining of the biomass for the preparation. Moreover, it is reasonable to include microorganisms of different genera for the purposes of less competition in metabolism of strains, so the strains Candida fluviatilis 24р-51, Rhodococcus erythropolis 24-44, Acinetobacter calcoaceticus 7-43, and Pseudomonas extremaustralis 7-31 were included into the consortium.
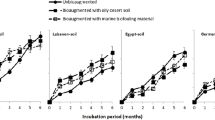
Selected strains were tested for the efficiency of decomposing different oil fractions. Gradual decrease in the decomposition activity of strains Acinetobacter calcoaceticus 7-43 and Candida fluviatilis 24р-51 was observed as the initial oil concentration increased (Fig. 1a). Decomposition activity of the strain Pseudomonas extremaustralis 7-31 sharply (by fiver times) decreased as oil concentration in the medium increased from 2 to 10%. Oil degradation by the strain Rhodococcus erythropolis 24-44 practically did not change, as oil concentration in the medium increased, but the proportion changed between the decrease of hydrocarbons of different fractions: The strain had a greater activity towards hydrocarbons of high-boiling oil fraction at high concentrations.
Degree of degradation of different fractions of hydrocarbons in (a) liquid mineral media with different oil contents and (b) liquid mineral media and sand with 10% oil relative to the control systems without microorganisms over 10 days of incubation at 24°C. Error bars designate standard deviation for three biological repetitions.
Qualitative and quantitative evaluation of the residual content of PAH was carried out in model systems with 2% oil (Table S5). The maximum decrease in concentrations of naphthalene and dibenzo[a, h]anthracene was observed in the system with strain 24р-51. Strain 7-31 actively consumed naphthalene, fluoranthene, dibenzo[а, h]anthracene, and benzo[g, h, i]perylene. Strain 7-43 demonstrated maximum efficiency in decomposing naphthalene, anthracene, fluoranthene, benzo[а]anthracene, benz[а]pyrene, and dibenzo[а, h]anthracene. There were no significant changes in PAH concentrations in the system with strain 24-44, whereas Rhodococcus decomposed significantly n-alkanes of both medium-boiling and high-boiling fractions of oil hydrocarbons (Table S6). On the basis of data on the residual concentrations of PAHs (Table S5), the maximum total decomposition of PAHs was found in model systems with strain 7-31 in comparison with the control (without microorganisms). It should be noted that medium-boiling fraction of oil hydrocarbons includes naphthalene, acenaphthene, fluorene, phenanthrene, and anthracene. The obtained results related to the presence of the genes of PAH degradation found in the strain Pseudomonas extremaustralis 7-31, and this apparently determined the maximum decrease in the content of aromatic hydrocarbons.
Living cells of microorganisms that preserved high activity of enzyme systems for biodegradation of oil components are the basis of most biopreparations for soil and water cleanup from oil contamination. The obtaining of biomass of available bacteria (culturing) is the main stage of biopreparation obtaining. The efficiency of bioremediation also depends on the quality of obtained biomaterial. At the same time, the stage of culturing of microorganisms is one of cost-intensive: expenses on culture media, electrical energy for bioreactors and so on form a larger part of production costs of the bioproduct. So, the refinement of the regimes of culturing the strains of microbial consortium was carried out at finishing stage. The results on separate submerged cultivation of the strains of microbial consortium are presented in Table 4. The process lasted from 16 to 22 h. The number of microorganisms in concentrated suspensions was more than 1 × 109 CFU/mL, and this attested to the balanced state of the chemical composition of nutrient media and optimally selected conditions of culturing the strains. Kuyukina et al. [6] demonstrated coculturing of microorganisms of the same genus. However, we cannot use this method in our work, because the conditions for optimal growth of bacteria and yeasts of microbial preparation differed significantly.
Preservation of the number of viable cells (CFU) in the course of drying is one of critical parameters determining the quality of bacterial preparations used in different economic sectors. The choice of optimal composition of protective media (cryoprotectors) plays a crucial role in successful decision of the problem of CFU stabilization. Two groups of substances play a pivotal role in the composition of protective media: carbohydrate-protein structures determining the crystalline structure of the frozen products and the group of antioxidants. Sodium glutamate in concentration 5% was used as antioxidant and demonstrated high efficiency [9]. The number of viable microorganisms in the obtained dry preparations ranged from 109 to 1011.
