Abstract
As a large conspicuous intertidal brown alga, individuals of Sargassum horneri can reach a length of more than 7 m with a fresh weight of 3 kg along the coasts of the Eastern China Sea. The biomass of this alga as a vital component in coastal water ecology has been well documented. In recent years, a steady disappearance of the algal biomass along the once densely populated coastal areas of the Eastern China Sea has drawn attention in China. Efforts have been made to reconstruct the subtidal algal flora or even to grow the alga by use of long-lines. As part of the efforts to establish an efficient technique for producing seedlings of S. horneri, in this investigation a series of culture experiments were carried out in indoor raceway and rectangular tanks under reduced solar irradiance at ambient temperature in 2007–2008. The investigation demonstrated that: (1) sexual reproduction of S. horneri could be accelerated in elevated temperature and light climates, at least 3 months earlier than in the wild; (2) eggs of S. horneri had the potential to be fertilized up to 48 h, much longer than that of known related species; (3) suspension and fixed culture methods were both effective in growing the seedlings to the long-line cultivation stage; and (4) the life cycle of S. horneri in culture could be shortened to 4.5 months, thus establishing this alga as an appropriate model for investigating sexual reproduction in dieocious species of this genus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The large conspicuous brown alga Sargassum horneri (Turner) C. Agardh is a native species of the northwest Pacific coasts (Yoshida et al. 1998). The alga is one of the principal members of the intertidal seaweed flora and plays important roles in providing an environment for a distinctive and specialized group of marine animals and plants, many of which are not found elsewhere (Nanba 1995; Choi et al. 2003). This large seaweed forms extensive floating biomass in both the intertidal and subtidal waters, as well as large-scale drifting biomass in surface waters (Komatsu et al. 2008). S. horneri has become one of the main algal species chosen to reconstruct seaweed beds in Korea and Japan (Yamauchi 1984; Choi et al. 2003). It also contributes significantly to nutrient uptake from mainland effluents, constituting an efficient algal biofilter along the near-shore coast due to the presence of large amounts of biomass in the near-shore coastal waters during its peak growing season and also its wide distribution across cold to subtropical regions.
Sargassum horneri is one of the few species in the genus of Sargassum that has received much attention from phycologists in the Far East. Uchida (1993) completed its life cycle in the laboratory for the first time and reported a long-day driven (not short-day) formation of receptacles. Choi et al. (2008) reported the optimal growing temperature of 25°C and an irradiance of 20 μmol photons m−2 s−1 for seedlings during their early stages. It is now known that most of the field populations of S. horneri reach peak reproduction in the early spring when surface water temperature starts to rise, though with slight geographical differences between countries. Adult plants were found to grow at a rate of 4.6% per day at 1-m water depth as measured in situ (Gao and Hua 1997). The phenology of the “spring fruiting” and “autumn fruiting” showed that two types of wild populations exist in the east–west Pacific coasts which may have different timings of reproduction in their natural habitats (Yoshida et al. 1998; Uchida and Arima 1993). No such observation has been made in China so far. However, new plant regeneration from vegetative branchlets from the previous year was observed in a field excursion at Nanji Islands, indicating that this alga might have a biannual life cycle (Sun et al. 2008).
Along the coast of Zhejiang Province (latitude: 25–27°N) in the East China Sea, the floating biomass of S. horneri which used to be distributed widely in near-shore waters or in waters around the islands has been observed to be disappearing steadily, starting from the end of 1990s, due to unknown reasons (Sun et al. 2008). In the meantime, Komatsu et al. (2008) reported the occurrence of large amounts of drifting biomass in the eastern area of the Eastern China Sea that was exclusively composed of S. horneri. Based on successive years of observation and calculation, the authors suspected that this drifting biomass came from the Zhejiang Islands of China. The disappearance of sessile populations along the near-shore coast in combination with the occurrence of the drifting biomass in the Eastern China Sea match each other and confirm the fact that the sessile populations of S. horneri have been steadily disappearing from the coasts of Zhejiang Province. The suspected reasons include the destruction of the coastal water environment due to rapid economic development and elevated levels of industrial pollution (Komatsu et al. 2008).
