Abstract
A similarity of electron ionization mass spectra of various alkyl esters of benzene-1,2-dicarboxylic (phthalic) acid places special demands on the reliability of the identification of monoalkyl phthalates, often recognized as dialkyl phthalates and vice versa. An original mass spectrometric criterion is proposed for the group identification of monoalkyl phthalates (assignment to a subgroup of homologues), which assumes the calculation of the total relative intensity of ion peaks at m/z > 170. In the mass spectra of monoalkyl esters, this value does not exceed 1%, whereas for dialkyl ethers, it varies from 1 to 172%. Within the set subgroups of isomers, it becomes effective to use the homologous increments of the retention indices, iRI = RI – 100x, where x = int (M/14), to refine the identification results. Using predefined iRI values and the ratio М ≈ 0.14(RI – iRI) + y, we can estimate the molecular weights of compounds in the mass spectra of which the molecular ion signals are not detected or are of low intensity (including monoalkyl phthalates). In addition, iRI values are in many cases informative for estimating the number of branches of the sp3 carbon skeleton of molecules (Z) based on regression relations of the form of iRI = aZ + b. The examination of homologous increments of the retention indices of dialkyl phthalates revealed an anomalous dependence of their values on the number of carbon atoms in the alkyl fragments of the molecules: from +125 (СН3) to –33 (С10Н21). In the subgroup of monoalkyl phthalates, such anomalies were not observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The basis of the effectiveness of present-day gas chromatography–mass spectrometry as a method for identifying the components of complex mixtures of organic compounds is the existence of detailed and well-systematized information support, namely, electron ionization (EI) mass spectra and gas chromatographic retention indices for standard nonpolar and polar phases. The NIST 2017 database [1] contains EI mass spectra of 267 376 compounds and gas chromatographic retention indices (RI) of 99 400 compounds. However, both individual compounds and their sub-groups (homologous series, groups of congeners, isomers, etc.) remain unrepresented in the databases for objective reasons. The main reasons for the lack of information are the following [2, 3]:
(1) The practical use of certain compounds is not obvious, which leads to a lack of interest in them from synthetic chemists.
(2) Compounds were not found in natural objects; no information on their biological activity, toxicity, etc. is available.
(3) Compounds are unstable and/or highly reactive.
(4) Direct chromatographic separation is not possible; derivatization is required.
(5) The synthesis is complicated or characterized by low yields.
(6) A large number of isomers of higher homologues of any series, an exhaustive characterization of the entire variety of which is impossible.
(7) Probably, the erroneous identification of various analytes found in real samples is sometimes caused by a lack of information support and the similarity of the mass spectra of compounds of different classes.
The lack of information in combination with the similarity of the mass spectra of compounds of different groups is the main cause of a large number of errors of the IInd order [4]Footnote 1. Partial esters of both organic and inorganic polybasic acids form one of the vast groups of poorly characterized compounds. Among inorganic acid derivatives, dialkylthiophosphates can be noted, the electrospray ionization mass spectra of which are difficult to distinguish from the spectra of trialkyl monothiophosphates [5]. For acidic esters of organic polycarboxylic acids, this problem was identified for benzene-1,2-dicarboxylic acid monoalkyl esters (monoalkyl phthalates) [6, 7], the EI mass spectra of which are similar to those of dialkyl phthalates. The reasons for the similarity of the mass spectra are similar patterns of fragmentation: in the mass spectra of the C6H4(CO2H)(CO2CnH2n + 1) monoesters, the signals of the primary fragmentation ions [M–CnH2n + 1O]+ → [C6H4(CO)2OH]+ are recorded at m/z 149, the signals of which are the maximum. An alternative two-stage way of the formation of these ions is possible, namely, [M– CnH2n – 1] (m/z 167) → [M–CnH2n – 1–H2O]+ → [C6H4(CO)2OH]+ (m/z 149). For C6H4(CO2CnH2n + 1)2 diesters described in more detail [8], similar ions at m/z 149 are formed in two stages: M+ → [M–CnH2n + 1O]+ → [M–CnH2n + 1O–CnH2n]+ → [C6H4(CO)2OH]+ (m/z 149); an alternative three-stage sequence of fragmentation processes is also possible: M+.→ [M–CnH2n – 1]+ → [M– CnH2n–1–CnH2n]+ (m/z 167) → [M–CnH2n–1–CnH2n– H2O]+ → [C6H4(CO)2OH]+ (m/z 149). The signals of hydrocarbon ions [CmH2m + 1]+, [CmH2m – 1]+ (m < n), and others in the mass spectra of all phthalate esters are not sufficiently characteristic. The above factors, combined with low signal intensities of molecular ions, explain the difficulties in the analytical differentiation of monoalkyl and dialkyl phthalates.
