Abstract
The homologous series of dialkyl phosphonates (CnH2n + 1O)2PH=O] (2 ≤ n ≤ 9) (dialkyl phosphites is their commonly used trivial name) was characterized by electron ionization mass spectra and retention indices (RIs) on an RTX-5 standard nonpolar stationary phase. It was established that, in the presence of even one chiral center in the alkyl fragments (for example, in sec-alkanol esters), dialkyl phosphonates were detected in the form of several chromatographic signals due to diastereomers with differences in the retention indices from 4 to 21. The four-coordinated phosphorus atom is the second chiral center. The homologous increments of the retention indices of di-n-alkyl phosphonates (iRI ± sRI = 29 ± 9) and esters having one (–4 ± 10) and two (–61 ± 14) branching points in the carbon skeletons of alkyl fragments were evaluated. These values, which were calculated based on data for a limited number of homologues, make it possible to characterize any compounds from the test series. It was found that the relationship М ≈ 0.14(RI – iRI) + y makes it possible to uniquely determine the molecular weights of analytes according to chromatographic data under the condition of rounding the results to the nearest value of M comparable with y = 12 to the modulus 14 [М ≡ 12(mod14)].
Similar content being viewed by others

Avoid common mistakes on your manuscript.
The main assignment of the currently available databases of mass spectra and gas-chromatographic retention indices is to serve as a source of reference information for the identification of organic compounds by gas chromatography–mass spectrometry (GC–MS) without their preparative separation from mixtures. The recent version (2017) of one of the most representative bases of the National Institute of Standards and Technology (NIST, the United States) contains more than 300 000 standard electron ionization (EI) mass spectra and more than 400 000 retention indices of almost 100 000 organic compounds [1]. However, the functions of these bases cannot be reduced to the simple summing up of analytical data because several additional types of problems can be solved with their aid.
First, the analysis of the contents of sufficiently large bodies of reference data makes it possible to reveal compounds from which classes were characterized in insufficient detail. An answer to this question makes it possible to refine reasons responsible for a deficiency in analytical data. Most frequently, these are difficulties in the synthesis of such compounds or the underestimation of the importance of such analytes for solving the problems of environmental, toxicological, etc., monitoring. The absence of reference information for comparatively simple compounds is of special interest. The monoesters of dicarboxylic and, in the general case, polybasic acids, including monoalkyl phthalates, can be given as an example [2]. The identification of such acid esters is difficult to perform because of the similarity of their mass spectra to the mass spectra of full esters so that it is possible only with the combined use of mass-spectrometric and chromatographic data. Therefore, the currently available databases should be supplied with appropriate information. The unsubstituted hydrazones RR'C=N–NH2 are one additional example; they are poorly understood because of their thermal instability, which leads to partial decomposition in a chromatographic column in the course of separation [3].
Second, the characterization of homologous series of organic compounds by reference data for a maximally large quantity of homologues is not necessary even theoretically. It is practically unreal because of a rapid increase in the number of the structural isomers (N) of compounds containing even the simplest alkyl fragments CnH2n + 1 [4]:
n | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
N | 1 | 1 | 2 | 4 | 8 | 17 | 39 | 89 | 211 | 507 |
In the characterization of homologous series, it is desirable to limit oneself to a certain minimum number of homologues so that the available data would allow their generalization to other members of these series.
Third, in the solution of the problems of GC–MS identification, it is necessary to maximally avoid the most dangerous IInd order errors (the incorrect identification of analytes caused by the absence of necessary information from the bases used, the coincidence of their analytical parameters with data for other compounds, and the neglect of chromatographic retention parameters).Footnote 1 The observed increase in the number of these errors makes it possible to suggest a dangerous tendency in current GC–MS analysis [5, 6]. The simplest and most effective method of averting them consists in increasing the number of objects in the bases and a maximum variety of their chemical nature. Among the particular examples of similar errors, it is possible to note the identification of the branched C12H26 isomer 2,2,4,6,6-pentamethylheptane as the constituent of the essential oils of different plants [7]. The detailed checking of more than a dozen of publications showed that the case in point is different hydrocarbons and the similarity of their mass spectra is the reason for errors. The detection of an exotic bicyclic hydrocarbon—spiro[2,4]hepta-4,6-diene—in different naturally occurring materials was reported; however, all of the examples were indicative of the incorrect identification of its C7H8 isomer—toluene [8].
