Abstract
This study compared the antioxidant activity of extracts from Dendropanax morbifera (D. morbifera) Levillis leaves. The concentrations of flavonoids and polyphenols were measured in extracts of D. morbifera leaves. The antioxidant activities were examined by ABTS and DPPH radical scavenging activity and ferric reducing antioxidant power (FRAP). Total flavonoid and polyphenol contents, and FRAP were highest in the 30% ethanol extract collected in May. The ABTS and DPPH radical scavenging activities were the highest in the 60% ethanol extract harvested in May. For investigating the relationship between antioxidant activity and specific polyphenols, rutin and chlorogenic acid of the polyphenol component were quantified by LC–MS/MS analysis. The concentrations of them were highest in the 60% ethanol extract collected in May, and showed positive correlations with antioxidant activities. The optimal extraction conditions to yield the most effective antioxidant activity were obtained using a 60% ethanol extraction solvent with samples collected in May.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reactive oxygen species (ROS), natural byproducts of metabolism, include hydrogen peroxide, superoxide, hydroxyl radical and peroxynitrite. However, ROS caused by environmental factors, and habitual factors such as stress and excessive exercise can attack proteins, DNA and membrane lipids [1, 2]. Oxidative stress leads to the production of ROS that damage cellular structures. Oxidative stress is associated with various medical disorders, including degenerative neuronal diseases [3], acquired degeneration of the retina [4], hypertension [5], metabolic syndrome [6] and progressive lung diseases such as chronic obstructive pulmonary disease [7]. A variety of antioxidants have been developed to prevent ROS induced damage; however, the safety of using synthetic antioxidants remains unclear. Moreover, natural antioxidants have been found because of the trend toward using natural rather than synthetic antioxidants [8, 9].
Dendropanax morbifera (D. morbifera) Léveille (Araliaceae) is an evergreen broad-leaved tree that is found in southwest Korea, e.g., Jeollanamdo or Jeju Island [10, 11]. The tree has been used as a non-official alternative medicine for the treatment of headaches, infections and skin problems [12]. The methanol extracts of leaves and debarked stems from D. morbifera have effective antioxidant and anticancer properties [13]. Polyacetylenes from D. morbifera, such as dendropanoxide, amyrin, α-glutinol, falcarinol, falcarindiol and diynene, have also been identified to have anti-complement activity [10, 14]. The dendropanoxide contained in D. morbifera was reported to show an anti-diabetic effect [15]. The antioxidant activities of extracts from D. morbifera have been reported to vary because of the ethanol concentration used in the extraction solvent [16]. According to Ahn et al. [17], the chemical composition of D. morbifera displays seasonal variation. Therefore, in this study, the antioxidant activity of D. morbifera leaves was evaluated by collecting samples from different seasons (May, August and November) and using different extraction solvent conditions (hot water, 30% ethanol and 60% ethanol). In addition to evaluating the antioxidant activity, we also compared the concentrations of major flavonoids such as rutin and chlorogenic acid.
Materials and methods
Materials
Sodium nitrite was purchased from ACROS (Geel, Belgium). Aluminum chloride and sodium carbonate were obtained from Junsei Chemical Co., Ltd. (Tokyo, Japan). Sodium hydroxide, potassium persulfate, ethanol and sodium acetate were obtained from Dae Jung (Seoul, Korea). Iron chloride was obtained from Duksan (Ansan, Gyeonggi). Folin-ciocalteu’s phenol reagent, catechin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid, chlorogenic acid, 2,2-azino-bis(3-ethylbenzothiazodine-6-sulfonic acid) diammonium salt (ABTS), HPLC grade acetic acid and rutin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Gallic acid monohydrate, acetic acid and hydrochloric acid were obtained from Samchun (Pyeongtaek, Gyeonggi). 2,4,6-Tris(2-pyridyl)-1,3,5-triazine (TPTZ) was purchased from Showa chemical Co., Ltd. (Tokyo, Japan). HPLC grade methanol was purchased from J.T. Baker Inc. (Deventer, Netherlands)
Sample preparation
The leaves of a 10-year-old D. morbifera tree were collected from Jeju Island in May, August and November. The collected leaves were air-dried at 50 °C for 15 h. Three solvents, water, 30% ethanol and 60% ethanol (v/v), were used to determine the optimal extraction conditions for antioxidant effects. Water extraction was performed in a water-bath at 85 °C for 3 h. The 30% ethanol extraction was carried out at 75 °C for 4 h, whereas the 60% ethanol extraction was performed at 70 °C for 4 h. The solutions were filtered under reduced pressure and the supernatant concentrated using a rotary evaporator and a vacuum drier. The extracts were lyophilized and stored at – 20 °C.