Dry preparation containing the strains of the consortium was made on the basis of obtained results. The number of active strains in the concentrated product reached 1.5 × 1010 CFU/g of dry preparation. The number of microorganisms decreased 1.2 times after 1 month of keeping the dry consortium preparation at 6°C, and this fact suggests high efficiency of storing the biomass of oil-decomposing microorganisms.
Model laboratory experiments were carried out in order to evaluate the quality of obtained dry preparation of microbial consortium. The results of analysis (Fig. 1b) demonstrated a more uniform consumption of carbohydrates of different oil fractions in liquid mineral medium as well as in sand in comparison with that shown in Fig. 1a (at contamination level of 10%). The loss of oil in liquid mineral medium reached 56%, and this exceeded values obtained for particular microorganisms (Fig. 1a). Oil was degraded by microbial consortium in liquid medium two times faster in comparison sand. It is likely that active agitation of liquid mineral medium increased oxygen availability and uniformity of distribution of hydrocarbons in the system, and this promoted accelerated consumption of hydrocarbons by microorganisms of the consortium. Periodical soil plowing and an increase in remediation time are necessary to provide the efficiency of degradation of oil hydrocarbons by microbial consortium in the soil systems.
CONCLUSIONS
An algorithm for the development of the microbial consortium for cleaning oil-contaminated territories in the Middle Ob region was obtained on the basis of the analysis of physiological, biochemical, and genetic properties of isolated and identified strains−decomposers of oil hydrocarbons. The obtained microbial consortium included the following strains: Candida fluviatilis 24р-51, Rhodococcus erythropolis 24-44, Acinetobacter calcoaceticus 7-43, and Pseudomonas extremaustralis 7-31.
REFERENCES
N. A. Avetov, O. L. Kuznetsov, and E. A. Shishkonakova, “Soils of oligomesotrophic and mesotrophic bogs in the boreal zone of West Siberia: possibilities of botanical diagnostics within the framework of the type of mesotrophic peat soils,” Eurasian Soil Sci. 54, 689–701 (2021). https://doi.org/10.1134/S1064229321030029
N. A. Avetov and E. A. Shishkonakova, “Oil pollution of soils in the taiga zone of Western Siberia,” Byull. Pochv. Inst. im. V.V. Dokuchaeva, No. 68, 45–55 (2011).
A. A. Vetrova, A. A. Ivanova, A. E. Filonov, V. A. Zabelin, A. B. Gafarov, S. L. Sokolov, I. A. Nechaeva, I. F. Puntus, and A. M. Boronin, “Biodegradation of oil by the strains and principles of composition of microbial consortiums for cleaning the environment from petroleum hydrocarbons,” Izv. Tul’sk. Gos. Univ., Estestv. Nauki, No. 2, 241–257 (2013).
Yu. S. Drugov and A. A. Rodin, Ecological Analysis of in Case of Spills of Oil and Petroleum Products: Practical Guide (BINOM. Laboratoriya Znanii, Moscow, 2007) [in Russian].
T. Yu. Izmalkova, O. I. Sazonova, S. L. Sokolov, I. A. Kosheleva, and A. M. Boronin, “The P-7 incompatibility group plasmids responsible for biodegradation of naphthalene and salicylate in fluorescent pseudomonads,” Microbiology (Moscow) 74, 290–295 (2005).
M. S. Kuyukina and I. B. Ivshina, RF Patent No. 2180276, Byull. Izobret., No. 7 (2002).
T. Maniatis, E. F. Fritsch and J. Sambrook, Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory, New York, 1982; Mir, Moscow, 1984).
PND F 16.1:2.2.22-98. Guide for Measurement of Mass Fraction of Petroleum Products in Mineral, Organogenic, Organomineral Soils and Bottom Sediments by IR Spectrometry (Moscow, 1998) [in Russian].
K. V. Petrikov, Candidate’s Dissertation in Chemistry (Moscow, 2011).
Practical Guide on Soil Science, Ed. by N. F. Ganzhara (Agrokonsalt, Moscow, 2002) [in Russian].
O. I. Sazonova, A. A. Vetrova, A. B. Gafarov, and S. L. Sokolov, “Isolation of epiphytic strains Aureobasidium pullulans producing high-molecular extracellular polysaccharides,” Izv. Tul’sk. Gos. Univ., Estestv. Nauki, No. 4, 24–31 (2017).
O. I. Sazonova, A. A. Vetrova, R. A. Streletskii, A. B. Gafarov, I. A. Kosheleva, A. E. Filonov, and S. L. Sokolov, “Strains Pseudomonas extremaustralis 7–31 and Pseudomonas fluorescens 7–41 degrading aliphatic and aromatic hydrocarbons,” Izv. Tul’sk. Gos. Univ., Estestv. Nauki, No. 3, 31–43 (2019).