The discovery of mono(2-ethylhexyl) phthalate in the composition of essential oils of some plants was reported [9–12]; however, such an ether is formed, most likely, as a result of partial hydrolysis of bis(2-ethylhexyl) phthalate, one of the most common plasticizers of polyvinyl chloride compositions [13]. The toxic effects of this plasticizer are reviewed in [14]. Other examples of the detection of monoalkyl phthalates in various objects are mentioned in [7]. However, checking the correctness of their identification in most cases reveals numerous inconsistencies and errors. According to the data [1], the RI of monobutyl phthalate is 1828 and is consistent with the RI values of other homologues. At the same time, an erroneous (underestimated) value of RI = 1520 of the same monoester was indicated in [9]. The retention index of monobutyl phthalate for the standard polar phase [10] is known (RI = 2552), but it seems too small for such a polar compound. The reason for this assumption is the insufficiently large RI difference for the standard polar and nonpolar (1849 [1]) phases, which is only 2552 – 1828 = 724, whereas, for example, for unsubstituted benzoic acid, this difference [1] can be estimated as (2427 ± 22) – (1170 ± 20) = 1247 ± 30.
Some essential properties of monoalkyl phthalates were found relatively recently. Such esters are unstable under gas chromatographic separation conditions and partially decompose in a chromatographic column to form the corresponding alcohols and phthalic anhydride [6, 7]. The possibility of such decomposition at lower temperatures and the structural analogy, in particular, of C6H4(CO2H)COOCH3 monomethyl phthalate and acetylsalicylic acid (aspirin) C6H4(CO2H)OCOCH3, suggests that it is precisely the instability of monoalkyl phthalates that can determine their toxicity [7].
A detailed examination of the reference values of the retention indices of dialkyl phthalates reveals the insufficient reliability of correlation of their values with the structures of compounds. Possible discrepancies may be due to both the erroneous identification of phthalates in real samples and the negligence of the nomenclature used. The same “iso” prefix in phthalate names may reflect different options of the carbon skeleton branching or refer to technical mixtures of several isomers. An additional reason for the scatter in the RIs may be differences in the polarity of standard nonpolar polydimethylsiloxane stationary phases and their analogues containing 5% of phenyl groups (they are classified as semistandard according to the classification adopted in [1]). The RI value of dioctyl phthalate C24H38O4 (no branching of the carbon skeleton) for the standard nonpolar phase is 2686 ± 3 index units [1] (Table 1), but for the isomeric decyl 2-ethylbutyl phthalate (one branching of the carbon skeleton) for the phase with 5% of phenyl groups, the known RI value is greater (2701), which is not consistent with the generally recognized effect of the number of such branches on retention indices. However, the additive estimate of the RI for the standard nonpolar phase [15] is even greater (2768), since it is based on the RI values of other homologues. Such noticeable discrepancies, first, make it ineffective to use such unreliable reference data for identification, and second, make it doubtful that the value 2686 ± 3 really belongs to di-n-octyl phthalate. At the same time, the RI of bis(2-ethylhexyl) phthalate is 2505 ± 4, which seems too low for the isomer with two branches in the carbon skeleton, whereas for bis(2,4,4-trimethylamyl) phthalate (six branches), it reaches 2587, which is too large and is not consistent with the previous value. The correction of such discrepancies requires either additional experimental verification or the use of special algorithms for “cross” control of reference data.
Thus, the gas chromatography–mass spectrometric identification of monoalkyl phthalates using reliable values of analytical parameters is among the urgent tasks. These parameters typically include standard electron ionization mass spectra and retention indices for standard nonpolar stationary phases. Improving the reliability of reference data and, as a consequence, the results of identification based on them make us turn to the consideration of “hybrid” parameters, such as the homologous increments of retention indices, combining mass spectrometric and chromatographic information.