A complete understanding of sexual reproduction in this alga in live culture under controlled conditions is one of the first steps towards the establishment of an artificial cultivation technique. Part of our efforts was to develop the technique for mass cultivation of S. horneri either on long-lines or in subtidal waters (to rebuild the populations). The goals of this investigation are: (1) to investigate the eco-physiological factors governing reproduction under reduced solar irradiance at ambient temperature conditions in indoor tanks; (2) to understand the temporal and spatial scale of gamete discharge; (3) to look for the optimal controlled conditions that would benefit the growth of young seedlings in indoor tanks; and finally (4) to establish a practical method to produce seedlings for the cultivation trials in the open sea.
Materials and methods
Adult male and female plants were sampled in April 2007 from the rocky shore at Xiaohuyu, Nanji Islands (27°27′N, 121°04′E; Fig. 1). Directly after sampling, the plants were cleaned, packed with wet cotton cloth and delivered to the laboratory in Qingdao in a cool box within 24 h by aeroplane. Preculture of the male and female plants was performed in 80-L polypropylene (PP) tanks in tumble culture under reduced solar irradiance from April to June 2008. Zygotes from female plants were collected on 6 June from one female individual plant and seeded onto a 0.5-m2 seedling collector (Pang et al. 2006). The seedlings were then grown until they reached a size of 5–7 cm and were then re-inserted into the strains of a rope composed of 50% nylon and 50% cotton with 2 cm in diameter. The attached seedlings were further grown in a 1,000-L raceway tank.
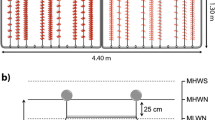
The structure of the indoor raceway tank was as described in Pang et al. (2006) and each lane was 400 × 40 × 40 cm in dimension. The flow-through 1,000-L indoor raceway tanks were mounted under a roof window through which 70% of solar irradiance penetrated. The maximal light measured at the water surface in April was 800 μmol photons m−2 s−1. Water was sand-filtered and ozone-treated before it was pumped into the tanks. Approximately 50% of the water was renewed daily. Water flow in three lanes of the tank was generated by an underwater pump. Every 2 days, KNO3 and KH2PO4 were added to reach a final concentration of 10 and 1 mg L−1, respectively. The long-lines with attached seedlings (5 cm) at 15-cm intervals were hung horizontally at the water surface. The culture ran from October 2007 to April 2008.
The 80-L flow-through PP tanks were rectangular in shape (65 × 45 × 35 cm; Fig. 2). An aeration tube (2-mm holes drilled at 10-cm intervals) was fixed at the bottom of the long side of the tank, such that the bubbles generated a strong upward current, causing the algal biomass to tumble. Isolated branchlets and the culture of seedlings on collectors were performed in these tanks. The light and temperature regimes were identical to that of the raceway tanks (Fig. 2). Water temperature was recorded daily (YSI, USA). Ambient light at the water surface was recorded every minute by use of a spherical photometer (Biospherical, USA).
Experiment 1: year-round cultivation from seedlings to adults in raceway and in tumble culture
In order to understand the growth and reproduction under indoor controlled conditions, 20 tagged 10-cm-long plants were individually attached to long-lines which were hung in three lanes of the raceway from 28 October 2007 to 10 March 2008 when spontaneous discharge of gametes occurred in most of the plants. Growth was represented as length increase at fixed time intervals.
Experiment 2: reproduction of isolated branchlets cultured in incubators at elevated temperature and irradiance as well as in tumble culture
Isolated branchlets with an average length of 11.2 ± 3.3 cm were sampled from ten individuals in the raceway on 17 January 2008. These branchlets were aerated in 2-L beakers in PES in temperature and light controlled photo-incubators at 300 μmol photons m−2 s−1 in 15°C under a 12:12 h light:dark regime. The culture water was renewed every 3 days and photographs taken periodically. Development of receptacles and discharge of eggs were observed.
Culture of the isolated branchlets was also performed in 80-L PP rectangular tanks. Ten branchlets (average length 15 cm) were isolated from each of the five selected individuals and cultured in five 80-L PP tanks from 16 February to 29 March 2008 when all the plants completed discharge of their gametes. The total length, fresh weight, and development of the branchlets were recorded at 10-day intervals.