Total flavonoid measurement
The total flavonoid content in each D. morbifera extract was measured by the aluminum chloride colorimetric method using catechin as the standard [18, 19]. The sample solution (100 μL) was added to 5% sodium nitrite (30 μL) and diluent water (400 μL). After 5 min at room temperature, 10% aluminum chloride (30 μL) was added for 6 min. After 1 M sodium hydroxide (200 μL) was added with diluent water (240 μL), the absorbance of the solution at 510 nm was measured immediately. Total flavonoid content was expressed as mg of catechin equivalent (CE)/g sample.
Total polyphenol measurement
The total polyphenol contents were determined using the Folin-Ciocalteu assay [19]. The sample solution (300 μL) was mixed with Folin–Ciocalteau (160 μL) and diluent water (250 μL) for 5 min. Ten percent sodium carbonate solution (300 μL) was then added to the reaction and the solution incubated for 30 min at room temperature. The absorbance was measured at 750 nm using a spectrophotometer (BioTekInstruments, Inc., Winooski, VT, USA). The results were expressed as mg of gallic acid equivalent (GAE)/g sample.
ABTS radical scavenging activity
The ABTS radical scavenging assay was carried out according to a previous method [20]. Ascorbic acid was used as the positive control. The ABTS stock solution was prepared from 7 mM ABTS (5 mL) and 140 mM potassium persulfate (88 μL). After 16 h in the dark at room temperature, the stock solution (250 μL) was diluted with ethanol (22 mL). The sample (50 μL) was mixed with the ABTS solution (1 mL) for 3 min. The absorbance was determined at 734 nm. The ABTS radical scavenging capacity was calculated using the following equation. Ac is the absorbance of the control, reacted with ethanol (50 μL) and the ABTS solution (1 mL). As is the absorbance of the sample.
DPPH radical scavenging activity
The DPPH radical scavenging assay was modified according to a previous method [21, 22], using ascorbic acid as the positive control. The 0.1 mM DPPH working solution diluted with ethanol (150 μL) was added to the sample (50 μL). After 30 min in the dark, change in absorbance (decrease) at 515 nm was measured using a spectrophotometer. The DPPH radical scavenging effect was calculated using the following equation. Ac is the absorbance of the control, reacted with ethanol (50 μL) and the DPPH working solution (1 mL). As is the absorbance of the sample.
Ferric reducing antioxidant power
The FRAP assay was performed according to a previous method [23]. As a FRAP reagent, 300 mM sodium acetate buffer (pH 3.6, 10 mL) was added to 10 mM TPTZ solution in 40 mM hydrochloric acid (1 mL) and 20 mM iron (III) chloride (1 mL). The FRAP reagent was used in a water bath at 37 °C. The sample (20 μL) was mixed with the FRAP reagent (150 μL). The absorbance was determined at 593 nm immediately. The FRAP value was recorded with the following equation. Ac is the absorbance of the positive control, reacted with ascorbic acid (20 μL) and the FRAP reagent (150 μL). As is the absorbance of the sample. Ab is the absorbance of the blank, reacted with distilled water (20 μL) and FRAP reagent (150 μL).
