Abstract
Two important brown algae, Sargassum horneri and Sargassum thunbergii are widely distributed along the coastal line of China. In this study, antioxidant, angiotensin-converting enzyme (ACE) inhibitory effects, and anticoagulant activities of the methanol extracts of S. horneri and S. thunbergii were investigated and compared. Total phenolic compounds and the fucoxanthin content were determined by the spectrophotometric and HPLC analyses, respectively, to relate phytochemical content to bioactivities of two Sargassum species. The phenolic compounds of S. horneri and S. thunbergii were also analyzed by LC-MS/MS. The results showed that S. horneri displayed higher antioxidant activities compared to S. thunbergii in all antioxidant bioassays evaluated, except the DPPH radical scavenging assay. Moreover, S. horneri showed a stronger ACE inhibitory effect (IC50 = 0.44 mg/mL) compared to S. thunbergii (IC50 = 1.81 mg/mL). Both S. horneri and S. thunbergii significantly prolonged the activated partial thromboplastin time (APTT) in blood plasma, and the APTT-prolonging effect of S. horneri was stronger than that of S. thunbergii. Salicylic acid was identified as the main phenolic compound in S. horneri, followed by m-coumaric acid, ferulic acid, and p-coumaric acid. Furthermore, S. thunbergii contained carnosic acid and salicylic acid. The total phenolic and fucoxanthin contents are positively correlated with antioxidant, ACE inhibitory and anticoagulant activities. These results indicated that S. horneri and S. thunbergii can be used as natural sources of functional foods because of their abundant bioactive compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Hypertension is a worldwide problem estimated to affect about 20% of the adult population of the world. It is one of the most common, chronic diseases and poses a potential hazard for heart disease, stroke, coronary artery disease, and premature death [9]. Hypertension is usually associated with other diseases. For instance, thrombotic lesions are always observed in chronic thromboembolic pulmonary hypertension and primary pulmonary hypertension [31]. Angiotensin-converting enzyme (ACE) is a central component of the renin-angiotensin system (RAS), which controls blood pressure by regulating the volume of fluids in the body [24]. Therefore, ACE inhibitors are widely used as pharmaceutical drugs for the treatment of cardiovascular diseases. Anticoagulants are also important for the treatment of thrombosis. Therefore, increased attention is being given to studies on plant-sourced constituents exhibiting antioxidant, ACE inhibitory and anticoagulant properties with little or no side effects.
Reactive oxygen species (ROS) are chemical compounds extremely harmful to human health. Excessive ROS production during cellular metabolism results in oxidative stress and cellular damage. In the past decades, several investigations demonstrated that ROS-mediated damage to lipoprotein could be an important factor in vascular wall oxidation [4, 8]. The pathogenesis of coronary heart disease can be better understood by studying its relationship with the factors which affect lipoprotein. Antioxidants decrease the risk of the mutations by scavenging ROS. Recourses to existing herbal remedies and continuous evaluation of plant extracts for antioxidant properties and other bioactive principles are becoming popular approaches to manage and ameliorate the risks posed to health by ROS. It is noteworthy that many natural products are being preferred as functional food ingredients due to their more pronounced biocompatibility, bioavailability, and safety in comparison to synthetic drugs.
Marine organisms comprising over half a million species produce numerous metabolic substances with diverse chemical structures and bioactivities because of their unique living environment when in comparison to terrestrial organisms. Secondary or primary metabolites produced from marine organisms have been identified as potential bioactive compounds for the food and nutraceutical industries. For over a thousand years, people in Eastern-Asian countries consume seaweeds as a part of their food intake. Phytochemical studies have demonstrated that seaweeds contain a variety of secondary metabolites, including polyphenols, flavonoids, terpenoids, phospholipids, and unsaturated fatty acids [14, 18, 20, 26, 32, 34].
Brown algae, comprising the class Phaeophyceae, are a large group of multicellular algae. Today, many brown algae have been recognized as an important nutritional and functional food sources [10]. Sargassum horneri Turner C. 1820 and Sargassum thunbergii ((Mertens ex Roth) Kuntze, 1880) are ecologically important seaweed species, which provide a crucial habitat for many marine organisms in the marine ecosystem [27]. These brown algae are also a good source of nutrients, including dietary fibers, vitamins, amino acids, and carbohydrates. Moreover, they have been used as traditional medicines in Asian countries for thousands of years. During the last few decades, several studies showed that the bioactivities of brown algae are attributed to polysaccharides [30, 35]. However, only few studies have been carried out to investigate the organic solvent extracts of these brown algae. In the present study, in vitro antioxidant, angiotensin converting enzyme (ACE) inhibitory effects, and anticoagulant activities of the methanol extracts of S. horneri and S. thunbergii were evaluated along with a determination of their phenolic and fucoxanthin contents.