Experiment 3: measurements of the potential time required by eggs to be fertilized by the sperm
To evaluate egg viability, newly discharged ova were gently removed from the surface of the female receptacles with a fine forceps on the morning when the discharged eggs were first observed. The eggs were separated into 5-cm petri dishes using pipettes (100–200 eggs per dish). At different time intervals, mature male receptacles were added into the petri dishes filled with the eggs. To determine whether a male receptacle was mature enough to discharge sperm, a male receptacle was isolated from the branchlets in culture and examined under an inverted microscope. Sperm discharge from the male conceptacles was usually observed to occur simultaneously from the receptacle surface constantly under the stimulation of strong light. Two days after addition of the male receptacles, the fertilization of eggs was checked and recorded under a dissection microscope. In another series, newly discharged eggs were isolated from the surface of the female receptacles and enclosed in a small fine-mesh bag of which the mesh diameter could not allow the eggs to go through. At 0, 12, 24, 48, and 72 h post-discharge, the bag was suspended in the 80-L PP tank in which the mature male branchlets were being tumbled. On day 3, the eggs inside the bag were removed, and inoculated into 9-cm petri dishes and grown at 12°C in 50 μmol photons m−2 s−1 under a 12:12 h light:dark regime. The total fertilized eggs were checked under a dissection microscope, and the fertilization rate was calculated.
Experiment 4: rope and suspension culture of seedlings in indoor tanks
Seedling culture was performed in two ways. In the first, zygotes were seeded into 9-cm petri dishes at a density of 100 zygotes cm−2, grown under 50 μmol photons m−2 s−1 irradiation for 10 days with 3-day intervals of medium renewal (PES) at room temperature (15°C). The seedlings were then transferred into 2-L beakers in tumbling culture for 20 days. Medium was renewed once per week. The seedlings were transferred further into the flow-through 80-L PP tanks when they reached 0.5 cm in length. Every 2 d, KNO3 and KH2PO4 were added to a level of 10 and 1 mg L−1, respectively. In another method, zygotes were seeded evenly on 0.4-mm-thick strands of the 0.35-m2 collectors. The strands, used traditionally for seedling production of seaweeds, were composed of 50% nylon and 50% cotton. The seeded collectors were kept still for 3 days before the rhizoids of the seedlings developed. Thereafter, the collectors were horizontally laid in the surface water of the 80-L PP tanks with gentle aeration for the first week, and strong aeration thereafter throughout the culture period of 3 months. Water was renewed daily. Every 2 days, KNO3 and KH2PO4 were added to a level of 10 and 1 mg L−1. The length of the seedlings was measured periodically.
Results
Performances of growth and reproduction in raceway tank from Oct., 2007 to Feb. 24, 2008 (Expt. 1)
The averaged length of 20 labeled individuals increased from 7 cm on 29 October to 161 cm on 24 February (Fig. 3a). The maximum length of the individuals was 217 cm with well-developed branchlets on the lateral sides. The water temperature declined from 18°C to 9°C and then increased to 13°C. The daily maximum irradiance at the water surface was about 200 μmol photons m−2 s−1. Solar irradiance at the surface of the water increased from mid-February (Fig. 3b), in parallel to the water temperature. The earliest occurrence of gamete discharge in 5 of the 20 plants was observed on 10 March (water temperature: 12.2°C, max. irradiance: 600 μmol photons m−2 s−1). At termination of the culture on 10 March, all plants had well developed receptacles and 70% of the plants discharged gametes. Zygotes from these plants were used to produce the seedlings required thereafter. After the zygotes shedding, all the plants became senescent and lost vesicles during the following week even though the water temperature was 15°C and was still in the optimal growth range.