Liquid chromatography-tandem mass spectrometry (LC–MS/MS) assay
The LC–MS/MS assay was based on a previous method [24, 25]. This analysis used a Vanquish UHPLC System (Thermo Fisher Scientific, Bremen, Germany) equipped with an Accucore aQ C18 column (2.1 × 100 mm; i.d., 2.6 µm) and a Q Exactive Plus mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The temperature of the column oven was maintained at 35°C. The mobile phase for LC consisted of 0.1% (v/v) formic acid in water (solvent A) and 0.1% (v/v) formic acid in acetonitrile (solvent B) and was applied as follows: 0–2 min, 5% (B); 2–13 min, 5–100% (B); 13–16 min, 100% (B); 16–17 min, 100–5% (B), followed by re-equilibration with 5% (B) for 17–20 min at a flow rate of 0.4 mL/min and an injection volume of 1 µL. Chlorogenic acid and rutin in the extracts of D. morbifera were verified by matching their retention times with those of external standards. A stock mixed standard solution of chlorogenic acid and rutin was prepared in methanol. Standard calibration curves were constructed over the concentration range of 0.0001–0.01 mg/mL. Lyophilized D. morbifera extracts were dissolved in methanol, filtered and injected into the UHPLC system. The compounds isolated from the D. morbifera extract were quantified by electrospray ionization (ESI) positive tandem MS.
Statistical analysis
Statistical analyses were performed using Duncan’s multiple range test one-way analysis of variance (ANOVA) (SPSS 20, SPSS Inc., Chicago, Illinois, USA). P values < 0.05 were considered statistically significant. Correlations were tested using Pearson’s correlation coefficient (R) by the IBM SPSS Statistics software. The data are presented as the mean ± standard error for triplicate determinations.
Results and discussion
Total flavonoid and polyphenol content
Analysis of the total flavonoid content revealed that the 30% ethanol extract harvested in May had the highest content at 88.6 mg CE/g (Fig. 1A). The lowest content was found in the 60% ethanol extract harvested in November with a value of 40.5 mg CE/g. In comparison, an Ulmus pumila extract showed a value of 0.615 mg CE/g [26] and the edible rose yielded a value less than 5 mg CE/g [27]. Thus, the flavonoid content of our extracts is relatively high when compared with that of previous samples from different plant extracts.
The total polyphenol content of the 30% ethanol extract harvested in May was also significantly higher than the other extracts (Fig. 1B). The lowest polyphenol content was found in the 60% ethanol extract collected in November with a value of 83.7 mg GAE/g. The polyphenol contents in water and ethanol extracts of sea buckthorn leaves were 120.06 and 147.78 μg/g, respectively [28]. A similar extraction method was used in these previous studies, indicating that the polyphenol content in extracts of D. morbifera are remarkably high. The polyphenol content in the 30% ethanol extract taken from samples in May was the highest, whereas the lowest polyphenol value was recorded when the May harvest was extracted by the hot water extract method. The hot water extract yielded the highest polyphenol content when samples were taken from the August harvest, but lowest when this harvest was extracted by 30% ethanol. In November, there was no significant difference (P < 0.05) among extraction solvents used.
Antioxidant activity
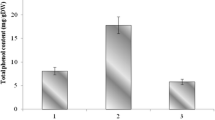
Radical scavenging activities and reducing power were analyzed to determine the antioxidant activity of the extracts. The DPPH assay measures a change in the stable radical DPPH by the electron donating ability of the sample [29]. Antioxidant compounds and ABTS react to form a radical cation, ABTS+ . Therefore, this assay can be used to measure the antioxidant activity [30]. Radical scavenging activities are represented by the inhibitory concentration 50 (IC50) and vitamin C was used as a positive control (Table 1). The sixty percent ethanol extract collected in August showed effective ABTS radical scavenging activity. The IC50 of the 60% ethanol extract collected in August was about three times higher than the positive control. For the DPPH radical scavenging activity, the 60% ethanol extract collected in May was the most active sample. Antioxidant activities increased as the ethanol concentration in the extraction solvent increased. The FRAP value measures the reduction of the ferric ion (Fe3+) to the ferrous ion (Fe2+) by donor electrons in the sample [31]. The most active extract in the FRAP assay was the 30% ethanol extract harvested in May (Fig. 2). The FRAP value did not follow an ethanol concentration-dependent correlation. In summary, extracts with excellent electron donating ability were the ethanol extracts collected in May. Electron donating ability was lowest for the extracts collected in November.