V. P. Seredina, E. V. Kolesnikova, V. A. Kondykov, A. I. Nepotrebnyi, and S. A. Ognev, “Specific impact of oil pollution on soils of the middle taiga of Western Siberia,” Neft. Khoz., No. 5, 108–112 (2017).
S. Ya. Trofimov, A. V. Arzamazova, R. R. Kinzhaev, N. A. Avetov, and M. M. Karpukhin, “Mineralization of organic matter in petroleum-polluted and background soils of the Middle Ob region in laboratory conditions,” Vestn. Mosk. Univ., Ser. 17: Pochvoved., No. 2, 51–56 (2021).
R. Alonso, A. Martín, T. Peláez, M. Marín, M. Rodríguez-Creixéms, and E. Bouza, “An improved protocol for pulsed-field gel electrophoresis typing of Clostridium difficile,” J. Medical Microbiol. 54 (2), 155–157 (2005). https://doi.org/10.1099/jmm.0.45808-0
E. Antoniou, S. Fodelianakis, E. Korkakaki, and N. Kalogerakis, “Biosurfactant production from marine hydrocarbon-degrading consortia and pure bacterial strains using crude oil as carbon source,” Front. Microbiol. 6, 274 (2015). https://doi.org/10.3389/fmicb.2015.00274
M. G. Barron, D. N. Vivian, R. A. Heintz, and U. H. Yim, “Long-term ecological impacts from oil spills: comparison of Exxon Valdez, Hebei Spirit, and Deepwater Horizon,” Environ. Sci. Technol. 54 (11), 6456–6467 (2020). https://doi.org/10.1021/acs.est.9b05020
BLAST. http://www.ncbi.nlm.nih.gov/BLAST.
R. J. W. Brooijmans, M. I. Pastink, and R. J. Siezen, “Hydrocarbon-degrading bacteria: the oil-spill clean-up crew,” Microb. Biotechnol. 2 (6), 587–594 (2009). https://doi.org/10.1111/j.1751-7915.2009.00151.x
L. Cabral, P. Giovanella, E. P. Pellizzer, E. H. Teramoto, C. H. Kiang, and L. D. Sette, “Microbial communities in petroleum-contaminated sites: structure and metabolisms,” Chemosphere 286 (2), 131752 (2022). https://doi.org/10.1016/j.chemosphere.2021.131752
A. Dasgupta, R. Saikia, and P. J. Handique, “Mapping the bacterial community in Digboi oil refinery, India by high-throughput sequencing approach,” Curr. Microbiol. 75 (11), 1441–1446 (2018). https://doi.org/10.1007/s00284-018-1541-x
J. Davies and W. Evans, “Oxidative metabolism of naphthalene by soil pseudomonads. The ring-fission mechanism,” Biochem. J. 91 (2), 251–261 (1964). https://doi.org/10.1042/bj0910251
L. Derguine-Mecheri, S. Kebbouche-Gana, and D. Djenane, “Biosurfactant production from newly isolated Rhodotorula sp. YBR and its great potential in enhanced removal of hydrocarbons from contaminated soils,” World J. Microbiol. Biotechnol. 37 (1), 18 (2021). https://doi.org/10.1007/s11274-020-02983-3
H. P. Doddamani and H. Z. Ninnekar, “Biodegradation of carbaryl by a Micrococcus species,” Curr. Microbiol. 43 (1), 69–73 (2001). https://doi.org/10.1007/s002840010262
P. Elumalai, P. Parthipan, M. Huang, B. Muthukumar, L. Cheng, M. Govarthanan, and A. Rajasekar, “Enhanced biodegradation of hydrophobic organic pollutants by the bacterial consortium: Impact of enzymes and biosurfactants,” Environ. Pollut. 289, 117956 (2021). https://doi.org/10.1016/j.envpol.2021.117956
C. G. T. Evans, D. Herbert, and D. W. Tempest, “The continuous cultivation of microorganisms: 2. Construction of a chemostat,” in Methods in Microbiology (Elsevier, Amsterdam, 1970), Vol. 2, Ch. 13, pp. 277–327.
W. Evans, H. Fernley, and E. Griffiths, “Oxidative metabolism of phenanthrene and anthracene by soil pseudomonads. The ring-fission mechanism,” Biochem. J. 95 (3), 819–831. 1965). https://doi.org/10.1042/bj0950819
GeneJET PCR Purification Kit. https://www.thermofisher.com/order/catalog/product/K0702.