MATERIALS AND METHODS
Chemicals
2,2-diphenyl-1-picryhydrazyl (DPPH), heparin, and fucoxanthin were purchased from Sigma-Aldrich (St. Louis, MO, USA). 2,6-di-tert-butyl-4-methylphenol (BHT) was purchased from Aladdin (Shanghai, China), and HPLC and HPLC/MS grade solvents were obtained from Fisher Chemical Company (Waltham MA, USA). All other chemicals and reagents used in this study were of analytical grade.
Plant Materials
S. horneri and S. thunbergii samples were collected from Zhoushan City, Zhejiang Province, China, in September 2018, and authenticated by Prof. Liu Changheng. Voucher specimens were deposited at Biology Institute, Qilu University of Technology (Shandong Academy of Sciences), Jinan, China. The collected samples were cleaned with distilled water to remove residues before drying at 50°C for 72 h.
Sample Extraction
Dried samples were pulverized into a fine powder and subsequently extracted three times with 100% methanol (1 : 10, g/mL) for three times under continuous shaking at room temperature for 24 h. The extracts were filtered through filter papers, and methanol was removed using a rotary evaporator to obtain the methanol extract of S. horneri and S. thunbergii, respectively.
Determination of Antioxidant Activities
There are literature reports on antioxidant activities including DPPH radical scavenging activity, superoxide radical activity [16], reducing power [19], and total antioxidant activity [17]. BHT was used as the positive control.
ACE Inhibitory Activity Assay
The ACE inhibitory effect was detected using an ACE inhibitory assay kit (ACE kit-WST, Dojindo Laboratory, Kumamoto, Japan). The measurement procedure was conducted in accordance with the protocol that was provided with the kit. The concentration of the ACE inhibitor, which inhibited 50% of ACE activity under the above assay conditions was defined as the IC50.
Anticoagulant Activity Assay
Activated partial thromboplastin time (APTT) clotting assay of normal sheep plasma was performed as described by Li et al. [15]. The assay medium contained the plasma sample (100 μL) and different concentrations of S. horneri and S. thunbergii (10 μL) prepared in DMSO. The medium was incubated at 37°C for 1 min, followed by the addition of 100 μL pre-warmed APTT assay reagent, and was incubated at 37°C for 2 min. A pre-warmed aliquot of 0.25 mol/L calcium chloride solution (100 μL) was then added, and the APTT was recorded using a coagulometer (SL-318 Coagulation Analyzer, Jinan Sen Blue Science and Trade Co. China). Heparin was used as the positive control.
Determination of Total Phenolic Content
The content of total phenolic compounds was determined by the Folin-Ciocalteu method. In brief, aliquots (0.5 mL) of each sample were blended with Folin-Ciocalteu reagent (0.5 mL) and 2% sodium carbonate (0.5 mL). The mixture was vortexed and kept at room temperature for 60 min. The absorbance was measured at 760 nm. Gallic acid was used as a standard, and the total phenolic content was expressed as μL gallic acid equivalents (GAE) per milligram.
Determination of Fucoxanthin
HPLC analysis was performed using an Agilent 1260 Infinity high performance liquid chromatograph (Agilent Technologies, Santa Clara, CA, USA) and chromatographic separations were achieved on a Waters symmetry C18 RP column (5 μm, 4.6 × 250 mm) (Waters, Milford, MA, USA). Based on the peak shape and resolution, a mixture of water (A) and acetonitrile (B) at a ratio of 18: 82 was used as the mobile phase with a flow rate of 1 mL/min. The injection volume was 20 μL, the column temperature was set at 25oC, and the detection wavelength was 450 nm. The column temperature was kept at 30oC. Samples were filtered through a 0.22 μm membrane filter.