Analysis of rutin and chlorogenic acid concentration
The leaves of D. morbifera have been identified to contain rutin, chlorogenic acid and rosmarinic acid [14]. The measured antioxidant activities may be due to these flavonoid compounds. Rutin, including quercetin, has been shown to display antioxidant activity by preventing chronic painful peripheral neuropathy in mice [32]. Chlorogenic acid and caffeic acid have been shown to have antioxidant activity in Caco-2 cells and a protective effect in an intestinal ischemia–reperfusion mouse model [33].
The results of the LC–MS/MS analysis showed that the 60% ethanol extract collected in May had the highest concentration of chlorogenic acid and rutin (Table 2). The concentration of rutin in the 60% ethanol extract collected in May was 116.71 mg/g and the concentration of chlorogenic acid was 36.44 mg/g. The extracts collected in May and August showed that as the concentration of ethanol used increased the amount of chlorogenic acid and rutin recovered increased. This result is consistent with a previous report, which showed that the optimal condition for extracting chlorogenic acid and cynaroside from flower buds of Lonicera japonica was an ethanol concentration of 60% [34]. In November, however, the concentrations were highest in the 30% ethanol extract. This extract from D. morbifera contained not only flavonoids but also nonpolar compounds such as sesquiterpene and sterol compounds [10, 15, 17]. However, in the present study, we could not measure these nonpolar compounds. Therefore, further effort is required to facilitate quantification of these nonpolar compounds.
Correlation between antioxidant compounds and activities
In the present study, the relationship between antioxidant effects and contents was investigated [3, 26, 27]. The correlation was calculated using a regression analysis (R = correlation coefficient; Fig. 3). The ABTS radical activity showed a weak correlation with the flavonoids and polyphenols (R2 = 0.599, R2 = 0.696). Additionally, the DPPH radical activity showed a weak correlation with the flavonoids and polyphenols (R2 = 0.585, R2 = 0.686). In contrast, the FRAP value correlated strongly with the flavonoids and polyphenols (R2 = 0.951, R2 = 0.953). Rutin and chlorogenic acid showed relatively low correlations between total polyphenols and flavonoids, respectively. Compared with rutin, chlorogenic acid showed higher correlations with total polyphenols and flavonoids (R2 = 0.788 and 0.683, respectively). Additionally, the correlation between antioxidant activities and each flavonoid, such as rutin and chlorogenic acid, was also analyzed (Fig. 3B). The ABTS radical activity showed a strong correlation with the contents of rutin and chlorogenic acid (R2 = 0.899 and 0.895, respectively). The DPPH radical activity also showed a strong correlation with the concentration of rutin and chlorogenic acid (R2 = 0.941 and 0.936, respectively). A weaker correlation was observed between the FRAP value and the concentrations of rutin and chlorogenic acid (R2 = 0.671 and 0.691, respectively). These results suggest that among the flavonoid components, rutin and chlorogenic acid may affect the radical scavenging activities of D. morbifera. Besides rutin and chlorogenic acid, leaves of D. morbifera contain numerous active flavonoid compounds. This is highlighted by the observation that the total flavonoid contents showed a strong correlation with FRAP values (Fig. 3A), whereas rutin and chlorogenic acid concentrations showed a weaker correlation with the FRAP values (Fig. 3B). Thus, it appears that other flavonoids contributed to the FRAP values. FRAP values and radical scavenging activity such as DPPH and ABTS have different mechanisms for measuring antioxidant capacity. The different mechanisms of antioxidant effects can be described by the singlet electron transfer (SET) based activity for FRAP values and the mixed mode with SET and hydrogen atom transfer based activity for DPPH and ABTS radical scavenging activities [35].
