Abstract
Bioremediation strategies apply environmental microbes to metabolize organic compounds and can be useful for the treatment of oil-contaminated soils. In this study, different approaches of bioremediation were compared on a scale-up treatment. The defined microbial consortium was formulated with degrading microorganisms previously selected (Pseudomonas mendoncina BPB 1.8, Bacillus cereus BPB 1.20, Bacillus cereus BPB 1.26, and Bacillus sphaericus BPB 1.35). Bioaugmentation/biostimulation, biostimulation, and natural attenuation strategies were evaluated after 60 days of treatment by gas chromatography. The contaminant level remained elevated after the treatments using natural attenuation and biostimulation. However, the bioaugmentation with biostimulation treatment showed a satisfactory ability to degrade petroleum hydrocarbons (85%). Interestingly, no correlation was observed with the presence of hydrocarbon-degrading microorganisms and CO2 production, and denaturing gradient gel electrophoresis exhibited no significant difference in the biodiversity of the treatments. Although, the results showed that the microbial consortium was imperative to the successful biodegradation of TPH-contaminated gas station soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Petroleum-based products are the principal energy source in the world. The main constituents belong to the saturated hydrocarbons, aromatic hydrocarbons, resins and asphaltenes classes (Liang et al. 2012; Mahjoubi et al. 2018). These compounds are among the most harmful to both the environment and human health. Leaks and accidental spills occur regularly during the oil exploration and processing (Teng et al. 2013; Chen et al. 2015; Wang et al. 2018). In addition, cases of large or small spills remain undetected for long periods, resulting in inflammable gasses in the soil and potentially dangerous conditions for the environment (Almeida et al. 2010).
Bioremediation is one alternative for the decontamination of polluted areas, offering the potential for the degradation of toxic contaminants, primarily by using microorganisms with the metabolic and physiological capacity to use hazardous organic compounds as a source of carbon and energy. Additionally, these technologies provide efficient results and simplified maintenance to reduce petroleum hydrocarbons to concentrations accepted by environmental agencies around the world, representing an attractive, cost-effective strategy (Maier and Gentry 2015; Napp et al. 2018; Roy et al. 2018). Indeed, the presence of microorganisms, which could potentially degrade petroleum hydrocarbons in contaminated and uncontaminated soil, has been well documented (Bento et al. 2005; Kaczorek and Olszanowski 2011; Fukuhara et al. 2013; Shankar et al. 2014), suggesting that the autochthonous soil microbiota can be useful for stimulated degradation. In this case, the biostimulation consists of correcting nutritional conditions in the contaminated environment, which accelerates the fuel degrading process through the activity and proliferation of the microbial population (Tyagi et al. 2011). An example of the application of the biostimulation strategy was that which occurred after the Exxon Valdez oil spill (1989). In an attempt to overcome low levels of N and P on the beaches of Prince William Sound in the Gulf of Alaska, large amounts of fertilizers (approximately 50,000 kg of N and 5000 kg of P) were added during the summers from 1989 to 1992 (Boufadel et al. 2010). Colla et al. (2014), investigated the effectiveness of successive bioaugmentation, conventional bioaugmentation, and biostimulation of biodegradation of blend diesel and biodiesel (B10) in soil. Nutrient introduction (biostimulation) promoted a positive effect on microbial populations and the total petroleum hydrocarbon (TPH) analysis indicated a biodegradation level of 35.7 and 32.2% for the biostimulation and successive bioaugmentation treatments, respectively.
On a laboratory scale, many isolated microorganisms can effectively degrade a single type of pollutant. However, when introduced into real field conditions with multiple types of contaminants, they often do not function as expected (Cerqueira et al. 2012; Sarkar et al. 2016; Wu et al. 2016; Ebadi et al. 2017; Roy et al. 2018). Associations of microorganisms increase their ability to utilize a large number of hydrocarbons as their sole source of carbon for survival (Napp et al. 2018). These microorganisms can completely mineralize the petroleum compounds through the metabolic actions of one or more strains. In this context, the application of mixed cultures in the environments contaminated with petroleum presents characteristics more advantageous than the pure cultures, due to the effect of synergic and co-metabolic interactions between the members of the association. The complete degradation of hydrocarbons involves the use of microbial consortia. Besides that, large-scale studies are limited concerning both the production of microorganisms and bioremediation treatment.
The present study aimed to evaluate the scale-up application of a defined microbial consortium in total petroleum hydrocarbon (TPH)-contaminated gas station soil from South Brazil, in order to analyze the effects of different remediation strategies applied in situ, to generate an environmentally safe alternative microbial formulation.
Materials and methods
Soil samples
Samples of soil contaminated with diesel oil were collected from the sand filters system of gas stations located in Porto Alegre, Rio Grande do Sul, Brazil. The filter system comprises a sandbox, oil separator and oil collector used to accumulate the oil and to fractionate the solid waste generated at gas stations. This system separates water from oil coming from the wash, supply, fuel discharge and exchange of lubricants for motor vehicles. The samples were placed in sterile bags, which had been hermetically closed and stored at 4 °C until further use. The physic–chemical soil characteristics were performed by Soils Analysis Laboratory at Federal University of Rio Grande do Sul (UFRGS-RS, Brazil).