Sargassum horneri in the indoor culture: increase of the length (squares in A), receptacle formation and discharge of gametes (stars in A) from October 2007 to April 2008. Temperature (circles in A) and reduced solar irradiance (circles in B) was recorded daily. Horizontal filled arrow refers to the period of branchlet culture in incubators in Expt. 2; dashed arrow refers to the period of branchlet culture in 80-L tank in Expt. 2 (Stars refer to the date of gamete discharge)
Earlier shift of receptacle development and reproduction in elevated light and temperature (Expt. 2)
In the 25-day culture from 17 January to 11 February at 15°C with an irradiation of 300 μmol photons m−2 s−1, vegetative branchlets isolated from ten individuals completed the development of receptacles (Fig. 4a–c). Six of the ten branchlets discharged gametes and completed fertilization on 11 February (Fig. 3a). This timing was 1 month earlier than that of plants in the raceway and 3 months earlier than the longline-cultured individuals at Nanji Island. In the 80-L tumble tanks in which the isolated branchlets were periodically exposed to reduced ambient solar irradiance (Fig. 2), the male and female branchlets discharged their gametes on 29 March, and this was 43 days later than the discharge in the incubators.
Sargassum horneri: isolated branchlets in Expt. 2, showing visible receptacles initially on the branchlets (A), conspicuous receptacle only 9 days later (B, arrow) and discharged eggs on receptacles 25 days later (C, arrow). Bars in A and B = 1 cm
The relative growth rate in length was greater during the period when temperature declined, i.e., 16–26 February, than when temperature increased from 26 February to 16 March. The changes in fresh weight were the opposite (Table 1).
Microscopic observations of the timing of male and female gamete discharging in culture (Expt. 3)
In several culture experiments, it was observed that female gametes were discharged usually in the early morning, appearing first at the basal conceptacles of the receptacle and steadily increasing to the apical conceptacles (Fig. 5a). At 17–24 h post-fertilization, zygotes were shed (Fig. 5b). By decantation, millions of zygotes could be collected for seeding from a few kg of the female plants (Fig. 5c). Egg discharge on a mature receptacle could last for a few hours. Strong light stimulated the discharge of eggs and sperm as was found when a receptacle was placed in the centre of a microscopic field with strong illumination. When discharged from a conceptacle, the antheridia disintegrated and then sperm swam swiftly away (Fig. 6 A-C). The typical process of individual egg discharge lasted only a few seconds (Fig. 6d–f). Fertilization was achieved when sperm were observed to enter an egg in a matter of seconds after release.
Time taken for eggs to be fertilized (Expt. 3)
The occurrence of cell division was taken as a sign that the egg was successfully fertilized and a zygote had begun to develop. The rate of fertilization dropped significantly in the eggs 24 h after discharged (Table 2). The critical time was found to be between 12–24 h during which the fertilization rate dropped from 94% to 50%. However, less than 1% of eggs were observed to have potential to be fertilized 48 h post-discharge. Young embryos that were derived from the same batch of fertilized eggs showed almost identical developmental stages as indicated by the timing of rhizoid appearance.
Growth performance of seedlings on collectors and in suspension cultures (Expt. 4)
In suspension culture, a free circulating 2-cm-long seedling underwent periodical changes in the irradiance as indicated in Fig. 2. These conditions were quite different from plants held at the water surface. Observation covering a period of more than 3 months revealed that the seedlings which were raised in suspension culture, as well as on the strands of collectors, all reached a size of about 1.5–2.5 cm with about 9 initial leaf blades well developed, a size that could allow manipulation by re-insertion into the strands of the main rope for open sea cultivation (Fig. 7). However, seedlings on collectors that were laid horizontally 5 cm below the water surface were generally larger than these seedlings in suspension culture but with no statistic significance (one-way ANOVA, P = 0.036905).
Sargassum horneri: Seedlings grown on strands of flat collectors (A left) or in suspension culture (B left), and the average length (A right) and numbers of blades of seedlings (B right) on collectors as well as in suspension culture in 80-L tumbling tanks. All cultures were performed under reduced solar irradiance at ambient temperature from Feb. 11 to the middle of May, 2008
Shortening of the life cycle of S. horneri to 4.5 months in indoor culture
In the first batch of seedlings that were fertilized on 11 February 2008, four out of a few hundred plants grown on the collector rope started to develop receptacles when they reached only 3 cm in length (main stem) on 20 May, even before the formation of any vesicles (Fig. 8). From 11 February to 20 May, surface water temperature increased from about 10 to 20°C and the daily maximal irradiance also increased from 200 to 800 μmol photons m−2 s−1. On 25 June, gametes were discharged which were successfully fertilized.