In addition to the partial esters of organic acids [2], the acidic esters of inorganic acids belong to the compounds inadequately represented in currently available databases. Their dissociation constants are higher by approximately two orders of magnitude than the corresponding values of organic acids; because of this, their gas-chromatographic separation is impossible [9] (the values of pKa for dialkyl phosphates are lower than the value of pKa,1 = 2.1 ± 0.1 of phosphoric acid). By analogy, the same conclusion can be extended to the acid esters of H2SO4, H2SeO4, H3AsO4, etc., but not to the diesters of phosphorous acid H3PO3, which can exist as two prototropic tautomers, dialkyl phosphites (I) and dialkyl phosphonates (II) [10]:
This equilibrium leads to the ambiguous interpretation of the structures of phosphorous acid derivatives. It is believed that their properties are predominantly determined by the presence of phosphonate tautomers II. However, a database [1] contains two structures with identical mass spectra for diisopropyl phosphate: (iso-C3H7O)2POH and (iso-C3H7O)2PH=O. It is most likely that the former is erroneous in this case. Phosphorous acid itself forms the tris-trimethylsilyl derivative P[OSi(CH3)3]3 [11], but the treatment of a reaction mixture containing diisopropyl phosphonate with N-trimethylsilylimidazole (the most active of well-known silylating reagents) did not result in the production of its trimethylsilyl derivative. Consequently, the hydrogen atom in tautomeric structure II is substantially less active than that in the structure of I. The problem of the uncertainty of the structures of these ions also occurs in mass spectrometry [12].
Table 1 illustrates in sufficient detail the level of characterization of the simplest C1–C4 dialkyl phosphonates by classical physicochemical properties (normal boiling points Tb, relative densities \(d_{4}^{{20}},\) and refractive indices \(n_{D}^{{20}}\)). The presence or the absence of their mass spectra in the base [1] and the reference values of their retention indices on standard nonpolar polydimethylsiloxane stationary phases was additionally noted.
Such data summaries are informative for the general evaluation of the degree of studying of compounds from various classes, and they were used for the characterization of dialkyl phosphates [9], unsubstituted hydrazones [2], etc. From the limited information given in Table 1, it follows the that dialkyl phosphonates were described by physicochemical data no worse than the corresponding full esters (trialkyl phosphites). Mass spectra are accessible for almost all of the simplest homologues, whereas the retention indices of both trialkyl phosphites and dialkyl phosphonates, starting with C3 esters, are unknown. This fact is responsible for possible errors in GC–MS identification caused by the above similarity of the mass spectra of full and partial esters. Acceptable methods for the calculation of the RIs of such esters are currently unknown.
As well as phosphates, the esters of phosphorous acid are used in industry as stabilizing agents (antioxidants) and effective fire-retardant additives to polymer compositions [13]. In addition to the compounds listed in Table 1, numerous unique representatives of this class, including tris(nonylphenyl)phosphite (Naugard P), tris-(2,4-di-tert-butylphenyl)phosphite (Irgafos 168), tris-(bis-nonylphenyl)phosphite), and 3,9-bis-(octadecylhydroxy)-2,4,8,10-tetraoxa-3,9-diphosphaspiro[5.5]undecane (additive 14), are well known. The mass spectrum and the retention index of the tris-trimethylsilyl derivative of phosphorous acid on a standard nonpolar phase (RI = 1115) are known [11]. The mass spectrum of the ionic series of alkyl phosphites was given in a monograph [4]; however, it was calculated based on a limited number of the mass spectra of esters from this class known at that time; therefore, it should be considered only as preliminary estimation. For this reason, it was given without separation into the spectra of the ionic series of di- and trisubstituted esters.
This work was dedicated to the systematic characterization of dialkyl phosphonates using analytical data for their GC–MS identification primarily by previously unknown gas-chromatographic retention indices and combined GC–MS parameters.
EXPERIMENTAL
Interaction of alcohols with phosphorus trichloride. Dialkyl phosphonates were synthesized by the interaction of 100 μL of aliphatic alcohols ROH (of pure or chromatographic grade) with a solution of 100 μL of phosphorus trichloride (Sigma-Aldrich, the United State; 99%) in 1 mL of pure chloroform (used without additional purification). The most probable mechanism of the formation of these esters is the interaction of intermediate trialkyl phosphites (RO)3P with HCl (a version of the Michaelis–Arbuzov reaction [14, 15]):
The reaction mixtures were stirred for 5 min at room temperature and directly used for gas-chromatographic analysis. The mixtures for GC–MS analysis were diluted with chloroform by a factor of 50‒100.