LC-MS Analysis
HPLC/ESI-MS analysis was performed with a Vanquish Flex UHPLC coupled with Q-Exactive Orbitrap Mass spectrometry and Chromeoleon Software v7.2.9 (Thermo Fisher, Wilmington, MA, USA). The column used was an Hypersil Gold C18(100 × 2.1 mm, 1.9 μm column. The mobile phase adopted for the separation was (A) 0.1% aqueous formic acid and (B) acetonitrile containing 0.1% formic acid with 0.20 mL/min, a column temperature of 25oC and the injection volume was 2 μL. The following elution gradients were used: 0–2 min, 2% B; 2–12 min, 2% B to100% B; 14–16 min, 100% B to 2% B.
Mass spectral data were recorded on ESI interface mode and the operating conditions were as follows: negative ionization mode, spray voltage 3.00 kV, capillary temperature 275oC. Ultra-pure nitrogen was used as the collision gas, the aux gas heater rate was 10 mL/min, and the temperature of the gas heater was 300°C. Mass spectra data were recorded in the mass range of m/z 70 to 2000.
Statistical Analysis
The data were expressed as the mean ± standard deviation (n = 3). One-way ANOVA was used to analyze the difference between the means of different groups. For comparative studies, the difference between the means and bioactivity data were considered statistically significant at P < 0.05.
RESULTS
Antioxidant Activities
Antioxidant activities of S. horneri and S. thunbergii were demonstrated in Fig. 1. As shown in Fig. 1a, both S. horneri and S. thunbergii exhibited scavenging activity against DPPH radicals in a concentration-dependent manner in the tested concentration range of 0.4–2.0 mg/mL. Results showed that the DPPH scavenging activity of S. thunbergii was higher than that of S. horneri at all concentrations tested. For example, at the concentration of 2.0 mg/mL, the DPPH radical scavenging activity of S. thunbergii (90.41%) was approximately 2-fold greater than that of S. horneri (46.53%). The scavenging rate of BHT was 90% at a concentration of 1 mg/mL. The scavenging activity of S. horneri and S. thunbergii increased with the increase in concentration, and S. horneri exhibited a higher scavenging activity than that of S. thunbergii at the tested concentrations (Fig. 1b). At a concentration of 2.0 mg/mL, the scavenging rate of S. horneri was more than 50% and nearly twice that of S. thunbergii. The scavenging activity of S. horneri and S. thunbergii is lower than that of BHT. Figure 1c shows that the reducing power of S. horneri and S. thunbergii increased in a concentration-dependent manner, thereby indicating that S. horneri and S. thunbergii are sufficient good electron and hydrogen donors. They can therefore be used to terminate radical chain reactions through the conversion of free radicals to more stable products. At a concentration of 2.0 mg/mL, the absorbance of S. horneri (0.75) was higher than that of S. thunbergii (0.50). Furthermore, S. horneri displayed a stronger reducing power compared to S. thunbergii at all tested concentrations. The total antioxidant activity of S. horneri and S. thunbergii was concentration-dependent at all concentrations tested (Fig. 1d). It is of interest however that S. horneri exhibited higher total antioxidant activity than S. thunbergii at all concentrations evaluated. These results are consistent with the results obtained by the reducing power assay. The total antioxidant activity of S. horneri was almost equivalent to 40% of that of BHT at the concentration of 0.4 mg/mL.
ACE Inhibitory Activity Assay
The results indicated that S. horneri exhibited a higher ACE inhibitory effect compared to S. thunbergii. S. horneri inhibited 50% of the ACE activity at a concentration of 0.44 mg/mL (i.e. IC50 = 0.44 mg/mL), while S. thunbergiidisplayed a 50% inhibitory effect against ACE activity at a concentration of 1.81 mg/mL (i.e. IC50 = 1.81 mg/mL).
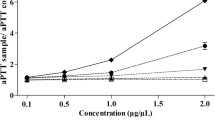
Anticoagulant Activity of S. horneri and S. thunbergii
As shown in Table 1, S. horneri displayed an increasing clotting time, which increased proportionately with an increasing sample concentration in the APTT assays. The clotting time of S. horneri was approximately twice longer (by about 60 s) or doubled that of the blank group at a concentration of 2 mg/mL. S. thunbergii also significantly prolonged APTT in a concentration-dependent manner. Compared to the blank group, the APTT of the S. thunbergii group increased by 31.5 s at a concentration of 2.0 mg/mL. Comparatively, under the experimental conditions, the APTT-prolonging effect of S. horneri was stronger compared to that of S. thunbergii under the experimental conditions. In addition, the APTT-prolonging effect of S. horneri (74.59 ± 1.76 s) was similar to that of heparin (75.49 ± 1.01 s) at a concentration of 0.5 mg/mL, however, the gap between S. horneri and heparin was obvious by increasing the sample concentration.