Formulation of microbial consortia
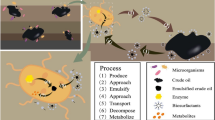
The microbial consortium used in bioaugmentation was previously selected based on the hydrocarbon degradation capacity (Allebrandt et al. 2015), comprising four degrading microorganisms: Pseudomonas mendoncina BPB 1.8, Bacillus cereus BPB 1.20, Bacillus cereus BPB 1.26 and Bacillus sphaericus BPB 1.35. The production of microbial cells was conducted using nutrient broth (Cerqueira et al. 2011) supplemented with soy protein (0.5%) under stirring at 37 °C. First, the pre-inoculum was generated in 10 mL of medium and incubated for 24 h. After, the pre-inoculum was transferred to 300 mL of the same medium and incubated for 12 h. In the third step, the inoculum was subsequently transferred to a 10 L reactor containing the culture medium with 0.02% of anti-emulsifying silicon (base poliglycol) and incubated for 15 h. In the last step, the content of the 10 L reactor was transferred to a 100 L reactor containing the medium with mineral solution (MgSO4 0.05%, CaCl2 0.01% and MnSO4 0.005%) and 0.05% of the same anti-emulsifying. After 24 h, the cells were centrifuged in an industrial centrifuge (5000 rpm for 2 h). Cell growth in the 100 L reactor was mixed and suspended in a solution containing sterile water, NaCl and xanthan gum. The final product (patent number BR 10 2014 024646) was stored at 4 °C until further use.
Scale-up bioremediation strategies
The scale-up bioremediation treatments were carried out in three wooden boxes, with dimensions of 1 m × 1 m × 0.60 m (L × W × H), which were used to accommodate TPH-contaminated soil. Each box was set up in duplicate containing 300 kg of soil, and the following remediation treatments were evaluated: natural attenuation (NA), biostimulation (BS) or bioaugmentation/biostimulation (BAS), adapted from Suja et al. (2014). The NA corresponds to condition with only the soil contaminated. The BS soil box was treated with 200 mL of nutrient solution (67 g NH4SO4 and 15 g KH2PO4), and BAS soil box was treated with 500 mL of the microbial consortium, containing 3.5 × 106 cells mL−1 of each microorganism and 200 mL of the nutrient solution. The inoculum and nutrient solution volumes added to the treatments were adjusted to maintain the soil field capacity at 80%, based on Suja et al. (2014). During 60 days of experimental analysis, the systems were performed at room temperature ranging from 15 to 30 °C.
Soil samples within the boxes were collected at four different depths, ranging from 0.2 to 0.5 m, forming a representative composite sample to determine pH, bacterial diversity and TPH concentration. The treatments were performed at room temperature ranging from 15 to 30 °C. The pH of the soil in the quadrants of each box was determined with a digital pH meter (Beckman PHI 71 model).
Microbial activity evaluation
The microbial respiratory activity was evaluated using the respirometric method of Bartha (Bartha and Pramer 1965) by the cumulative release of CO2. Microcosms with 300 g of the soil obtained from the bioremediation treatments (NA, BAS and BS) were monitored for 60 days. The amount of carbon dioxide produced was calculated using Eq. (1):
where VB and VA are the volumes of 0.1 M HCl used to titrate the blank and the treatment in mL, respectively; MC is the molar mass of carbon dioxide in g/mol; MHCl is the molar concentration of HCl standard solution in mol/L; FC is the correction factor for acid/base molarity (MHCl/MKOH); and m is the mass in kg of dry soil in the flask.
Quantitative analysis of hydrocarbons
TPHs were determined using the US EPA 8015 technique to analyze different fractions of organic compounds: C8–C11 (gasoline range organics), C11–C14 (kerosene range organics), C14–C20 (diesel range organics) and C20–C40 (lubricating oil range organics). The soil sample was collected and extracted with methylene chloride at different treatment times (0, 30 and 60 days) and injected into a gas chromatography with flame ionization detector (GC-FID). A capillary column HP Basic Wax (30 m, 0.53 mm i.d. × 1 μm film thickness) was used, and the samples were injected (1 µL) in split mode (10:1) and carrier gas (nitrogen) at a constant flow of 1 mL min−1. The injector and detector temperatures were 270 and 350 °C, respectively. The initial oven temperature was 50 °C for 0.5 min. The heating ramp was at the rate of 50 °C min−1 to 350 °C, kept at this temperature for 15 min. The hydrocarbons identification of each range was based on their respective retention times based on analytical standards. Percentage of degradation was calculated by the following Eq. (2):
Soil DNA extraction, sequencing and DGGE analysis
Total DNA was extracted from 300 mg of soil samples using the PowerSoil DNA Isolation Kit (MO BIO Inc., USA), according to the manufacturer’s instructions. A fragment of the V3 region of the bacterial 16S rRNA gene was amplified from metagenomic DNA using the primers BA338 F-GC (5′-278CGCCCGCCGCGCGCGGCGGGCGGGGCGGGGCACGGGGGGACTCCTACGGGAGGCAGCAG-3′) and UN518R (5′-ATTACCGCGGCTGCTGG-3′) (Ovreas et al. 1997).