To check whether the trimethylsilyl esters of the reaction products of isopropyl alcohol with PCl3 can be formed, we added 50 μL of N-trimethylsilylimidazole (Sigma-Aldrich, the United States; 97%) to 300 μL of the reaction mixture.
Conditions for gas-chromatographic analysis. The chromatographic analysis of reaction mixtures was carried out on a Kristall 5000.2 chromatograph with a flame-ionization detector on a BPX-1 column (with a standard nonpolar polydimethylsiloxane phase) 10 m in length with an inside diameter of 0.53 mm and a stationary phase film thickness of 2.65 μm. The analytical conditions were the following: temperature programming from 50 to 200°C at a rate of 5 K/min; injector temperature, 180°C; detector temperature, 200°C; carrier gas, nitrogen; flow rate, 5.5 mL/min (linear velocity, 44.7 cm/s); split ratio, 3 : 1; and injected sample volume, 0.5 μL. For the determination of RIs, a mixture of C8–C16 and C20 reference hydrocarbons with the even numbers of carbon atoms in molecules was added to the samples. Standard deviations are given as the reproducibility characteristics of the retention indices. The number of the simultaneous determinations of the indices was varied from two to five.
GC–MSanalysis. The GC–MS analysis was carried out on a Shimadzu QP 2010 SE instrument with EI at an interface and ion source temperature of 200°C (the temperature was increased to 250°C for 1-octanol and 1-nonanol) on an RTX-5 MS column (polydimethylsiloxane containing 5% phenyl groups) with a length of 30 m, an inside diameter of 0.32 mm, and a stationary phase film thickness of 0.25 μm. The analytical conditions were the following: temperature programming from 50 to 200°C (250°C for 1-octanol and 1-nonanol) at a rate of 5 K/min; injector temperature, 180°C; detector temperature, 200°C; carrier gas, helium; flow rate, 1.84 mL/min (linear velocity, 49 cm/s); split ratio, 1 : 10; and injected sample volume, 0.5 μL.
Processing of the results. Excel (Microsoft Office 2010) and QBasic were used for the calculation of the linear-logarithmic retention indices.
RESULTS AND DISCUSSION
Table 2 summarizes the mass spectra and retention indices of C2‒C9 dialkyl phosphonates in the composition of the mixtures of the reaction products of aliphatic alcohols with phosphorus trichloride and other chemical impurities that were present in the reaction mixtures. For brevity sake, the mass spectra of the repeatedly mentioned components were excluded and the following statement was given for these components: Identified based on the RI value; the mass spectrum is given above. In addition to the target products, some reaction mixtures contained the traces of alkyl chlorides, 1-alkanols, and alkyl carboxylates. In the case of benzyl alcohol, the expected phosphonates were not detected; benzyl chloride was found the main product. The results of the single determinations of retention indices were given without standard deviations.
Of phosphorous acid derivatives, only dialkyl phosphonates were detected in all of the samples. This was confirmed by the identification of diethyl phosphonate (RI = 942 ± 2), whose reference RI value is known (933 ± 12). Diethyl phosphonate (C2H5O)2PH=O and the mixed alkyl ethyl esters (C2H5O)(RO)PH=O were present in almost all of the reaction mixtures in addition to the expected dialkyl phosphonates (RO)2PH=O. The appearance of these products was caused by the presence of an ethanol admixture in the chloroform used for preventing its oxidation with the formation of phosgene [16]. The alcohol admixture can be removed from chloroform with the use of polar sorbents, for example, silica gels; however, we decided to preserve information on such mixed alkyl ethyl phosphonates for expanding the range of characterized esters.
In addition to dialkyl phosphonates, alkyl dichlorophosphates ROP(O)Cl2, which were not characterized previously, were detected in many reaction mixtures of aliphatic alcohols with PCl3; it is most likely that they were formed because of the presence of a phosphoryl trichloride POCl3 impurity in PCl3 and its interaction with alcohols ROH. Trialkyl phosphites in the reaction mixtures were detected in no case.