Determination of Total Phenolic Content
Phenolic compounds, including simple phenols, flavonoids, lignans, coumarins, and phlorotannins, are important metabolites that ubiquitously acting as antioxidant [3]. Similarly, the total phenolic content of S. horneri and S. thunbergii can reasonably be taken as a measure of their antioxidant capacity. The linear regression coefficient for the standard Gallic acid calibration curve was calculated from the equation, absorbance = 0.0141 × Gallic acid (μg/mL) + 0.0769 to obtain, the regression coefficient, R2= 0.9999. The total phenolic contents of S. horneri and S. thunbergii were expressed as GAE, 212.95 ± 7.65 μg/g and 180.48 ± 3.40 μg/g, respectively. Taken together, the results indicated that the difference in the antioxidant effects of S. horneri and S. thunbergii might be attributable to their different total phenolic content.
Determination of Fucoxanthin
Fucoxanthin is one of the most common carotenoids in brown algae, which exhibits various therapeutic effects on health, including antioxidant effects, anticarcinogenic and antimutagenic activities [21, 36]. S. horneri and S. thunbergii were qualitatively and quantitatively analyzed for fucoxanthin by HPLC. Identification of column resolved components was achieved by comparing their retention times (RT) and ultraviolet absorption spectra with the standard fucoxanthin profile. The RT of fucoxanthin in S. horneri and S. thunbergii was 18.7 min, which was consistent with the RT of standard fucoxanthin, as shown in Fig. 2. Peak area of eluted sample fucoxanthin were converted to micro gram quantities using the following standard equation as follows: Peak area = 82.097 × fucoxanthin (μg/mL) + 5.7698, R2 = 0.9999. It was observed that the fucoxanthin content of S. horneri was 70.56 ± 0.37 (μg/g, which was about 15 times higher than than the fucoxanthin content ofS. thunbergii (see Table 2).
LC-MS Analysis
Phenolic acids have been reported as important secondary metabolites in brown algae [23]. In this study, LC-MS/MS was performed to characterize the major phenolic compounds in S. horneri and S. thunbergii. Several phenolic acids were tentatively identified using MS and compared with data presented in the literature. As shown in Table 3, six and seven phenolic acid compounds were identified from S. horneri and S. thunbergii, respectively. Three phenolic acid compounds including gallic acid, chlorogenic acid, and salicylic acid were present in both S. horneri and S. thunbergii. In previous studies, these three phenolic acids were also reported for different seaweeds [13, 25, 28]. Moreover, p-coumaric acid, m-coumaric acid, and trans-isoferulic acid were observed in S. horneri, and gentisic acid, rosmarinic acid, 2,4-dihydroxybenzoic, and carosic acid were detected in S. thunbergii. Salicylic acid is the most abundant phenolic acid in S. horneri (1.75%), followed by m-coumaric acid, ferulic acid, and p-coumaric acid. Unlike S. horneri, carnosic acid had the highest relative content (1.67%), and salicylic acid had the second highest content (0.22%) in S. thunbergii. Thus, the above-mentioned results indicated that the difference in phenolic acid profile in S. horneri and S. thunbergii might contribute to the difference in bioactivities.
DISCUSSION
Antioxidants scaveng ROS in body cells and reduce potential mutations, thereby preventing cancer and heart diseases. Seaweeds could be a potential source of antioxidants, which would increase its commercial value as food or additives, thereby boosting their demand on the market. For this purpose, the antioxidant properties of two brown algae, S. horneri and S. thunbergii were evaluated by multiple assays. Both S. horneri and S. thunbergii demonstrated considerable but different levels of antioxidant activities in multiple assays in vitro. S. horneri displayed a higher antioxidant activity compared to S. thunbergii in superoxide oxygen radical scavenging capacity, reducing power, and total antioxidant activity assays. According to previous studies, several brown algae species displayed different level antioxidant activities. In this study, DPPH scavenging activity of S. thunbergii was higher than those of Anthophycus longifolius (IC50 = 2.08 ± 0.15 mg/mL), S. plagiophyllum (IC50 = 2.27 ± 0.23 mg/mL), and S. myriocystum(IC50 = 6.57 ± 0.59 mg/mL) [6]. These results might be corroborated by the occurrence of high contents of phenolics and fucoxanthin in the brown algae. Gallic acid, chlorogenic acid, salicylic acid, and coumaric acid were reported as typical phenolics possessing antioxidant activity in seaweeds [6]. The above-mentioned results confirm the positive correlation between the phenolic compounds, and fucoxanthin content and antioxidant activity [33].