EXPERIMENTAL
The synthesis of monoalkyl phthalates from phthalic anhydride and the corresponding alcohols in the presence of catalytic amounts of phosphoric acid, the conditions for their gas chromatography–mass spectrometric determination, and the full EI mass spectra (70 eV) of monoalkyl phthalates are reported in [6, 7]. The mass spectra of dialkyl phthalates for calculating the ion series spectrum were both borrowed from available databases and obtained experimentally. Below we discuss the gas chromatographic retention indices of monoalkyl phthalates and their combined gas chromatography–mass spectrometric characteristics in comparison with dialkyl phthalates. Statistical data processing and graphing were performed using ORIGIN software (vers. 4.1 and 8.2).
RESULTS AND DISCUSSION
Determination and application of homologous increments of retention indices. Mass spectrometric and gas chromatographic characteristics of analytes can be interpreted jointly, including in the form of homologous increments of retention indices (iRI),
where x = int (M/14), M is the molecular mass number (integer), int is a function denoting the integer part of a number (equivalent to expression M = 14x + yM), yM is the number of the homologous group of the compound, yM ≡ M(mod14).
Homologous increments of retention indices, along with similar increments of other properties, were proposed in the mid-1980s [16] for the gas chromatography–mass spectrometric identification of analytes. Like other differential analytical parameters [17], they have the property of invariance within homologous series (with some reservations discussed below) and, therefore, are applicable at the stage of group identification (assignment to the corresponding series).
By definition, chromatographic retention indices are additive quantities: moving to the next homologue by adding a homologous difference—a CH2 fragment—increases the RI by approximately 100 index units, which makes their direct comparison for different homologues impossible. However, invariants such as iRI offer a comparison of the chromatography–mass spectrometric characteristics of different members of the series. Another important application of the homologous increments of retention indices follows, that is, the identification of erroneous or doubtful RI values and, in general, the increase in the reliability of reference information.
A feature of iRI, as well as isomer retention indices themselves, is their dependence on the number of branches in the carbon skeleton of the molecules (Z). As a first approximation, we can differentiate the topological nonequivalence of the various branches of the sp3 carbon skeleton of the molecules, but it should be borne in mind that tertiary carbon atoms correspond to one branch, and quaternary carbon atoms are for two branches. The dependence iRI = f(Z) complicates the group identification procedure, since several alternative values of Z have to be taken into account; however, such a comparison of hypotheses refers to typical methods of interpreting mass spectrometric information.
The absence or low intensity of molecular ion signals in the mass spectra (most typical for EI mass spectra) makes it impossible to determine the values of M or calculate iRI. In such cases, the solution of the “inverse” problem becomes relevant: estimating the molecular weights of analytes, in the mass spectra of which the signals of molecular ions are not recorded or are of low intensity, based on predefined iRI values. Substitution of the equation x = (M – yM)/14 into Eq. (1) and the solution of the resulting equation for M lead to the following expression for estimating molecular mass numbers:
The specific of rounding of the calculated M values is that it is performed, rather than to the nearest integer number, to the nearest value M comparable to yM modulo 14 or, in the symbolism of the theory of residues, М ≡ уМ(mod14). Such a problem was considered using homologues of the series of dialkyl phosphonates and trialkyl phosphites [18, 19].
If the information on the molecular mass numbers of analytes is available, then the existence of the iRI = f(Z) dependences enables the estimation of the total number of branches of the sp3 carbon skeleton of the alkyl fragments in the molecules using chromatography–mass spectrometric data. For the convenience of estimates, such dependences can be used in the form of inverse functions Z = φ(iRI). The dependences iRI = f(Z) and Z = φ(iRI) are decreasing; that is, the larger the total number of branches in the carbon skeleton of the molecules, the smaller the iRI values (taking into account the signs), which can be approximated with acceptable accuracy by linear regression equations
Coefficients a and b are calculated by the least-squares method.
For many homologous series, the domains of iRI values at different Z do not overlap with each other, which corresponds to an unambiguous determination of the number of branches by iRI values. A solution to this problem is illustrated [3] by the example of a homologous series of 2,2-dialkyl-substituted 1,3-dioxolanes.