The amplification was performed in a reaction containing 20 ng μL−1 of DNA template, 1 U Platinum Taq DNA Polymerase (Invitrogen, São Paulo, Brazil), 3 mM MgCl2, 0.2 mM dNTP, and 5 pmol of each primer at a final volume of 25 μL. The amplification conditions were 5 min at 95 °C, followed by 30 cycles for 1 min at 95 °C, 1 min at 55 °C, and 1 min at 72 °C with a final extension at 72 °C for 10 min. The polymerase chain reaction (PCR) amplicons were analyzed through denaturing gradient gel electrophoresis (DGGE) (Ovreas et al. 1997). An 8% polyacrylamide (acrylamide/bis-acrylamide mix 37.5:1 wt.v−1) gel containing a 15–55% urea-formamide gradient was used. The electrophoresis was performed in TAE 1 × buffer at a constant 200 V for 3 h and 30 min at 60 °C using the DCode TM System (Bio-Rad Inc., Hercules, CA, USA). After electrophoresis, the gel was stained with 3 μL Syber Green I (Invitrogen) in 300 mL of deionized water for 30 min. The images were acquired using a Kodak GL2200 photodocumentation system. The DGGE profiles were compared using Gel Compare software, followed by visual analysis. The resulting patterns were used to estimate the diversity via the Shannon–Weaver (H′) index. Each band was considered an operational taxonomic unit (OTU). The data analysis was performed using the DivEs Species Diversity program, version 2.0. Dendrograms were produced after evaluating the binary array generated from the band profiles and subjected to statistical analysis using the Dice coefficient.
Statistical analysis
Descriptive statistics were based on biological triplicates using ANOVA, followed by Boneferroni’s test with a confidence level of 95%.
Results and discussion
Microbial consortium formulation
The microorganism selection to formulate the microbial consortium used on the BAS approach was based on the reduction of 2,3,5-triphenyl tetrazolium chloride (TTC) and rhamnolipids production (Allebrandt et al. 2015). The 16S rRNA gene sequencing revealed that two bacteria exhibited similarity to Pseudomonas mendoncina (98%) and Bacillus sphaericus (99%) and the other two microorganisms presented 99% similarity to Bacillus cereus.
These genera are commonly found in hydrocarbon-contaminated soils and have been described to potential bioremediation processes (Bento et al. 2005; Das and Chandran 2011; Yu et al. 2014; Chaudhary et al. 2015; Obi et al. 2016; Napp et al. 2018). According to Yu et al. (2014), a strain of Bacillus spp. isolated from petroleum-contaminated soil has been used for the bioremediation of the Shengli Oil Field, showing a crude oil removal rate of 67.7% after 2 months.
Furthermore, Bento et al. (2005) and Dörr de Quadros et al. (2016) also isolated Bacillus sp. from soil contaminated with diesel oil and petrochemical oily sludge, respectively.
Meyer et al. (2018) evaluated natural attenuation, biostimulation and bioaugmentation as bioremediation strategies in a controlled microcosm simulating a surface spill over soil with diesel/biodiesel mixtures, during 60 days of incubation. The bacterial inoculum employed for biostimulation/bioaugmentation strategy consisted of Bacillus megaterium, Bacillus pumilus, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia. The addition of the bacterial consortium and macronutrients contributed to 74.53% of biodiesel degradation (B100) in soil after 60 days.
Additionally, Pacwa-Płociniczak et al. (2014) used a strain of Pseudomonas sp. isolated from soil contaminated with hydrocarbons and showed the degradation of crude oil fractions from components of light fuel oils, components of heavy fuel oils and hexadecane (27, 39, 27 and 13% of hydrocarbons were degraded, respectively), while other authors also demonstrated the ability of Pseudomonas spp. to degrade a broad range of hydrocarbons, including crude oils, refined fuels, alkanes and polycyclic aromatic hydrocarbons (PAHs) (Arun et al. 2008; Kumar et al. 2008; Sopeña et al. 2013).
In this study, the microorganisms used in bioaugmentation treatment were isolated from TPH-contaminated gas station soils (diesel and gasoline), which probably enabled microbial adaptation to environments with high hydrocarbon indices (Maier and Gentry 2015; Abbasian et al. 2016). The microorganisms were separately cultivated in industrial-scale reactors (Fig. 1) and used to formulate the microbial consortium.