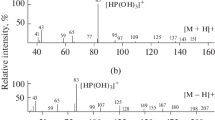
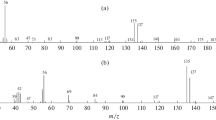
Mass-spectrometric fragmentation of dialkyl phosphonates. Formally, these processes do not require detailed commentaries because the mass spectra of many of these compounds are well known [1]. However, compounds from this class rarely occur in chemical practice; therefore, their fragmentation is usually not discussed in general manuals, for example [17]. Only Hamming and Foster [18] attempted to consider their mass-spectrometric classification. From the spectrum of ionic series reported previously [4], it follows that the main signals in the mass spectra of these compounds (homologous group y = 12) relate to the homologous groups y = 9 and 13. The signals of the molecular ions of dialkyl phosphonates were almost not detected with the exception of a mass spectrum of the simplest homologue (dimethyl phosphonate).
The first step of the fragmentation of the molecular ions of dialkyl phosphonates, which is analogous to the fragmentation of the molecular ions of trialkyl phosphates, alkylphosphonic acid esters, and other phosphorus(V) derivatives, consists in the elimination of an alkenyl radical CnH2n – 1, which corresponds to a greater alkyl fragment (n ≥ m), as a result of a so-called double hydrogen rearrangement [4, 18]. The second step implies the elimination of an olefin CmH2m with the number of carbon atoms in the second alkyl fragment:

The formation of [PH(OH)3]+ ions with m/z 83, which are characteristic of alkyl phosphonates, is the result of these two consecutive processes. They do not contain carbon; therefore, the intensities of the corresponding isotopic peaks with m/z 84 are lower than 1%. Analogous ions are characteristic of the derivatives of other phosphorus-containing acids, for example, phosphoric ([P(OH)4]+ with m/z 99) and methylphosphonic ([CH3P(OH)3]+ with m/z 97) acids [1, 4, 18]. In accordance with reaction scheme (1), the mass spectra of, for example, mixed alkyl ethyl esters are characterized by a combination of the signals of fragmentation ions with m/z 111 and 83 (Table 2).
In addition to the isotopic peaks of the chlorine-containing ions, the characteristic feature of the mass spectra of alkyl dichlorophosphates ROP(O)Cl2 is also caused by the double hydrogen rearrangement, which leads to the formation of ions with mass numbers of 135, 137, and 139 and an intensity ratio of 9 : 6 : 1 (Table 2):
Thus, dialkyl phosphonates exhibit mass spectra sufficiently characteristic for group identification (reference to a homologous series). However, the absence of the signals of molecular ions under EI conditions complicates the individual mass-spectrometric identification of these esters. For this purpose, gas-chromatographic RIs should be additionally used.
Chromatographic parameters of dialkyl phosphonates.Table 3 compares the RIs of 27 C2‒C9 dialkyl phosphonates on standard nonpolar polydimethylsiloxane phases. These data can be directly used for their identification, and this does not require special commentaries. It is reasonable to note the good additivity of RIs with increasing number of carbon atoms in n-alkyl fragments. Theoretically, differences between the RIs of diesters (Cn)2 and (Cn + 1)2 should be close to 200; this is consistent, for example, with the data for C2‒C6 diesters:
Di-n-alkyl phosphonate: | (С2Н5O)2 | (C3H7O)2 | (C4H9O)2 | (C5H11O)2 | (C6H13O)2 | ||||
RI | 942 | 1132 | 1317 | 1527 | 1722 | ||||
ΔRI | 190 | 185 | 210 | 195 |
A similar regularity can be considered as a demonstration criterion for the correctness of the determination of the RI values. The retention indices of branched isomers are expectedly lower than the RIs of normal linear homologues, and those of cyclic (cyclohexyl) derivatives are higher.