Discussion
Early shift of sexual reproduction in S. horneri and its implications
One of the important findings of this investigation was the identification of an independent fruiting process of the isolated branchlets in elevated temperature and light regimes. Recognition of the temporal and spatial scale of sexual reproduction in S. horneri is a crucial step for understanding the seasonal dynamic changes of the biomass within near-shore waters and the recruitment of field populations, as well as the eco-physiological factors that might have an impact on such processes. S. horneri has been shown in this investigation to grow to 2 m in indoor culture tanks under reduced solar irradiance and to complete sexual reproduction in 25 days when exposed to elevated light and temperature. These features make this algal species an ideal candidate for detailed study of synchronization of sexual reproduction under artificial growing conditions. Changes in temperature, irradiance, and photoperiod as external eco-physiological triggers are well known in coordinating sexual reproduction in different seaweeds (Lüning et al. 2008). Early shift in sexual reproduction at elevated water temperature and irradiance levels has been observed in several other seaweeds including Sargassum fulvellum (Hwang et al. 2006), Hizikia fusiformis (Pang et al. 2005), Laminaria saccharina (Pang and Lüning 2004), and Ulva pseudocurvata (Lüning et al. 2008). In S. horneri, the shift of 3 months in comparison with field long-line farmed individuals was achieved in this investigation. It is noteworthy that 10-cm isolated branchlets bearing immature conceptacles (0.2 mm) could complete the maturation of receptacle and discharge of gametes at 25 days old. This indicates a rapid physiological adjustment between growth and reproductive phases when the plant is exposed to suitable conditions. The results of this study suggest that individuals of S. horneri in drifting biomass in the ocean will undergo reproduction and shed zygotes whilst in the drift, thus expanding the potential distribution of the populations. This was thought to be one of the principal ways of invasion of other reported species in this genus, especially those that have a monoecious life cycle such as S. muticum (Stæhr et al. 2000; Arenas et al. 1995). In most of the near-shore waters along the Eastern China Sea, S. horneri grows in subtidal waters and is seldom exposed during low tide. Sexual reproduction in sessile populations usually peaks in April–May (Choi et al. 2008). However, maturation of receptacles could be accelerated due to higher temperatures and elevated irradiance in surface water when the biomass is drifting; thus early shedding of zygotes would be expected.
The opportunistic nature of sexual reproduction in S. horneri
Sexual reproduction in S. horneri often occurs in nature after vegetative growth from a few centimeters-long seedlings to several meters-long adults over 8–10 months (Sun et al. 2008). In this investigation, two lines of evidence support the view that S. horneri is an opportunistic alga with regard to sexual reproduction. Firstly, 3- to 5-cm-long young plants at the age of just 4.5 months could produce receptacles and undergo sexual reproduction giving rise to mature gametes when temperature and light regimes were appropriate. This process was initiated even before complete maturity of the vegetative thallus. On the other hand, a vegetative branchlet could complete transition from vegetative to reproductive state after 25 days, while the original plant from which the branchlets were isolated remained vegetative (Expt. 2). In seaweeds, one of the best examples of opportunistic sexual reproduction is found in the genus Ulva where a weekly sporulation rhythm was discovered in the field populations during high temperature seasons, while for the remainder of the year a biweekly rhythm was observed (Lüning et al. 2008). Elevation of the irradiance or temperature could speed up the discharge of gametes in isolated discs of U. pseudocurvata. A transition from vegetative growth to reproduction in S. horneri involved the formation of the special reproductive organ, the receptacle, which therefore requires a much longer time than that of the simple membranous alga U. pseudocurvata. Opportunistic sexual reproduction was found to be most prevalent in algal species that favored high levels of nutrients and had a relatively simple and short life cycle. Such species could often produce large-scale blooms, or have the much larger potential to become invasive species.