Evaluation of scale-up bioremediation treatments
Contaminated soils used in scale-up trials were analyzed based on their physical–chemical characteristics (Table 1). The analyses were performed separately for each treatment used and the soils showed similar features. The soil used in the experiment was slightly acidic with a pH range of 6.4 to 6.7. The most prevalent metals were K (78–158 mg dm−3), S (88–134 mg dm−3), Zn (60–187 mg dm−3) and Cu (32–106 mg dm−3). Moreover, the sample of soil collected exhibited low contents of organic matter (2.8–5%) and clay (7–12%). The soil collected at the gas station can be characterized as a soil that suffered weathering (variations in temperature, pressure, humidity) losing part of its original characteristics. Weathering works through mechanisms that modify the physical properties of minerals and rocks (morphology, resistance, texture) and its chemical characteristics (chemical composition and crystalline structure). In our case, the soil samples composed of analyzed soils reflected the different contents of clay and organic matter. According to Moschini et al. (2005), the environmental diagnosis of risks associated to potential leaking in underground storage tanks is highly dependent on the way fuels behave in different types of soils, which is influenced by the physical and chemical properties of liquid fuels (density, viscosity, solubility and vapor pressure) and by the characteristics of the soils through which these fuels migrate.
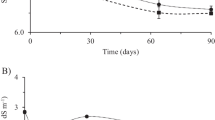
The pH was monitored during the 60 days in the boxes. The values remained between 6.2 and 7.2 in all bioremediation strategies. However, these differences were not statistically significant. The pH directly affects the activity of microorganisms through the effect of H + ions on enzymatic activity and cellular permeability, thereby indirectly influencing the availability of macro- and micronutrients (Al-Hawash et al. 2018). The small variations observed in the present study did not show a negative effect on microbial growth because the pH values remained neutral as a favorable condition for microbial growth. These results corroborate with Horel and Schiewer (2009), which previously reported adequate physical and environmental conditions for microbial growth and activity.
The total hydrocarbon degradation in contaminated soils is shown in Table 2. The initial values of the contaminants presented variations between treatments due to the origin of the contaminated soil and the difficulties in homogenization, reflecting the high volume of soil used in the treatments (300 kg). In the NA treatment, the TPH was 3.621 mg kg−1, whereas in BS and BAS treatments, contamination was 1.586 mg kg−1 and 6.703 mg kg−1, respectively.
As shown in Table 2, TPHs were markedly reduced in the BAS treatments from 6.703 to 969 mg kg−1 (85%). In addition, the light, intermediate and heavy fractions (C8–C14, C14–C20 and C20–C40, respectively) were also analyzed at 0 and 60 days (Table 3). Regarding the initial time, the light and intermediate fractions were detected at a higher concentration in BAS soil (1146 and 1605 mg kg−1), potentially reflecting the high initial TPH concentration present in the soil used for the BAS treatment when compared with the other treatments. For the NA and BS treatments, the initial concentrations in these fractions were similar (134 and 76 mg kg−1; 480 and 357 mg kg−1, respectively) (Table 3). The concentration of light fraction was reduced to levels lower than the quantification limit after 60 days of treatment in all remediation strategies, demonstrating that all remediation strategies used were reasonable in decreasing the concentration of hydrocarbons.
The results concerning the intermediate fraction (C14–C20) showed the highest reduction after 60 days in BAS treatment (88.7%) (Table 3). The BS treatment decreased the intermediate fraction level by 59%. However, the NA treatment showed only 11% of biodegradation. For the heavy hydrocarbon fraction (C20–C40), no reduction was detected through NA or BS treatments. Interestingly, BAS soil treatment showed again higher hydrocarbon reduction of 3.465–788 mg kg−1 (77.3%) (Table 3).
Independent of the hydrocarbons fraction assessed (light, intermediate or heavy) and bioremediation treatments applied, changes to the level of soil contamination were observed. In summary, these results demonstrate that BAS treatment obtained a higher degradation rate of contaminants in all fractions compared with other treatments.
The light fractions were degraded after 30 days, while the heavy fractions were better degraded within 60 days, inferring that the successive addition of the consortium favored degradation.
The sequential biostimulation with bioaugmentation strategy was also described by Tahhan et al. (2011) as the most effective treatment. The indigenous population did not overcome the exogenous inoculum, and the periodic BAS addition increased the bioremediation activity. In addition, the BS treatment was not as effective as a bioremediation process, demonstrating that the addition of adapted microorganisms was indispensable to hydrocarbon degradation. Bento et al. (2005) reported that treatment with concomitant bioaugmentation and biostimulation for three months obtained 72.7% and 75.2% of degradation of light and heavy fractions, respectively. Also, natural attenuation treatment was more effective than biostimulation.
However, Couto et al. (2010) demonstrated that natural attenuation was more effective as bioaugmentation and biostimulation strategy in an oil refinery. This observation could be associated with the age of the soil and physiologically adapted indigenous microorganisms, which can be useful in the degradation of the pollutants (Sabaté et al. 2004; Bento et al. 2005).
The initial biodegradation of organic compounds usually follows a period of adaptation or acclimatization of the microorganisms, in which the duration depends on the structure of the pollutant. Previous exposure to a contaminant by repeated applications or frequent oil spills consequently provides an environment in which the degradation pathways are maintained within an adapted community (Maier and Gentry 2015; Abbasian et al. 2016). The adaptation of microbial populations occurs most commonly through the induction of enzymes required for biodegradation, followed by an increase in the population of biodegradable organisms (Peixoto et al. 2011; Maier and Gentry 2015). Different microorganisms provide distinct degrees of hydrocarbon degradation. In this context, the application of mixed cultures in environments contaminated with petroleum presents more advantageous characteristics, when compared to pure cultures (Kostka et al. 2011; Napp et al. 2018). Thus, BAS treatment showed the potential for large-scale use and could be tested in hydrocarbon-contaminated environments.