Hypertension is an important public health concern and a potential risk factor for cardiovascular diseases and the related complications, including heart attack, aneurysm, dementia, and metabolic syndrome. ACE inhibitors are considered as an important class of antihypertensive drugs. Recent studies also demonstrated the presence of effective ACE inhibiting phenolic compounds derived from different plant sources. For example, caffeic acid and chlorogenic acid exhibited inhibitory effects on key enzymes linked with hypertension and prevented prooxidant-induced oxidative damage in the rat heart [1]. In addition, ferulic acid and chlorogenic acid may synergistically contribute to the ACE inhibitory effects [29]. Although phenolic compounds have poor solubility and subsequent restriction in bioavailability, different experiments with spontaneously hypertensive rats confirmed that these compounds exerted antihypertensive effect in vivo [2]. Our chemical analyses showed that S. horneri displayed a higher phenolic content, which may contribute to its stronger ACE inhibitory effect.
Blood coagulation plays a significant role in the cellular process of hemostasis. Coagulation disorders cause hemorrhage or thrombosis [7]. In general, determination of blood clotting and bleeding time are employed to evaluate anticoagulant properties. S. horneri showed higher anticoagulant activity than S. thunbergii, which was consistent with the results of antioxidant and ACE inhibitory activities. However, the APTT-prolonging effect of S. horneri (127.35 ± 3.43 s at the level of 2.0 mg/mL) and S. thunbergii (99.72 ± 1.48 s at the level of 2.0 mg/mL) are lower than sulfated polysaccharide isolated from the green alga Monostroma latissimum (158.4 ± 4.7 s at the level of 8.0 μg/mL), indicating that the anticoagulant activity of polysaccharides especially the sulfated polysaccharide might be stronger than that of phenolic acid compound [15]. According to the previous studies, chlorogenic acid delayed the APTT, prothrombin time, and thrombin time by degrading blood clots and inhibiting the enzymatic activity of proteases [11]. The anticoagulant effects of ferulic acid were achieved by the prolongation of the intrinsic or/and extrinsic pathways, and by delaying the recalcification time in plasma coagulation [12]. Moreover, several studies demonstrated that plant extracts with high content of phenolic acids were more likely to display anticoagulant activity [5, 22]. It is therefore likely that the phenolic compounds of S. horneri induced the anticoagulant activity by inhibiting the platelet aggregation. Further investigations are required to comprehensively determine the phytochemical constituents of S. horneriand S. thunbergii and to evaluate them against all essential biochemical properties.
CONCLUSIONS
In summary, in the present study, two brown algae (S. horneri and S. thunbergii) were investigated for ACE inhibitory effect, antioxidant and anticoagulant activities in a comparative analytical approach. S. horneri showed higher antioxidant, ACE inhibitory, and anticoagulant activities than those found in S. thunbergii. A higher content of phenolic acid and fucoxanthin, and the different phenolic profile of S. horneri may contribute to its higher functional properties. Given the significant antioxidant, ACE inhibitory and anticoagulant properties that were exhibited in the study, the two brown algae are recommended for future development as marine algal resources for further technological advancement.
REFERENCES
Agunloye, O.M. and Oboh, G., Caffeic acid and chlorogenic acid: Evaluation of antioxidant effect and inhibition of key enzymes linked with hypertension, J. Food Biochem., 2018, vol. 42, e12541. https://doi.org/10.1111/jfbc.12541
Al Shukor, N., Van Camp, J., Gonzales, G.B., et al., Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: A study of structure activity relationships, J. Agric. Food Chem., 2013, vol. 61, pp. 11832−11839.
Balboa, E.M., Conde, E., Moure, A., et al., In vitro antioxidant properties of crude extracts and compounds from brown algae, Food Chem., 2013, vol. 138, pp. 1764−1785.