Synchronization of discharge of gametes under identical light and temperature regimes
The fertilization of eggs in S. horneri in nature habitat is quite similar to that in other species of Sargassum that have a diecious life cycle (Pang et al. 2006). The spatial distance between individuals and high currents might dramatically decrease the probability of fertilization. The temporal difference in discharge of male and female gametes could even increase this discrepancy. However, for an individual living in a population, sperm might not be a limiting factor (Berndt et al. 2002). The 48-h fertilization potential of an egg implied a possible greater number of surviving zygotes in nature than in other species that might have a shorter duration, i.e. Hizikia fusiformis. Eggs of the latter that were 24 h old were found to have lost viability (Pang et al. 2006). A longer duration of egg viability might exist in a number of other species of Sargassum and could be closely related to the invasion potential of species in this genus in general. However, this point has been largely neglected in nearly all the previous investigations of invasive species. Egg discharge in S. horneri was also observed in many of the trials to occur independently of the presence of male receptacles, similar to that in H. fusiformis. This again gives rise to the question of synchronization of fertilization to facilitate the practical production of seedlings if high efficiency of fertilization condition were required.
Hatchery for S. horneri seedlings
The fact that plants of S. horneri grew to 3–4 cm in length from zygotes in tumble culture in indoor tanks over 3 months in this investigation demonstrated the feasibility of growing up the seedlings in massive culture, rather than using traditional flat collectors. The density of plants on the strands of a collector used for S. horneri could not be as high as other species, e.g., Laminaria japonica, due to the special flowerlike structure of the young seedlings of Sargassum. This feature limits the production efficiency in illuminated areas in hatchery practice if seedlings are collected. However, in the tumble culture of seedlings, an illuminated water surface can be fully employed to produce more young plants. Seeding spores or zygotes on collectors has been a traditional way for the production of seaweed seedlings in mass cultivation in the genera of Laminaria, Undaria, Porphyra, and Ulva as well as Hizikia (Critchley and Ohno 1998). Tumble culture is often employed to grow up vegetative thalli such as Chondrus crispus in Canada as well as Palmaria palmata (Pang and Lüning 2006). It is noteworthy that the growth of seedlings of Sargassum horneri does not need to be in the sea due to the special low light requirement of growth as found in this investigation and others (Choi et al. 2008). This is different from Hizikia fusiformis which needs at least 3 months on surface long-lines in the sea and requires a lot of labor for removing epiphytes and mud from the collectors (Pang et al. 2007). Based on the findings of this investigation, a large-scale trial of production of S. horneri seedlings would be required to further establish a practical technique, under full-scale production conditions.
References
Arenas F, Fernadéz C, Rico JM, Fernandéz E, Haya D (1995) Growth and reproductive strategies of Sargassum muticum (Yendo) Fensholt and Cystoseira nodicaulis (Whit.) Roberts. Sci Mar 59:1–8
Berndt ML, Callow JA, Brawley SH (2002) Gamete concentrations and timing and success of fertilization in a rocky shore seaweed. Mar Ecol Prog Ser 226:273–285. doi:10.3354/meps226273
Choi CG, Kim HG, Sohn CH (2003) Transplantation of young fronds of Sargassum horneri for construction of seaweed beds. J Korean Fish Soc 36:469–473
Choi HG, Lee KH, Yoo HI, Kang PJ, Kim YS, Nam KW (2008) Physiological differences in the growth of Sargassum horneri between the germling and adult stages. J Appl Phycol. doi:10.1007/s10811-007-9281-5
Critchley AT, Ohno M (1998) Seaweed resources of the world. Japan International Cooperation Agency, Yokosuka
Gao KS, Hua WQ (1997) In situ growth rates of Sargassum horneri (Fucales, Phaeophyta). Phycol Res 45:55–57. doi:10.1111/j.1440-1835.1997.tb00062.x