Microbial activity analysis
Along with an evaluation of the pollutant’s biodegradation levels, a microcosm experiment was simultaneously performed in order to detect the release of CO2. The CO2 production is a parameter for quantifying the microbial activity in the soil, demonstrating that microorganisms are capable of assimilating hydrocarbons or other carbon source (Cerqueira et al. 2011). Figure 2 shows the values of CO2 accumulated during 60 days in the NA, BS, and BAS treatments.
The behavior of microbial communities showed higher levels of CO2 production in all treatments. Notably, the NA treatment showed similar levels of CO2 emissions as the soils subjected to the addition of inoculants and nutrients (BS and BAS), suggesting that adequate abiotic soil conditions stimulated the growth and metabolism of the resident flora. It is also reasonable to conclude that these treatments showed a similar profile in the oscillation of the values and experienced an adaptive phase during the initial period of the experiment, namely between 1 and 5 days. The similarities between the BAS and NA treatments demonstrated that the addition of autochthonous microorganisms in contaminated soil did not increase the rate of cellular respiration in the contaminated soil. Interestingly, no correlation was found between hydrocarbon-degrading microorganisms and CO2 production.
Colla et al. (2014) demonstrated that during the bioremediation experiments in soil contaminated with diesel–biodiesel, the CO2 evolution did not show no significant difference in soil microbial activity between biostimulation and bioaugmentation treatments.
In addition, the BS treatment obtained a higher microbial activity when compared with other treatments, in contrast with other studies, where the rate of CO2 production was higher in BAS treatments (Colla et al. 2014; Meyer et al. 2014; Szulc et al. 2014).
Bacterial community dynamics and structure analysis
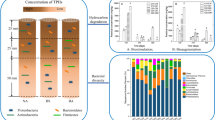
To understand how bioremediation influences the microbial community composition and the diversity of microorganisms DGGE analyses were performed for all treatments during different periods (0, 30 and 60 days). Four samples were additionally analyzed through DGGE, representing the enrichment cultures used in bioaugmentation (BPB 1.8, BPB 1.20, BPB 1.26 and BPB 1.35) (Fig. 3).
Bacterial dendrogram based on the DGGE banding profile generated from soil treated through bioaugmentation/biostimulation (BAS), biostimulation (BS) or natural attenuation (NA) at time 0. Bioaugmentation/biostimulation (BAS30), biostimulation (BS30), natural attenuation (NA30), after 30 days and bioaugmentation/biostimulation (BAS60), biostimulation (BS60), natural attenuation (NA60), after 60 days. The enrichment cultures are represented as BPB 1.8, BPB 1.20, BPB 1.26 and BPB 1.35. The x-axis shows community similarities based on Dice coefficient
The BAS treatment initially formed a cluster with the four microorganisms added in the consortium (Fig. 3). This cluster can be associated with the addition of the microbial consortium (10 × cell mL−1), justifying the stronger presence of the consortium in relation to indigenous microorganisms already presents in the contaminated soil. Thus, during the process of DNA extraction, and subsequent PCR amplification highlighted the consortium of microorganisms, for the detriment of the native microorganisms.
The BE and NA treatments were similar (72.57%) throughout the experiment (0, 30 and 60 days). However, for the BAS treatment with successive additions of the consortium for 30 days, the inoculum of microorganisms showed higher growth compared with the natural soil community. The Shannon–Wiener diversity index, presented in Table 4, shows that there was no significant difference in the biodiversity of the treatments, suggesting that the native microbiota acted synergistically with the microorganisms inoculated in the bioaugmentation treatment. The combination between the addition of microorganisms and the biostimulation strategy to affect the removal of TPH is shown to be an effective form in terms of bioremediation. In the present study, DGGE analysis (Fig. 3) shows that the addition of microorganisms reinforced the microbial population, does not replace or negatively interfere in the competition process, and together with nutrient supplement promotes further degradation of TPH.
Colla et al. (2014) evaluated the structure of bacterial community by DGGE, during bioremediation experiments in soil contaminated with blend B10. The results showed bacterial community changes in soil bioremediated with different strategies over 32 days.
Zeneli et al. (2019) have found that the combination of bioaugmentation-biostimulation and native microbial communities accelerates the bioremediation process of oil refinery sludge, while promoting a superior performance of degradation of PAHs. Roy et al. (2018) correlated the higher TPH bioremediation in refinery sludge with an increase in bacteria from genera described as hydrocarbon-degrading, such as Bacillus, Achromobacter, Rhodobacter, Pseudomonas, when they applied the bioaugmentation and biostimulation strategies in combination. The results observed in the present study, and the annotations already made in the literature show that the bioaugmentation with microorganisms that have the potential to degrade hydrocarbons and their stimulation with nutrients were the critical determinants for the successful removal of TPHs.