In addition to the listed examples, iRI values are recommended as a new criterion for the polarity of organic compounds [20].
Homologous increments of retention indices for dialkyl and monoalkyl phthalates. Consideration of iRI values for a series of monoalkyl phthalates leads to essential conclusions regarding the features of their identification. Given the above similarity of the mass spectra of various phthalate esters, it is advisable to consider the data for monoalkyl phthalates in constant comparison with dialkyl phthalates. Table 1 lists the gas chromatographic retention indices of some dialkyl phthalates [1] and their homologous increments depending on the total number of branches in the carbon skeleton of the molecular alkyl fragments, iRI(Z). In the lower part of Table 1, the average 〈iRI(Z)〉 values the same Z are compared, and the iRI(Z) dependence is represented graphically in Fig. 1a. The straight line corresponds to the linear regression equation; the parameters of the equation are given in the caption to Fig. 1.
Dependences of the homologous increments of retention indices (iRI) on the total number of branches in the carbon skeleton of alkyl fragments of molecules for (a) dialkyl phthalates and (b) monoalkyl phthalates. Parameters of linear regression equations: (а) a = 21.2 ± 9.7, b = –37.9 ± 6.1, R = –0.813, S0 = 33; (b) a = 336 ± 6, b = –74 ± 7, R = –0.925, S0 = 19.
We noted above that an increase in the total number of branches in the carbon skeleton of alkyl fragments leads to a noticeable decrease in iRI values: at Z = 2, 3, and 4, the average iRI values are –49, –100, and –162. Here, the absence of significant differences in the iRI values for Z = 0 and 1 (Fig. 1) attracts attention. Such a discrepancy manifests itself in the relatively low absolute value of the correlation coefficient (R = –0.813). The most probable reason for this may be the discrepancies noted above for the reference values of the retention indices of dialkyl phthalates to their molecular structures, caused by both the difficulties in identifying such esters and the ambiguity in interpreting the “iso” prefix in the names of compounds of this class. Another reason may be the anomalously expressed dependence of iRI on the number of carbon atoms in alkyl substituents: their values naturally decrease from +125 at R = CH3 to –33 at R = С10Н21. In other words, if the simplest homologue—dimethyl phthalate—is a sufficiently polar compound, more polar than, for example, 2-alkanols (for 2-butanol, M = 74, RI = 586 ± 5 [1]; therefore, iRI = 86) and only slightly poorer in polarity than 2,3-alkanediols (for 2,3-butanediol M = 90, RI = 756 ± 9 [1], iRI = 156), didecyl phthalate is less polar than n-alkanes, for which the iRI values are zero by definition. At the same time, the absence of differences in iRI values for Z = 0 and 1 suggests that at least a part of the reference data, especially, for dialkyl phthalates with asymmetric alkyl fragments, deserves verification and refinement. On the other hand, there is a good correlation between the retention indices of monoalkyl and dialkyl phthalates (with the same number of carbon atoms in the alkyl substituents) with each other [7]; this enables us to estimate the missing RI values from data for another subgroup of homologues.
Similar information for monoalkyl phthalates, including gas chromatographic retention indices and their homologous increments depending on the total number of branches in the carbon skeleton of alkyl substituents, iRI(Z), is presented in Table 2. To increase reliability, the results of [6, 7] are compared with the values of the RI from [1]; the average value of their difference (taking into account the sign for 11 pairs of homologues) is +3 ± 10 index units, which confirms the reliability of both independent sources of information. This immediately affects the values of iRI(Z), which, in contrast to the data of Table 1, no longer show any anomalies at Z = 0 and 1. The graphical interpretation of this dependence in Fig. 1b confirms the possibility to determine the number of branches of Z by iRI values unambiguously, since the intervals of scattering of iRI values for different Z, in contrast to Fig. 1a, do not overlap. The best correlation of iRI(Z) also affects the absolute value of the correlation coefficient, which, in this case, is –0.925. The large positive values of the homologous increments of the retention indices of monoalkyl phthalates (339 ± 13 at Z = 0) confirm their high polarity, which is comparable to the polarity of arenecarboxylic acids (for benzoic acid, M = 122, RI = 1170 ± 20 [1], iRI = 320) or phenols (for p-cresol, M = 108, RI = 1052 ± 4 [1], iRI = 352). For monoalkyl phthalates, there is no noticeable decrease in iRI values with an increase in the number of carbon atoms in alkyl substituents: their values only slightly decrease from 366 at R = CH3 to only 331 at R = C10H21. Therefore, such an effect for dialkyl phthalates deserves more detailed consideration.