Bian, F., Cui, J., Zheng, T., et al., Reactive oxygen species mediate angiotensin II-induced transcytosis of low-density lipoprotein across endothelial cells, Int. J. Mol. Med., 2017, vol. 39, pp. 629−635.
Bijak, M., Bobrowski, M., Borowiecka, M., et al., Anticoagulant effect of polyphenols-rich extracts from black chokeberry and grape seeds, Fitoterapia, 2011, vol. 82, pp. 811−817.
Chakraborty, K., Maneesh, A. and Makkar, F., Antioxidant activity of brown seaweeds, J. Aquat. Food Prod. Technol., 2017, vol. 26, pp. 406–419.
Chapin, J.C. and Hajjar, K.A., Fibrinolysis and the control of blood coagulation, Blood Rev., 2015, vol. 29, pp. 17–24.
Chen, Q., Wang, Q., Zhu, J., et al., Reactive oxygen species: Key regulators in vascular health and diseases, Br. J. Pharmacol., 2018, vol. 175, pp. 1279–1292.
Chen, Y., Gao, X., Wei, Y., et al., Isolation, purification and the anti-hypertensive effect of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from Ruditapes philippinarum fermented with Bacillus natto, Food Funct., 2018, vol. 9, pp. 5230–5237.
Cho, M.L., Lee, H.S., Kang, I.J., et al., Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed, Food Chem., 2011, vol. 127, pp. 999–1006.
Choi, J.H., and Kim, S., Investigation of the anticoagulant and antithrombotic effects of chlorogenic acid, J. Biochem. Mol. Toxicol., 2017, vol. 31, e21865.
Choi, J.H., Park, J.K., Kim, K.M., et al., In vitro and in vivo antithrombotic and cytotoxicity effects of ferulic acid, J. Biochem. Mol. Toxicol., 2018, vol. 32, e22004.
Hossain, M.B., Rai, D.K., Brunton, N.P., et al., Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS, J. Agric. Food Chem., 2010, vol. 58, pp. 10576–10581.
Kendel, M., Wielgosz-Collin, G., Bertrand, S., et al., Lipid composition, fatty acids and sterols in the seaweeds Ulva armoricana, and Solieriachordalis from Brittany (France): An analysis from nutritional, chemotaxonomic, and antiproliferative activity perspectives, Mar. Drugs, 2015, vol. 13, pp. 5606–5628.
Li, H., Mao, W., Zhang, X., et al., Structural characterization of an anticoagulant-active sulfated polysaccharide isolated from green alga Monostroma latissimum, Carbohydr. Polym., 2011, vol. 85, pp. 394–400.
Liu, X., Sun, Z.L., Jia, A.R., et al., Extraction, preliminary characterization and evaluation of in vitro antitumor and antioxidant activities of polysaccharides from Mentha piperita, Int. J. Mol. Sci., 2014, vol. 15, pp. 16302–16319.
Liu, X., Sun, Z., Zhang, M., et al., Antioxidant and antihyperlipidemic activities of polysaccharides from sea cucumber Apostichopus japonicus, Carbohydr. Polym., 2012, vol. 90, pp. 1664–1670.
Liu, X., Wang, C.Y., Shao, C.L., et al., Chemical constituents from Sargassum pallidum (Turn.) C. Agardh, Biochem. Syst. Ecol., 2009, vol. 37, pp. 127–129.
Liu, X., Zhang, M., Guo, K., Jia, A., et al., Cellulase-assisted extraction, characterization, and bioactivity of polysaccharides from Polygonatum odoratum, Int. J. Biol. Macromol., 2015, vol.75, pp. 258–265.
Liu, Y.J., Deng, Z.Z., Geng, L.H., et al., In vitro evaluation of the neuroprotective effect of oligo-porphyran from Porphyra yezoensis in PC12 cells, J. Appl. Phycol., 2019, vol. 31, pp. 2559–2571.
Liu, Z., Sun, X., Sun, X., et al., Fucoxanthin isolated from Undaria pinnatifida can interact with Escherichia coli and lactobacilli in the intestine and inhibit the growth of pathogenic bacteria, J. Ocean Univ. China, 2019, vol. 18, pp. 926–932.