The dialkyl phosphonates that are the derivatives of racemic secondary alcohols (2-methylbutanol and 2-octanol), in the reaction mixtures of which two (alkyl ethyl) or three (bis-alkyl) components with identical mass spectra were detected, should be specially noted. In the former case, their quantities are equal, whereas a ratio between them in the latter case is about 1 : 2 : 1, which is typical of diastereomers. Because the alkyl fragments of 2-alkyl ethyl esters contain only one asymmetrical carbon atom, the second chiral center, which is necessary for the formation of diastereomers, is the four-coordinated atom of phosphorus (structure III). In bis-(2-alkyl)phosphonates, there are three chiral centers, two of which are identical (structure IV); this manifests itself in the appearance of three signals in the chromatograms:

Similarly, chromatographic signals of two diastereomers were recorded for O-pinacolyl methylphosphonofluoridate (soman, structure V) bearing two chiral centers, one of which is the phosphorus atom in the molecule [1]. For compound V, the difference in RI of diastereomers on standard nonpolar phases is about 3 index units (1011 ± 3 and 1014 ± 2), whereas for dialkyl phosphonate it is 8 index units ((2-methylbutyl)ethyl ester), 10 index units (2-octylethyl ester), 2 × 4 unites (bis-(2-methylbutyl ester)), and 2 × 21 index unites (bis-(2-octyl ester)] For structures IV, pair RI differences of the three peaks are equal, which is an additional feature of diastereomers.The retention indexes of N-alkyl dichlorophosphates ROP(O)Cl2 also well obey the additivity rule (RI differences for consecutive homologues are close to 100):
R | C2H5 | С3Н7 | С4Н9 | С5Н11 | С6Н13 | С7Н15 | С8Н17 | С9Н19 | ||||||||
M | 162 | 176 | 190 | 204 | 218 | 232 | 246 | 260 | ||||||||
RI | 897 ± 2 | 991 ± 2 | 1087 ± 2 | 1182 ± 3 | 1282 ± 5 | 1391 | 1494 ± 3 | 1597 ± 5 | ||||||||
ΔRI | 94 | 96 | 95 | 100 | 109 | 103 | 103 | |||||||||
Thus, the addition of gas-chromatographic retention indices to mass spectra makes it possible to perform the unambiguous identification of dialkyl phosphonates. The united GC–MS characteristics of these compounds provide additional opportunities.
United GC–MS parameters of dialkyl phosphonates. For the representation of the signals of ions in the mass spectra, their mass numbers (Da) are used. As for the retention indices, a scale based on reference components (most frequently, n-alkanes) is conventional for them and the conditional values of RI = 100n, where n is the number of carbon atoms in the molecule, are given to them. Consequently, a comparison of the mass-spectrometric and chromatographic parameters requires their representation in a united scale, which can be performed by two methods. In the first of them, the retention indices of reference n-alkanes are taken equal to their molecular weights to determine the system of molecular retention indices (MI = 0.14 RI + 2); on the contrary, in the second method, the so-called homological increments of indices (iRI) are calculated on the basis of the molecular weights of analytes [4, 19]:
where x = int(M/14); int is the function that designates the integer part, which is equivalent to the expression M = 14x + y, and y is the number of the homologous group of the compound, y ≡ M(mod14) [4]. An approach based on the values of iRI is more preferable. The values of iRI for n-alkanes are zero by definition; iRI < 0 for isoalkanes, and iRI > 0 for compounds that are retained better than alkanes. Table 3 summarizes the values of iRI together with the RIs of dialkyl phosphonates.
The concept based on the homological increments of the retention indices makes it possible to optimize the examination of the chromatographic behaviors of homologues. Thus, the values of iRI for di-n-alkyl phosphonates vary in a range from 13 to 43, and they can be represented as an average value and its standard deviation, iRI ± sRI, that is, 29 ± 9. For the homologues containing one branching point in the alkyl fragments, the value of iRI decreases to –4 ± 10 or to –61 ± 14 for two branching points. The presence of a ring in the molecule increases the value of iRI to +75 or to +129 in the presence of two rings; the two last values relate to single esters (cyclohexyl derivatives), and this fact excludes the possibility of their statistical processing.
It is important to note that the absence of information on the molecular weight of an analyte makes impossible the calculation of the values of iRI; however, these parameters can be used for solving the inverse problem of evaluating the molecular weights of analytes from chromatographic data. Indeed, the substitution of the expression M = 14x + y (more precisely, x = (M – y)/14) into Eq. (2) and the solution of the resulting equation for the molecular weight M leads to the following relationship:
Because the dialkyl phosphonates in question belong to the homologous group y = 12, general relationship (3) for the compounds of this series is converted into the form
For checking this relationship based on the example of di-n-alkyl phosphonates, we use the average value iRI ± sRI = 29 ± 9 and (selectively) the data of Table 3. Table 4 summarizes the results obtained for seven homologues; they indicate that, in all cases, the calculated values of M differ from the true values by no more than 1 Da. The standard deviation of iRI (9 index units) is consistent with the expected accuracy of the determination of M about ±1 Da. If we consider the specific additional rounding of obtained data (to the nearest value of M comparable with 12 to the modulus 14 or, in the symbolism of calculus of residues, М ≡ 12(mod14) rather than to the nearest integral value of M), the results of the determination of molecular weights from gas-chromatographic data become unambiguous.