The signals of the molecular ions in the mass spectra of both monoalkyl and dialkyl phthalates are low-intensity, except for methyl and ethyl esters; therefore, the problem of estimating molecular mass numbers is relevant for them. A simple application of Eq. (2) for these purposes can be illustrated by the following examples:
Diamyl phthalate: RI = 2122 ± 3, iRI = +11 ± 31 (assuming Z = 0). Then, М ≈ 0.14 × (2122 – 11) + 12 ≈ 307.5 → 306 ≡ 12(mod14) (correct answer).
Monoamyl phthalate: RI = 1929 ± 11, iRI = 339 ± 13 (assuming Z = 0). Now М ≈ 0.14 × (1929 – 339) + 12 ≈ 234.6 → 236 ≡ 12(mod14) (correct answer).
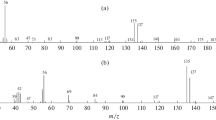
Mass spectrometric differences of monoalkyl and dialkyl phthalates. The above possibilities of applying the homologous increments of retention indices can most effectively be used only when the homologous series of analytes are uniquely identified (otherwise, several alternative hypotheses must be verified). In the case under consideration, the gas chromatographic retention parameters, useful at the stage of refinement of the analyte structure, are only partially informative for group identification, and it is necessary to refer to mass spectrometric data. To the greatest extent, the problem of group identification is relevant precisely for monoalkyl phthalates, since the assignment of diesters to the corresponding subgroup of phthalates is quite reliable. This position is easily confirmed by the results of a library search, for example, using the NIST database [1]. For example, we select the mass spectra of diisodecyl and monodecyl phthalates and compare them with other mass spectra contained in the same database for the coincidence factor Q > 0.800 (Table 3). Of the 38 library search results found for diisodecyl phthalate at the group identification level, 37 are correct (refer to a number of dialkyl phthalates), while for monodecyl phthalate the correct answers are less than half. A similar situation is observed for other monoalkyl and dialkyl phthalates. The reason for this is the similarity noted above for the fragmentation processes of molecular ions of compounds of these series under EI conditions. Thus, the problem of assigning homologues to the corresponding subgroup is most relevant for monoalkyl phthalates.
The use of mass spectrometric invariants such as the ion series spectra [16] for group identification does not offer reliable identification of monoalkyl phthalates. The ion series mass spectra are a combination of 14 numbers {I(y)} (0 ≤ y ≤ 13), each of which is the total intensity of the peaks of ions of homologous groups (0 ≤ y ≤ 13) in the total ion current, ΣIy = 100, y ≡ m/z(mod14). After averaging such sets of numbers for several homologues, we obtain statistically processed data sets {I(y)s(y)} that are applicable for assigning analytes to the corresponding homologous series (standard deviations are indicated in subscripts):
Dialkyl phthalates: 11, 76, 0, 0, 0, 0, 53, 22, 0, 677, 82, 0, 0, 105.
Monoalkyl phthalates: 65, 32, 0, 22, 11, 11, 44, 62, 22, 5111, 83, 43, 0, 126.
The ion series spectra confirm that the signals of the homologous group y = 9 have the highest intensity in the mass spectra of all esters, since they include the maximum peaks at m/z 149. It follows from the same spectra that there are no fundamental differences for mono- and diesters in them; the ranges of most Iy values overlap within double standard deviations, so that the group identification of phthalate mono- and diesters is possible only as an exception. Only for individual dialkyl phthalates, the total intensities of the signals of the y = 1 homologous group (7 ± 6%) are statistically significantly different from their intensities in the mass spectra of monoalkyl phthalates (3 ± 2%), which is quite natural due to the unequal number of alkyl fragments in the molecules. The same can be said about the signals of the y = 7 group (5 ± 3 and 6 ± 2%, respectively), as well as the y = 11 groups (0 and 4 ± 3%). However, it is advisable to consider such a criterion only as an addition to more reliable features. Attempts to differentiate monoalkyl and dialkyl phthalates by relative peak intensities of individual ions using chemometric methods (linear discriminant analysis, LDA) showed their low efficiency and were found to be unacceptable. In particular, the model of recognition of monoalkyl phthalates by the criterion of decreasing peak intensities in the sequence of mass numbers 105 > 77 > 69 > 57 leads to erroneous results for 5 out of 19 homologues.