Mohamed, A.A., Ali, S.I., Sameeh, M.Y., et al., Effect of solvents extraction on HPLC profile of phenolic compounds, antioxidant and anticoagulant properties of Origanum vulgare, Res. J. Pharm. Technol., 2016, vol. 9, pp. 2009–2016.
Montero, L., del Pilar Sánchez-Camargo, A., Ibáñez, E., et al., Phenolic compounds from edible algae: Bioactivity and health benefits, Curr. Med. Chem., 2018, vol. 25, pp. 4808–4826.
Muñoz, A., Esgueva, M., Gómez-Diez, M., et al., Modulation of acute transient exercise-induced hypertension after oral administration of four angiotensin-converting enzyme inhibitors in normotensive horses, Vet. J., 2016, vol. 208, pp. 33–37.
Nagy, T. O., Solar, S., Sontag, G., et al., Identification of phenolic components in dried spices and influence of irradiation, Food Chem., 2011, vol. 128, pp. 530–534.
Nguyen, V.T., Ueng, J.P., and Tsai, G.J., Proximate composition, total phenolic content, and antioxidant activity of seagrape (Caulerpa lentillifera), J. Food Sci., 2011, vol. 76, pp. C950–C958.
Pang, S.J., Liu, F., Shan, T. F., et al., Cultivation of the brown alga Sargassum horneri: sexual reproduction and seedling production in tank culture under reduced solar irradiance in ambient temperature, J. Appl. Phycol., 2009, vol. 21, pp. 413–422.
Rajauria, G., Foley, B., and Abu-Ghannam, N., Identification and characterization of phenolic antioxidant compounds from brown Irish seaweed Himanthalia elongata using LC-DAD–ESI-MS/MS, Innov. Food Sci. Emerg., 2016, vol. 37, pp. 261–268.
Salem, M.A., Michel, H.E., Ezzat, M.I., et al., Optimization of an extraction solvent for angiotensin-converting enzyme inhibitors from Hibiscus sabdariffa L. based on its UPLC-MS/MS metabolic profiling, Molecules, 2020, vol. 25, pp. 2307.
Sanjeewa, K. A., Lee, J. S., Kim, W. S., et al., The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran, Carbohydr. Polym., 2017, vol. 177, pp. 451–459.
Schölzel, B.E., Snijder, R.J., Mager, J.J., et al., Chronic thromboembolic pulmonary hypertension, Neth. Heart J., 2014, vol. 22, pp. 533–541.
Ye, H., Wang, K., Zhou, C., et al., Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum, Food Chem., 2008, vol. 111, pp. 428–432.
Youn, J.S., Kim, Y.J., Na, H.J., et al., Antioxidant activity and contents of leaf extracts obtained from Dendropanax morbifera LEV are dependent on the collecting season and extraction conditions, Food Sci. Biotechnol., 2019, vol. 28, pp. 201–207.
Yu, X., Xiong, C., Jensen, K.B., et al., Mono-acyl arsenosugar phospholipids in the edible brown alga Kombu (Saccharina japonica), Food Chem., 2018, vol. 240, pp. 817–821.
Zaporozhets, T., and Besednova, N., Prospects for the therapeutic application of sulfated polysaccharides of brown algae in diseases of the cardiovascular system, Pharm. Biol., 2016, vol. 54, pp. 3126–3135.
Zhao, P., Zang, Z., Xie, X., et al., The influence of different flocculants on the physiological activity and fucoxanthin production of Phaeodactylum tricornutum, Process Biochem., 2014, vol. 49, pp. 681–687.
ACKNOWLEDGMENTS
This study was supported by the National Key R&D Program of China (2018YFC0311206), Key R & D plan of Shandong Province (Medical Food) (2019YYSP001), and Industry university research collaborative innovation project of Shandong Academy of Sciences (2019-CXY22 and 2019-CXY23).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests. The author declares that there is no conflict of interest.
Statement on the welfare of animals. All applicable international, national and/or institutional guidelines for the care and use of animals have been followed.
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Caijiao, C., Leshan, H., Mengke, Y. et al. Comparative Studies on Antioxidant, Angiotensin-Converting Enzyme Inhibitory and Anticoagulant Activities of the Methanol Extracts from Two Brown Algae (Sargassum horneri and Sargassum thunbergii). Russ J Mar Biol 47, 380–387 (2021). https://doi.org/10.1134/S1063074021050035
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063074021050035