Conclusion
The application of a microbial consortium (BAS) showed potential for bioremediation when compared with the other strategies in 60 days. The results suggest that the degradation of hydrocarbons can effectively be achieved through the formulation and application of autochthonous microorganisms, depending on the treatment time and characteristics of the contaminated area. Also, the scale-up strategy showed satisfactory results in real field conditions. According to the present knowledge, this is one of the first studies to report scale-up successful bioaugmentation of TPH-contaminated soil in Brazil.
References
Abbasian F, Lockington R, Megharaj M, Naidu R (2016) A review on the genetics of aliphatic and aromatic hydrocarbon degradation. Appl Biochem Biotechnol 178:224–250. https://doi.org/10.1007/s12010-015-1881-y
Al-Hawash AB, Dragh MA, Li S, Alhujaily A, Abbood HA, Zhang X, Ma F (2018) Principles of microbial degradation of petroleum hydrocarbons in the environment. Egypt J Aquat Res 44:71–76. https://doi.org/10.1016/j.ejar.2018.06.001
Allebrandt SR, Napp AP, Mitidieri S, Bento FM, Vainstein MH (2015) Bioremediation of diesel oil-contaminated soil using native hydrocarbon-degrading bacterial strains under microcosm study. Int J Adv Res 3:732–746
Almeida JA, Augusto F, Jardim ICSF (2010) Biorremediação de solos contaminados por petróleos e seus derivados. Eclet Quím 35:17–43. https://doi.org/10.1590/S0100-46702010000300002
Arun A, Raja PP, Arthi R, Ananthi M, Kumar KS, Eyini M (2008) Polycyclicaromatic hydrocarbons (PAHs) biodegradation by basidiomycetes fungi, Pseudomonas isolate, and their cocultures: comparative in vivo and in silico approach. Appl Biochem Biotechnol 151:132–142. https://doi.org/10.1007/s12010-008-8160-0
Bartha R, Pramer D (1965) Features of flask and method for measurement of the persistence and biological effects of pesticides in soil. Soil Sci 100:68–70. https://doi.org/10.1097/00010694-196507000-00011
Bento FM, Camargo FAO, Okeke BC, Frankenberger WT (2005) Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresour Technol 96:1049–1055. https://doi.org/10.1016/j.biortech.2004.09.008
Boufadel MC, Sharifi Y, Van Aken B, Wrenn BA, Lee K (2010) Nutrient and oxygen concentrations within the sediments of an Alaskan beach polluted with the Exxon Valdez oil spill. Environ Sci Technol 44:7418–7424. https://doi.org/10.1021/es102046n
Cerqueira VS, Hollenbach EB, Maboni F, Vainstein MH, Camargo FAO, Peralba MCR, Bento FM (2011) Biodegradation potential of oily sludge by pure and mixed bacterial cultures. Biores Technol 102:11003–11010. https://doi.org/10.1016/j.biortech.2011.09.074
Cerqueira VS, Hollenbach EB, Maboni F, Camargo FA, Peralba Mdo C, Bento FM (2012) Bioprospection and selection of bacteria isolated from environments contaminated with petrochemical residues for application in bioremediation. World J Microbiol Biotechnol 28:1203–1222. https://doi.org/10.1007/s11274-011-0923-z
Chaudhary P, Sahay H, Sharma R, Pandey AK, Singh SB, Saxena AK, Nain L (2015) Identification and analysis of polyaromatic hydrocarbons (PAHs) biodegrading bacterial strains from refinery soil of India. Environ Monit Assess 187:1–9. https://doi.org/10.1007/s10661-015-4617-0
Chen M, Xu P, Zeng G, Yang C, Huang D, Zhang J (2015) Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: applications, microbes and future research needs. Biotechnol Adv 33:745–755. https://doi.org/10.1016/j.biotechadv.2015.05.003
Colla TS, Andreazza R, Bücker F, De Souza MM, Tramontini L, Prado GR, Frazzon APG, Camargo FAO, Bento FM (2014) Bioremediation assessment of diesel–biodiesel-contaminated soil using an alternative e bioaugmentation strategy. Environ Sci Pollut Res 21:2592–2602. https://doi.org/10.1007/s11356-013-2139-2
Couto MN, Monteiro E, Vasconcelos MT (2010) Mesocosm trials of bioremediation of contaminated soil of a petroleum refinery: comparison of natural attenuation, biostimulation and bioaugmentation. Environ Sci Pollut Res 17:1339–1346. https://doi.org/10.1007/s11356-010-0318-y
Das N, Chandran P (2011) Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol Res Int 2011:1–13. https://doi.org/10.4061/2011/941810
Dörr de Quadros P, Cerqueira VS, Cazarolli JC, Peralba MCR, Camargo FO, Giongo A, Bento FM (2016) Oily sludge stimulates microbial activity and changes microbial structure in a landfarming soil. Int Biodeterior Biodegrad 115:90–101. https://doi.org/10.1016/j.ibiod.2016.07.018