If the molecule contains branched alkyl fragments, it is necessary to use other values of homological increments in place of iRI ± sRI = 29 ± 9 (see above). In the interpretation of the results of the GC–MS analysis of unknown compounds, this can indicate the need of checking several alternate hypotheses, which is a standard approach in mass spectrometry.
An analogous characterization of n-alkyl dichlorophosphates CnH2n + 1OP(O)Cl2 (homologous group y = 8) by the homological increments of retention indices affords iRI ± sRI = –210 ± 6. The determination of their molecular weights based on relationship (3) has no limitations.
Notes
Ist order errors include the incorrect identification of compounds data for which are present in the used bases.
REFERENCES
The NIST 17 Mass Spectral Library (NIST17/2017/EPA/NIH). Software/Data Version (NIST17); NIST Standard Reference Database no 69, National Institute of Standards and Technology, Gaithersburg, MD, 2017. http://webbook.nist.gov. Accessed August 2017.
Zenkevich, I.G. and Fakhretdinova, L.N., J. Anal. Chem., 2016, vol. 71, no. 12, p. 1204.
Zenkevich, I.G. and Podol’skii, N.E., Analitika Kontrol’, 2017, vol. 21, no. 2, p. 125.
Zenkevich, I.G. and Ioffe, B.V., Interpretatsia mass-spektrov organicheskikh soedinenii (Interpretation of Mass Spectra of Organic Compounds), Leningrad: Khimia, 1986.
Zenkevich, I.G., J. Anal. Chem., 2013, vol. 68, no. 13, p. 1158.
Zenkevich, I.G., Anal. Bioanal. Chem., 2013, vol. 405, p. 3075.
Zenkevich, I.G., J. Struct. Chem., 2009, vol. 50, no. 5, p. 895.
Menchikov, L.G., Nefedov, O.M., and Zenkevich, I.G., Russ. Chem. Bull., 2017, vol. 66, no. 3, p. 491.
Zenkevich, I.G. and Nosova, V.E., Analitika Kontrol’, 2016, vol. 20, no. 4, p. 307.
Janesko, B.G., Fisher, H.C., Bridle, M.J., and Montchamp, J.-L., Org. Chem., 2015, vol. 20, no. 20, p. 10 025.
Butts, W.C. and Rainey, W.T., Anal. Chem., 1971, vol. 43, no. 4, p. 538.
Kenttanaa, H.J. and Cooks, R.G., J. Am. Chem. Soc., 1985, vol. 107, p. 1881.
Bolgar, M., Hubball, J., Goeger, J., and Meronek, S., Handbook for the Chemical Analysis of Plastic and Polymer Additives, Boca Raton, FL: CRC, 2016, 2nd ed.
Bhattacharya, A.K. and Thyagarahan, G., Chem. Rev., 1981, vol. 81, no. 4, p. 415.
Rajeshwaran, G.G., Nandakumar, M., Sureshbabu, R., and Mohanakrishnan, A.K., Org. Lett., 2011, vol. 13, no. 6, p. 1270.
Turk, E., Chem. Eng. News, 1998, vol. 76, no. 9, p. 6.
Lebedev, A.T., Mass-spektrometria v organicheskoi khimii (Mass Spectrometry in Organic Chemistry), Moscow: Tekhnosfera, 2015.
Hamming, M.C. and Foster, N.G., Interpretation of Mass Spectra of Organic Compounds, New York: Academic, 1979.
Zenkevich, I.G., Russ. J. Gen. Chem., 2017, vol. 87, no. 4, p. 795.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Makhlyarchuk
Rights and permissions
About this article
Cite this article
Zenkevich, I.G., Nosova, V.E. Characterization of Dialkyl Phosphites by Gas Chromatography–Mass Spectrometry. J Anal Chem 73, 1162–1176 (2018). https://doi.org/10.1134/S1061934818090150
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934818090150