J. J. Germida, C. M. Frick, and R. E. Farrell, “Phytoremediation of oil-contaminated soils,” in Developments in Soil Science (Elsevier, Amsterdam, 2002), Vol. 28, Part 2, pp. 169–186. https://doi.org/10.1016/S0166-2481(02)80015-0
J. B. Herrick, K. G. Stuart-Keil, W. C. Ghiorse, and E. L. Madsen, “Natural horizontal transfer of a naphthalene dioxygenase gene between bacteria native to a coal tar-contaminated field site,” Appl. Environ. Microbiol. 63 (6), 2330–2337 (1997). https://doi.org/10.1128/aem.63.6.2330-2337.1997
H. Kiyohara, S. Torigoe, N. Kaida, T. Asaki, T. Iida, H. Hayashi, and N. Takizawa, “Cloning and characterization of a chromosomal gene cluster, pah, that encodes the upper pathway for phenanthrene and naphthalene utilization by Pseudomonas putida OUS82,” J. Bacteriol. 176 (8), 2439–2443 (1994). https://doi.org/10.1128/jb.176.8.2439-2443.1994
E. Koshlaf and A. Ball, “Soil bioremediation approaches for petroleum hydrocarbon polluted environments,” AIMS Microbiol. 3 (1), 25–49 (2017). https://doi.org/10.3934/microbiol.2017.1.25
M. J. Larkin and M. J. Day, “The metabolism of carbaryl by three bacterial isolates, Pseudomonas spp. (NCIB 12042 & 12043) and Rhodococcus sp. (NCIB 12038) from garden soil,” J. Appl. Bacteriol. 60 (3), 233–242 (1986). https://doi.org/10.1111/j.1365-2672.1986.tb01078.x
A. D. Laurie and G. Lloyd-Jones, “Quantification of phnAc and nahAc in contaminated New Zealand soils by competitive PCR,” Appl. Environ. Microbiol. 66 (5), 1814–1817 (2000). https://doi.org/10.1128/AEM.66.5.1814-1817.2000
Ł. Ławniczak, M. Woźniak-Karczewska, A. P. Loibner, H. J. Heipieper, and Ł. Chrzanowski, “Microbial degradation of hydrocarbons—basic principles for bioremediation: a review,” Molecules 25 (4), 856 (2020). https://doi.org/10.3390/molecules25040856
X. Li, L. Zhao, and M. Adam, “Biodegradation of marine crude oil pollution using a salt-tolerant bacterial consortium isolated from Bohai Bay, China,” Mar. Pollut. Bull. 105 (1), 43–50 (2016). https://doi.org/10.1016/j.marpolbul.2016.02.073
A. R. Markande, D. Patel, and S. Varjani, “A review on biosurfactants: properties, applications and current developments,” Bioresour. Technol. 330, 124963 (2021).
G. T. Mehetre, S. G. Dastager, and M. S. Dharne, “Biodegradation of mixed polycyclic aromatic hydrocarbons by pure and mixed cultures of biosurfactant producing thermophilic and thermo-tolerant bacteria,” Sci. Total Environ. 679, 52–60 (2019).
S. Mnif, M. Chamkha, and S. Sayadi, “Isolation and characterization of Halomonas sp. strain C2SS100, a hydrocarbon-degrading bacterium under hypersaline conditions,” J. Appl. Microbiol. 107 (3), 785–794 (2009). https://doi.org/10.1111/j.1365-2672.2009.04251.x
Y. Nie, C.-Q. Chi, H. Fang, J.-L. Liang, S.-L. Lu, G.‑L. Lai, Y.-Q. Tang, and X.-L. Wu, “Diverse alkane hydroxylase genes in microorganisms and environments,” Sci. Rep. 4 (1), 4968 (2014).