Ebadi A, Sima NAK, Olamaee M, Hashemi M, Nasrabadi RG (2017) Effective bioremediation of a petroleum-polluted saline soil by a surfactant producing Pseudomonas aeruginosa consortium. J Adv Res 8:627–633. https://doi.org/10.1016/j.jare.2017.06.008
Fukuhara Y, Horii S, Matsuno T, Matsumiya Y, Mukai M, Kubo M (2013) Distribution of hydrocarbon-degrading bacteria in the soil environment and their contribution to bioremediation. Appl Biochem Biotechnol 170:329–339. https://doi.org/10.1007/s12010-013-0170-x
Horel A, Schiewer S (2009) Investigation of the physical and chemical parameters affecting biodegradation of diesel and synthetic diesel fuel contaminating Alaskan soils. Cold Reg Sci Technol 58:113–119. https://doi.org/10.1016/j.coldregions.2009.04.004
Kaczorek E, Olszanowski A (2011) Uptake of hydrocarbon by Pseudomonas fluorescens (P1) and Pseudomonas putida (K1) strains in the presence of surfactants: a cell surface modification. Water Air Soil Pollut 214:451–459. https://doi.org/10.1007/s11270-010-0436-7
Kostka JE, Prakash O, Overholt WA, Green SJ, Freyer G, Canion A, Delgardio J, Norton N, Hazen TC, Huettel M (2011) Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the Deepwater Horizon oil spill. Appl Environ Microbiol 77:7962–7974. https://doi.org/10.1128/AEM.05402-11
Kumar M, De Sisto LV, A, Ilzins OA, Luis L, (2008) Biosurfactant production and hydrocarbon-degradation by halotolerant and thermotolerant Pseudomonas sp. World J Microbiol Biotechnol 24:1047–1057. https://doi.org/10.1007/s11274-007-9574-5
Liang YT, Zhang X, Wang J, Li GH (2012) Spatial variations of hydrocarbon contamination and soil properties in oil exploring fields across China. J Haz Mat 241:371–378. https://doi.org/10.1016/j.jhazmat.2012.09.055
Mahjoubi M, Cappello S, Souissi Y, Jaouani A, Cherif A (2018) Microbial bioremediation of petroleum hydrocarbon–contaminated marine environments. Recent Insights Petrol Sci Eng. https://doi.org/10.5772/intechopen.72207
Maier RM, Gentry TJ (2015) Microorganisms and organic pollutants. In: Environmental microbiology: Third Edition, pp 377–413. Elsevier Inc, Amsterdam. https://doi.org/10.1016/b978-0-12-394626-3.00017-x
Meyer DD, Beker SA, Bücker F, Peralba MCR, Frazzon APG, Osti JF, Andreazza R, Camargo FAO, Bento FM (2014) Bioremediation strategies for diesel and biodiesel in oxisol from southern Brazil. Int Biodeterior Biodegr 95:356–363. https://doi.org/10.1016/j.ibiod.2014.01.026
Meyer DD, Beker SA, Heck K, Peralba MCR, Bento FM (2018) Simulation of a surface spill of different diesel/biodiesel mixtures in an ultisol, using natural attenuation and bioaugmentation/biostimulation. An Acad Bras Ciênc 90:2741–2752. https://doi.org/10.1590/0001-3765201820170268
Moschini LE, Santos JE, Pires JSR (2005) Environmental diagnosis of risk areas related to gas stations. Braz Arch Biol Technol 48:657–666. https://doi.org/10.1590/S1516-89132005000500019
Napp AP, Pereira JES, Oliveira JS, Silva-Portela RCB, Agnez-Lima LF, Peralba MCR, Bento FM, Passaglia LMP, Thompson CE, Vainstein MH (2018) Comparative metagenomics reveals different hydrocarbon degradative abilities from enriched oil-drilling waste. Chemosphere 209:7–16. https://doi.org/10.1016/j.chemosphere.2018.06.068
Obi LU, Atagana HI, Adeleke RA (2016) Isolation and characterisation of crude oil sludge degrading bacteria. Springerplus 5:1–13. https://doi.org/10.1186/s40064-016-3617-z
Ovreas L, Forney L, Dae FL, Torsvik V (1997) Distribution of bacterioplankton in meromictic lake saelenvannet, as determined by denaturing gradient gel eletrophoresis of PCR-amplified gene fragment coding for 16S rRNA. Appl Environ Microbiol 63:3367–3373. https://doi.org/10.1128/AEM.63.9.3367-3373.1997
Pacwa-Płociniczak M, Płaza GA, Poliwoda A, Piotrowska-Seget Z (2014) Characterization of hydrocarbon-degrading and biosurfactant-producing Pseudomonas sp. P-1 strain as a potential tool for bioremediation of petroleum-contaminated soil. Environ Sci Pollut Res 21:9385–9395. https://doi.org/10.1007/s11356-014-2872-1
Peixoto RS, Vermelho AB, Rosado AS (2011) Petroleum-degrading enzymes: bioremediation and new prospects. Enzyme Res 2011:1–7. https://doi.org/10.4061/2011/475193