Nevertheless, a method enabling a more reliable differentiation of monoalkyl and dialkyl phthalates precisely at the level of group identification (attribution to subgroups of homologues) does exist. It is based on the above-mentioned features of the fragmentation of molecular ions of these esters under EI conditions. The fact is that the most characteristic ions at m/z 149 (correspond to the maximum signals of the mass spectra) of monoalkyl phthalates are obtained mainly in two stages, that is, М+ → [M– CnH2n + 1O]+ → [M–CnH2n + 1O]+ → [C6H4(CO)2OH]+ (m/z 149), and, for dialkyl phthalates, there is one more step: М+.→ [M–CnH2n + 1O]+ → [M–CnH2n + 1O–CnH2n]+ → [C6H4(CO)2OH]+ (m/z 149). It can be assumed that additional “intermediate” ions must somehow manifest themselves in the mass spectra, as a result of which the total intensities of ions with larger mass numbers for dialkyl phthalates should be higher than that of monoalkyl phthalates. If we formalize this condition in the form of “total relative intensity of peaks at m/z > 170” acceptable for practical use, we obtain the results presented in Table 4.
For most monoalkyl phthalates, regardless of the size of the alkyl fragments, the total relative intensities of the signals at m/z > 170 are close to zero. The only exception is monoethyl phthalate, but this should not be considered a significant limitation of the chosen criterion. First, the signal of molecular ions (Irel ≈ 1%) is reliably recorded in the mass spectrum of this ester. Second, the only phthalate compound isobaric to monoethyl phthalate is dimethyl phthalate; the mass spectra and retention indices of these esters differ significantly. In the mass spectra of dialkyl phthalates, the total relative intensities of ion peaks at m/z > 170 vary in the range of 1–172%. Such a criterion is characterized by sufficient reliability and does not require additional calculations for, for example, the expression of the peak intensities in the total ion current.
Among the additionally verified criteria for the group identification of monoalkyl and dialkyl phthalates, we can mention the relative intensities of ion signals at m/z 167 and 149. For monoalkyl phthalates, the peak intensities at m/z 167 are higher (13 ± 5%) than those for dialkyl phthalates (7 ± 5%). However, such a criterion cannot be considered statistically significant, since the difference in average values is less than the sum of their standard deviations. The same applies to another criterion, that is, the intensities of signals at m/z 149 (most often they are maximum in the mass spectra) in the total ion current: for monoalkyl phthalates, the average value is 0.49 ± 0.14, and for dialkyl esters, it is 0.69 ± 0.07.
CONCLUSIONS
Despite the significant similarity of EI mass spectra of various alkyl phthalates, the special processing of the gas chromatography–mass spectrometric data ensures the exclusion of the erroneous identification of monoalkyl phthalates. The mass spectrometric criterion for assigning analytes to a subgroup of monoesters is the low total relative intensity of ion peaks at m/z > 170 (less than 1%). After being assigned to a subgroup of homologues, the use of homologous increments of retention indices makes it possible to evaluate the molecular weights of analytes and the total number of branches in the alkyl fragments of the molecules. Alkyl phthalates can also be identified directly by their retention indices for standard nonpolar phases.
Notes
Identification errors of the Ist order is the impossibility of correct identification of the compounds characterized and presented in the reference information arrays, of the IInd order is an erroneous identification of previously uncharacterized objects.
REFERENCES
The NIST 17 Mass Spectral Library (NIST17/2017/EPA/NIH), NIST Standard Reference Database no. 69, June 2017, Gaithersburg, MD: Natl. Inst. Stand. Technol. http://webbook.nist.gov. Accessed January 2020.