Relationship between antioxidant activities and antioxidants taken from different extracts of D. morbifera. Correlations are presented as the Pearson’s correlation coefficient (R2). (A) Correlation between antioxidants (flavonoids and polyphenols) and ABTS activity, DPPH activity and FRAP. (B) Correlation between polyphenols (rutin and chlorogenic acid) and ABTS activity, DPPH activity and FRAP
In conclusion, by considering the antioxidant capacities and flavonoid contents, the most suitable extraction procedure of compounds from D. morbifera leaves is 60% ethanol with harvesting of material in May.
References
Kornfeld OS, Hwang S, Disatnik MH, Chen CH, Qvit N, Mochly-Rosen D (2015) Mitochondrial reactive oxygen species at the heart of the matter: new therapeutic approaches for cardiovascular diseases. Circ Res 116:1783–1799
Cho M, Ko SB, Kim JM, Lee OH, Lee DW, Kim JY (2016) Influence of extraction conditions on antioxidant activities and catechin content from bark of Ulmus pumila L. Appl Biol Chem 59:329–336
Moniczewski A, Gawlik M, Smaga I, Niedzielska E, Krzek J, Przegalinski E, Pera J, Filip M (2015) Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 1. Chemical aspects and biological sources of oxidative stress in the brain. Pharmacol Rep 67:560–568
Hanus J, Zhang H, Wang Z, Liu Q, Zhou Q, Wang S (2013) Induction of necrotic cell death by oxidative stress in retinal pigment epithelial cells. Cell Death Dis 4:e965
Sinha N, Dabla PK (2015) Oxidative stress and antioxidants in hypertension–a current review. Curr Hypertens Rev 11:132–142
Perez-Cornago A, Lopez-Legarrea P, de la Iglesia R, Lahortiga F, Martinez JA, Zulet MA (2014) Longitudinal relationship of diet and oxidative stress with depressive symptoms in patients with metabolic syndrome after following a weight loss treatment: the RESMENA project. Clin Nutr 33:1061–1067
Kirkham PA, Barnes PJ (2013) Oxidative stress in COPD. Chest 144:266–273
Hamishehkar H, Khani S, Kashanian S, Ezzati Nazhad Dolatabadi J, Eskandani M (2014) Geno- and cytotoxicity of propyl gallate food additive. Drug Chem Toxicol 37:241–246
Taghvaei M, Jafari SM (2015) Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J Food Sci Technol 52:1272–1282
Park BY, Min BS, Oh SR, Kim JH, Kim TJ, Kim DH, Bae KH, Lee HK (2004) Isolation and anticomplement activity of compounds from Dendropanax morbifera. J Ethnopharmacol 90:403–408
Kim LS, Youn SH, Kim JY (2014) Comparative Study on Antioxidant Effects of Extracts from Rubus coreanus and Rubus occidentalis. J Korean Soc Food Sci Nutr 43:1357–1362
Kim W, Yim HS, Yoo DY, Jung HY, Kim JW, Choi JH, Yoon YS, Kim DW, Hwang IK (2016) Dendropanax morbifera Leveille extract ameliorates cadmium-induced impairment in memory and hippocampal neurogenesis in rats. BMC Complement Altern Med 16:452
Hyun TK, Kim MO, Lee H, Kim Y, Kim E, Kim JS (2013) Evaluation of anti-oxidant and anti-cancer properties of Dendropanax morbifera Leveille. Food Chem 141:1947–1955
Chung IM, Song HK, Kim SJ, Moon HI (2011) Anticomplement activity of polyacetylenes from leaves of Dendropanax morbifera Leveille. Phytother Res 25:784–786
Moon HI (2011) Antidiabetic effects of dendropanoxide from leaves of Dendropanax morbifera Leveille in normal and streptozotocin-induced diabetic rats. Hum Exp Toxicol 30:870–875
Nakamura M, Ra JH, Kim JS (2016) The comparative analysis of antioxidant and biological activity for the Dendropanax morbifera LEV. Leaves extracted by different ethanol concentrations. Yakugaku Zasshi 136:1285–1296
Ahn JC, Kim SH, Kim MY, Kim OT, Kim KS, Hwang B (2003) Seasonal variations in yields of Hwangchil lacquer and major sesquiterpene compounds from selected superior individuals of Dendropanax morbifera Lev. J Plant Biol 46:38–40
Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG (2005) Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem 91:571–577
Kim W, Kim DW, Yoo DY, Jung HY, Nam SM, Kim JW, Hong SM, Kim DW, Choi JH, Moon SM, Yoon YS, Hwang IK (2014) Dendropanax morbifera Leveille extract facilitates cadmium excretion and prevents oxidative damage in the hippocampus by increasing antioxidant levels in cadmium-exposed rats. BMC Complement Altern Med 14:428
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Mensor LL, Menezes FS, Leitão GG, Reis AS, Dos Santos TC, Coube CS, Leitão SG (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15:127–130
Dudonne S, Vitrac X, Coutiere P, Woillez M, Merillon JM (2009) Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem 57:1768–1774
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Reisz JA, Wither MJ, Dzieciatkowska M, Nemkov T, Issaian A, Yoshida T, Dunham AJ, Hill RC, Hansen KC, D’Alessandro A (2016) Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood 128:e32–42
Rampler E, Coman C, Hermann G, Sickmann A, Ahrends R, Koellensperger G (2017) LILY-lipidome isotope labeling of yeast: in vivo synthesis of 13C labeled reference lipids for quantification by mass spectrometry. Analyst 142:1891–1899
Kim YJ, Shin Y (2015) Antioxidant profile, antioxidant activity, and physicochemical characteristics of strawberries from different cultivars and harvest locations. J Korean Soc Appl Bi 58:587–595
Yang H, Shin Y (2017) Antioxidant compounds and activities of edible roses (Rosa hybrida spp.) from different cultivars grown in Korea. Appl Biol Chem 60:129–136
Kim KY, Park MH, Kim KH, Im SH, Park YH, Kim YN (2009) Analysis of chemical composition and in vitro anti-oxidant properties of extracts from sea buckthorn (Hippophae rhamnoides). J Appl Biol Chem 52:58–64
Rahman MM, Islam MB, Biswas M, Khurshid Alam AH (2015) In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes 8:621
Tai A, Ohno A, Ito H (2016) Isolation and Characterization of the 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Radical Cation-Scavenging Reaction Products of Arbutin. J Agric Food Chem 64:7285–7290
Chen TS, Liou SY, Wu HC, Tsai FJ, Tsai CH, Huang CY, Chang YL (2010) New analytical method for investigating the antioxidant power of food extracts on the basis of their electron-donating ability: comparison to the ferric reducing/antioxidant power (FRAP) assay. J Agric Food Chem 58:8477–8480
Azevedo MI, Pereira AF, Nogueira RB, Rolim FE, Brito GA, Wong DV, Lima-Júnior RC, de Albuquerque Ribeiro R, Vale ML (2013) The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol Pain 9:53
Sato Y, Itagaki S, Kurokawa T, Ogura J, Kobayashi M, Hirano T, Sugawara M, Iseki K (2011) In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int J Pharm 403:136–138
Hu W, Guo T, Jiang WJ, Dong GL, Chen DW, Yang SL, Li HR (2015) Effects of ultrahigh pressure extraction on yield and antioxidant activity of chlorogenic acid and cynaroside extracted from flower buds of Lonicera japonica. Chin J Nat Med 13:445–453
Kareem HS, Ariffin A, Nordin N, Heidelberg T, Abdul-Aziz A, Kong KW, Yehye WA (2015) Correlation of antioxidant activities with theoretical studies for new hydrazone compounds bearing a 3,4,5-trimethoxy benzyl moiety. Eur J Med Chem 103:497–505
Acknowledgements
The results have been undertaken with the support from the Agency for Jeju Plant Resources Dendropanax.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Youn, J.S., Kim, YJ., Na, H.J. et al. Antioxidant activity and contents of leaf extracts obtained from Dendropanax morbifera LEV are dependent on the collecting season and extraction conditions. Food Sci Biotechnol 28, 201–207 (2019). https://doi.org/10.1007/s10068-018-0352-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-018-0352-y