Hwang EK, Park CS, Baek JM (2006) Artificial seed production and cultivation of the edible brown alga, Sargassum fulvellum (Turner) C. Agardh: developing a new species for seaweed cultivation in Korea. J Appl Phycol 18:251–257. doi:10.1007/s10811-006-9021-2
Komatsu T, Matsunaga D, Mikami A, Sagawa T, Boisnier E, Tatsukawa K, Aoki M, Ajisaka T, Uwai S, Tanaka K, Ishida K, Tanoue H, Sugimoto T (2008) Abundance of drifting seaweeds in eastern East China Sea. J Appl Phycol. doi:10.1007/s10811-007-9302-4
Lüning K, Kadel P, Pang SJ (2008) Control of reproduction rhythmicity by environmental and endogenous signals in Ulva pseudocurvata (Chlorophyta). J Phycol 44:866–873. doi:10.1111/j.1529-8817.2008.00535.x
Nanba N (1995) Egg release and germling development in Sargassum horneri (Fucales, Phaeophyceae). Phycol Res 43:121–125. doi:10.1111/j.1440-1835.1995.tb00014.x
Pang SJ, Lüning K (2004) Breaking seasonal limitation: year-round induction of sporogenesis of Laminaria saccharina by blocking the transport of sporulation inhibiting factors. Aquaculture 240:531–541. doi:10.1016/j.aquaculture.2004.06.034
Pang SJ, Lüning K (2006) Tank cultivation of the red alga Palmaria palmata: year-round induction of tetrasporangia, tetraspore release in darkness and mass cultivation of vegetative thalli. Aquaculture 252:20–30. doi:10.1016/j.aquaculture.2005.11.046
Pang SJ, Chen LT, Zhuang DG, Fei XG, Sun JZ (2005) Cultivation of the brown alga Hizikia fusiformis (Harvey) Okamura: enhanced seedling production in tumbled culture. Aquaculture 245:321–329. doi:10.1016/j.aquaculture.2004.12.011
Pang SJ, Gao SQ, Sun JZ (2006) Cultivation of the brown alga Hizikia fusiformis (Harvey) Okamura: controlled fertilization and early development of seedlings in raceway tanks in ambient light and temperature. J Appl Phycol 18:723–731. doi:10.1007/s10811-006-9078-y
Pang SJ, Zhang ZH, Shan TF, Gao SQ, Sun JZ (2007) Cultivation of the brown alga Hizikia fusiformis (Harvey) Okamura: stress resistance of artificially raised young seedlings revealed by chlorophyll fluorescence measurement. J Appl Phycol 19:557–565. doi:10.1007/s10811-007-9170-y
Stæhr PA, Pedersen MF, Thomsen MS, Wernberg T, Krause-Jensen D (2000) Invasion of Sargassum muticum in Limfjorden (Denmark) and its possible impact on the indigenous macroalgal community. Mar Ecol Prog Ser 207:79–88. doi:10.3354/meps207079
Sun JZ, Chen WD, Zhuang DG, Zheng HY, Li L, Pang SJ (2008) In situ ecological studies of the subtidal brown alga Sargassum horneri at Nanji Island of China. S China Fish Sci 4(3):59–64. in Chinese with English abstract
Uchida T (1993) The life cycle of Sargassum horneri {Phaeophyta) in laboratory culture. J Phycol 29:231–235. doi:10.1111/j.0022-3646.1993.00231.x
Uchida T, Arima S (1993) Crossing experiments between autumn- and spring-fruiting types of Sargassum horneri (Phaeophyta). Bull Jpn Soc Sci Fish 59:1685–1688
Yamauchi K (1984) The formation of Sargassum beds on artificial substrata by transplanting seedlings of S. horneri (Turner) C. Agardh and S. muticum (Yendo) Fensholt. Bull Jpn Soc Sci Fish 50:1115–1123
Yoshida G, Arima S, Terawaki T (1998) Growth and maturation of the “autumn-fruiting type” of Sargassum horneri (Fucales, Phaeophyta) and comparisons with the “spring-fruiting type”. Phycol Res 46:183–189. doi:10.1111/j.1440-1835.1998.tb00112.x
Acknowledgements
The authors wish to thank C.H. Song for her technical assistance in maintaining the algal culture in the lab. This work is financially supported by: (1) the 863 Hi-Tech Research and Development Program of China (2006AA10A412, 2006AA10A416); (2) a project from Natural Science Foundation of China (30671596); (3) a project from the Chinese Academy of Sciences (KSCX2-YW-N-47–07); and (4) a project from the Ministry of Science and technology of China (2006GB24910469).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pang, S.J., Liu, F., Shan, T.F. et al. Cultivation of the brown alga Sargassum horneri: sexual reproduction and seedling production in tank culture under reduced solar irradiance in ambient temperature. J Appl Phycol 21, 413–422 (2009). https://doi.org/10.1007/s10811-008-9386-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-008-9386-5