S. Patel, A. Homaei, S. Patil, and A. Daverey, “Microbial biosurfactants for oil spill remediation: pitfalls and potentials,” Appl. Microbiol. Biotechnol. 103 (1), 27–37 (2019). https://doi.org/10.1007/s00253-018-9434-2
V. Patel, A. Sharma, R. Lal, N. A. Al-Dhabi, and D. Madamwar, “Response and resilience of soil microbial communities inhabiting in edible oil stress/contamination from industrial estates,” BMC Microbiol. 16 (1), 50 (2016). https://doi.org/10.1186/s12866-016-0669-8
R. Perdigão, C. M. R. Almeida, F. Santos, M. F. Carvalho, and A. P. Mucha, “Optimization of an autochthonous bacterial consortium obtained from beach sediments for bioremediation of petroleum hydrocarbons,” Water 13 (1), 66 (2020). https://doi.org/10.3390/w13010066
A. V. Poliakova, I. I. Chernov, and N. S. Panikov, “Yeast biodiversity in hydromorphic soils with reference to grass-Sphagnum swamp in Western Siberia and the hammocky tundra region (Barrow, Alaska),” Mikrobiologiia 70 (5), 714–20 (2001). https://doi.org/10.1023/A:1012328710111
R. A. Rosselló-Mora, J. Lalucat, and E. García-Valdés, Comparative biochemical and genetic analysis of naphthalene degradation among Pseudomonas stutzeri strains,” Appl. Environ. Microbiol. 60 (3), 966–972 (1994). https://doi.org/10.1128/aem.60.3.966-972.1994
J. Sambrook and D. W. Russell, Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, New York, 2001).
J. Sanseverino, B. M. Applegate, J. M. King, and G. S. Sayler, “Plasmid-mediated mineralization of naphthalene, phenanthrene, and anthracene,” Appl. Environ. Microbiol. 59 (6), 1931–1937 (1993). https://doi.org/10.1128/aem.59.6.1931-1937.1993
K. N. Timmis, P. R. Lehrbach, S. Harayama, R. H. Don, N. Mermod, S. Bas, R. Leppik, A. J. Weightman, W. Reineke, and H. J. Knackmuss, “Analysis and manipulation of plasmid-encoded pathways for the catabolism of aromatic compounds by soil bacteria,” in Plasmids in Bacteria, Ed. by D. R. Helinski, S. N. Cohen, (Plenum, New York, 1985), pp. 719–739.
M. Tyagi, M. M. R. da Fonseca, and C. C. C. R. de Carvalho, “Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes,” Biodegradation 22 (2), 231–241 (2011). https://doi.org/10.1007/s10532-010-9394-4
S. Viggor, J. Juhanson, M. Jõesaar, M. Mitt, J. Truu, E. Vedler, and A. Heinaru, “Dynamic changes in the structure of microbial communities in Baltic Sea coastal seawater microcosms modified by crude oil, shale oil or diesel fuel,” Microbiol. Res. 168 (7), 415–427 (2013). https://doi.org/10.1016/j.micres.2013.02.006
V. Walter, C. Syldatk, and R. Hausmann, “Screening concepts for the isolation of biosurfactant producing microorganisms,” in Biosurfactants, Ed. by R. Sen (Springer-Verlag, New York, 2010), pp. 1–13. https://doi.org/10.1007/978-1-4419-5979-9_1
Y. Yang, J. Wang, J. Liao, S. Xie, and Y. Huang, “Abundance and diversity of soil petroleum hydrocarbon-degrading microbial communities in oil exploring areas,” Appl. Microbiol. Biotechnol. 99 (4), 1935–1946 (2015). https://doi.org/10.1007/s00253-014-6074-z
Z.-F. Zhou, M.-X. Wang, X.-H. Zuo, and Y.-H. Yao, “Comparative investigation of bacterial, fungal, and archaeal community structures in soils in a typical oilfield in Jianghan, China,” Arch. Environ. Contam. Toxicol. 72 (1), 65–77 (2017). https://doi.org/10.1007/s00244-016-0333-1
S. Zinjarde, M. Apte, P. Mohite, and A. R. Kumar, “Yarrowia lipolytica and pollutants: Interactions and applications,” Biotechnol. Adv. 32 (5), 920–933 (2014). https://doi.org/10.1016/j.biotechadv.2014.04.008
Funding
This work was carried out within the framework of state assignment of the Ministry of Science and Higher Education of the Russian Federation no. № 121041300098-7 and, partly, within the framework of the development program of the Interdisciplinary Scientific and Educational School of Lomonosov Moscow State University “Future of the Planet and Global Environmental Changes.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by T. Chicheva
Supplementary Information
Rights and permissions
About this article
Cite this article
Vetrova, A.A., Trofimov, S.Y., Kinzhaev, R.R. et al. Development of Microbial Consortium for Bioremediation of Oil-Contaminated Soils in the Middle Ob Region. Eurasian Soil Sc. 55, 651–662 (2022). https://doi.org/10.1134/S1064229322050106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229322050106