Roy A, Dutta A, Pal S, Gupta A, Sarkar J, Chatterjee A, Saha A, Sarkar P, Sar P, Kazy SK (2018) Biostimulation and bioaugmentation of native microbial community accelerated bioremediation of oil refinery sludge. Bioresour Technol 253:22–32. https://doi.org/10.1016/j.biortech.2018.01.004
Sabaté J, Viñas M, Solanas AM (2004) Laboratory-scale bioremediation experiments on hydrocarbon-contaminated soils. Int Biodeterior Biodegrad 54:19–25. https://doi.org/10.1016/j.ibiod.2003.12.002
Sarkar J, Kazy SK, Gupta A, Dutta A, Mohapatra B, Roy A, Bera P, Mitra A, Sar P (2016) Biostimulation of indigenous microbial community for bioremediation of petroleum refinery sludge. Front Microbiol 7:1–20. https://doi.org/10.3389/fmicb.2016.01407
Shankar S, Kansrajh C, Dinesh MG, Satyan RS, Kiruthika S, Tharanipriya A (2014) Application of indigenous microbial consortia in bioremediation of oil contaminated soils. Int J Environ Sci Technol 11:367–376. https://doi.org/10.1007/s13762-013-0366-1
Sopeña F, Laiz L, Morillo E, Sanchez-Trujillo MA, Villaverde J, Jurado V, Saiz-Jimenez C (2013) Phenanthrene biodegradation by Pseudomonas xanthomarina isolated from an aged contaminated soil. Clean: Soil, Air, Water 42:785–790. https://doi.org/10.1002/clen.201300247
Suja F, Rahim F, Taha MR, Hambali N, Razali RM, Khalid A, Hamzah A (2014) Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. Int Biodeterior Biodegradation 90:115–122. https://doi.org/10.1016/j.ibiod.2014.03.006
Szulc A, Ambrozewicz D, Sydow M, Lawniczak L, Piotrowska-Cyplik A, Marecik R, Chrzanowski L (2014) The influence of bioaugmentation and biosurfactant addition on bioremediation efficiency of diesel-oil contaminated soil: feasibility during field studies. J Environ Manag 132:121–128. https://doi.org/10.1016/j.jenvman.2013.11.006
Tahhan RA, Ammari TG, Goussous SJ, Al-Shdaifat HI (2011) Enhancing the biodegradation of total petroleum hydrocarbons in oily sludge by a modified bioaugmentation strategy. Int Biodeterior Biodegrad 65:130–134. https://doi.org/10.1016/j.ibiod.2010.09.007
Teng Y, Feng D, Song L, Wang J, Li J (2013) Total petroleum hydrocarbon distribution in soils and groundwater in Songyuan oilfield, Northeast China. Environ Monit Assess 185:9559–9569. https://doi.org/10.1007/s10661-013-3274-4
Tyagi M, Da Fonseca MR, De Carvalho CCCR (2011) Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegrad 22:231–241. https://doi.org/10.1007/s10532-010-9394-4
Wang Y, Liang J, Wang J, Gao S (2018) Combining stable carbon isotope analysis and petroleum-fingerprinting to evaluate petroleum contamination in the Yanchang oilfield located on loess plateau in China. Environ Sci Pollut Res 25:2830–2841. https://doi.org/10.1007/s11356-017-0500-6
Wu M, Dick WA, Li W, Wang X, Yang Q, Wang T, Xu L, Zhang M, Chen L (2016) Bioaugmentation and biostimulation of hydrocarbon degradation and the microbial community in a petroleum-contaminated soil. Int Biodeterior Biodegrad 107:158–164. https://doi.org/10.1016/j.ibiod.2015.11.019
Yu Y, Zhang W, Chen G, Gao Y, Wang J (2014) Preparation of petroleum-degrading bacterial agent and its application in remediation of contaminated soil in Shengli Oil Field, China. Environ Sci Pollut Res 21:7929–7937. https://doi.org/10.1007/s11356-014-2707-0
Zeneli A, Kastanaki E, Simantiraki F, Gidarakos E (2019) Monitoring the biodegradation of TPH and PAHs in refinery solid waste by biostimulation and bioaugmentation. J Environ Chem Eng 7:2213–3437. https://doi.org/10.1016/j.jece.2019.103054
Acknowledgements
The authors would like to thank Bioplus Desenvolvimento Biotecnológico Ltda for the use of facilities and financial support. This work was financially supported through grants from the following Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior (CAPES). MHV have been supported by Grant Numbers [302637/2017-6] and [307191/2016-8], respectively.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the writing of this manuscript. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: BV Thomas.
Rights and permissions
About this article
Cite this article
Napp, A.P., Allebrandt, S.R., Pereira, J.E.S. et al. Scale-up treatment of petroleum hydrocarbon-contaminated soil using a defined microbial consortium. Int. J. Environ. Sci. Technol. 19, 6023–6032 (2022). https://doi.org/10.1007/s13762-021-03467-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03467-z