Zenkevich, I.G. and Lukina, V.M., Analitika Kontrol’, 2019, vol. 23, no. 3, p. 410. https://doi.org/10.15826/analitika.2019.23.3.009
Eliseenkov, E.V. and Zenkevich, I.G., Mass-Spektrom., 2020, vol. 20 (in press).
Zenkevich, I.G., Anal. Bioanal. Chem., 2013, vol. 405, p. 3075. https://doi.org/10.1007/s00216-013-6751-2
Zenkevich, I.G., Pushkareva, T.I., and Karakashev, G.V., Analitika Kontrol’, 2019, vol. 23, no. 3, p. 425. https://doi.org/10.15826/analitika.2019.23.3.012
Zenkevich, I.G. and Fakhretdinova, L.N., Analitika Kontrol’, 2015, vol. 19, no. 2, p. 175. https://doi.org/10.15826/analitika.2015.19.2.013
Zenkevich, I.G. and Fakhretdinova, L.N., J. Anal. Chem., 2016, vol. 71, no. 12, p. 1204. https://doi.org/10.7868/S004445021612015X
Hamming, M.G. and Foster, N.G., Interpretation of Mass Spectra of Organic Compounds, New York: Academic, 1979.
Kim, N.-S. and Lee, D.S., Anal. Sci., 2001, vol. 17, no. 1 (suppl.), A383.
Pasenzotto, L., Gracco, L., and Conte, L., J. Sci. Food Agric., 2001, vol. 83, p. 1037.
Syeda, F.A., Habib-ur-Rehman, Choudahry, M.I., and Ata-ur-Rahman, Int. J. Genet. Mol. Biol., 2011, vol. 3, no. 7, p. 95.
Bagavathi, P.E. and Ramasamy, N., Pharmacogn. Res., 2012, vol. 14, no. 1, p. 11. https://doi.org/10.4103/0974-8490.91028
Kawakami, T., Isama, K., and Matsuoka, A., J. Environ. Sci. Health, 2011, vol. 46, no. 8, p. 855. https://doi.org/10.1080/10934529.2011.579870
Rowdhwal, S.S.S. and Chen, J., BioMed Res. Int., 2018, 1750368. https://doi.org/10.1155/2018.1750368
Stein, S.E., Babushok, V.I., Brown, R.L., and Linstrom, P.J., J. Chem. Inf. Model., 2007, vol. 47, p. 975. https://doi.org/10.1021/ci600548y
Zenkevich, I.G. and Ioffe, B.V., Interpretatsiya mass-spektrov organicheskikh soedinenii (Interpretation of Mass Spectra of Organic Compounds), Leningrad: Khimiya, 1986.
Zenkevich, I.G., Russ. J. Gen. Chem., 2017, vol. 87, no. 4, p. 795. https://doi.org/10.1134/S1070363217040211
Zenkevich, I.G. and Nosova, V.E., J. Anal. Chem., 2019, vol. 74, no. 13, p. 1297. https://doi.org/10.1134/S1061934819130124
Zenkevich, I.G. and Nosova, V.E., J. Anal. Chem., 2019, vol. 74, no. 14, p. 1413. https://doi.org/10.1134/S1061934819140120
Zenkevich, I.G., Russ. J. Gen. Chem., 2019, vol. 89, no. 3, p. 369. https://doi.org/10.1134/S1070363219030010
ACKNOWLEDGMENTS
The experimental data discussed in this work were obtained using the equipment of the Resource Center in the field of “Chemistry” at the Institute of Chemistry, St. Petersburg State University. The author is grateful to his colleagues working at the Center for assistance. The author acknowledges Prof. Károly Héberger (Research Center for Natural Sciences, Hungarian Academy of Sciences, Budapest) for his conclusions regarding the possibilities of using chemometric methods for the differentiation of alkyl phthalates.
Funding
This work was partially supported by the Russian Foundation for Basic Research, project no. 18-03-00151/A.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Zhukova
Rights and permissions
About this article
Cite this article
Zenkevich, I.G. Specific Features of the Gas Chromatography–Mass Spectrometry Identification of Monoalkyl Phthalates. J Anal Chem 75, 1322–1329 (2020). https://doi.org/10.1134/S1061934820100160
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934820100160