Abstract
The interaction of aliphatic alcohols ROH with phosphorus trichloride in the absence of bases leads to the formation of exclusively dialkyl phosphonates (RO)2PHO. To obtain trialkyl phosphites (RO)3P, insufficiently described to date, the pH of the reaction mixtures was adjusted by adding N,N-dimethylaniline (pKa 5.1 ± 0.1). The identification of trialkyl phosphites directly in reaction mixtures without preparative isolation implies a joint consideration of their mass spectra and gas chromatographic retention indices, provided that all other components of such mixtures are identified. The need to comply with this condition has led to the identification of several homologues of a previously uncharacterized series of 2-alkoxy-1,3,2-dioxaphospholanes, formed from a random admixture of ethylene glycol. The use of such combined mass spectrometric parameters as homologous increments of retention indices helps to distinguish trialkyl phosphites from isobaric dialkyl phosphonates; the compounds of both series belong to the homologous group of y = 12, y ≡ M(mod14). Despite the absence of molecular ion signals in the electron ionization (EI) mass spectra, the molecular weights of trialkyl phosphites can be estimated. In addition, a comparison of the homologous increments of retention indices shows that, compared with dialkyl phosphonates, trialkyl phosphites are significantly less polar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
In contemporary organic mass spectrometry, compounds of some even relatively simple classes remain insufficiently characterized. This is most often due to the limited preparative and analytical “demands” for such compounds or to some difficulties of their synthesis. As was recently shown, one example of such compounds is provided by monosubstituted esters of polybasic acids, including monoalkyl phthalates [1]. The mass spectrometric identification of such monosubstituted esters is complicated by the considerable similarity of their electron ionization (EI) mass spectra with the spectra of the full esters of the corresponding acids; it is possible, for example, with the application of gas chromatographic retention parameters (retention indices, RIs). Similar problems are typical for the partial esters of polybasic inorganic acids.
The identification of insufficiently characterized classes of compounds is possible by viewing the content of complete databases of mass spectra, for example, the database of the National Institute of Standards and Technology (NIST, United States) [2]. For example, it can be found that, among the trialkyl phosphates (CmH2m + 1O)3PO (with a total number of carbon atoms of 3 ≤ nC ≤ 15) represented in it, 27 homologues are characterized by EI mass spectra, but only six of them (with the same simplest alkyl substituents) have the values of retention indices on standard nonpolar polydimethylsiloxane stationary phases. However, given that they are the simplest homologues that are most often found in analytical practice, this level of knowledge can be considered acceptable. As for the characterization of phosphoric acid dialkyl esters [dialkyl phosphates (CmH2m + 1O)2P(O)OH], none of these compounds (2 ≤ nC ≤ 15) are presented in the database [2]. A particular discussion of their mass spectrometric characteristics [3] showed that the gas chromatographic separation of such esters is impossible. The most likely reason for this is in their acidic properties: the pKa values of dialkyl phosphates are lower than those of the phosphoric acid itself, that is, pKa(1) = 2.1 ± 0.1.
Both normal and acidic esters of phosphorous acid H3PO3 have not been described in sufficient detail. In the case of dialkyl phosphonates (CmH2m + 1O)2PHO (often called dialkyl phosphites) with 2 ≤ nC ≤ 15, the mass spectra of only eight homologues are presented in the database [2], and the retention index is known only for one of them (diethyl phosphite). Improving of this situation was the subject of a special publication [4]. A similarity of the EI mass spectra of normal and monosubstituted esters, typical of various polybasic acids, which leads to identification errors, compels us to consider in detail trialkyl phosphites (CmH2m + 1O)3P, for homologues of which with 3 ≤ nC ≤ 15, eight mass spectra and three retention indices are presented in the database [2]. In the current publications in the field of mass spectrometry, phosphorous acid derivatives are mentioned only in [5].
Of various phosphorus-containing substances, the most detailed data were obtained for toxic compounds and products of their transformations, mainly, derivatives of alkyl phosphonic acids [6–8]. The level of knowledge of gas-chromatographic retention indices of such compounds makes it possible to predict with sufficiently high accuracy their values for representatives of these series, which are not yet known, using additive approaches [9–12].
A particular interest to trialkyl phosphites is associated with the features of their synthesis: the interaction of phosphorus trichloride (PCl3) with the corresponding alcohols without controlling the pH of the reaction mixtures leads exclusively to dialkyl phosphonates [4], which is caused by the Michaelis–Arbuzov rearrangement in an acidic medium [13, 14], that is,
In order to stop the reaction at the stage of the formation of trialkyl phosphites (RO)3P, it is necessary to fix the pH of the reaction mixtures by adding bases. It is undesirable to use aliphatic amines (for example, triethylamine with pKa of 10.7 ± 0.1), since this can lead to the hydrolysis of the esters formed. It is acceptable to use weaker bases, for example, dialkylaryl amines C6H5NR2, including N,N-dimethylaniline (pKa 5.1 ± 0.1), N,N-diethylaniline (pKa 6.4 ± 0.2), and others [15, 16]. In this case, the reaction can be written as follows:
This paper is devoted to the determination and consideration of the mass spectrometric characteristics of the simplest trialkyl phosphites as an essential addition to the similar characteristic of more complex phosphorus-containing acids.
EXPERIMENTAL
Interaction of alcohols with phosphorus trichloride in the presence of N,N-dimethylaniline. Trialkylphosphites were synthesized by mixing 100 μL of aliphatic alcohols ROH (cp grade or for chromatography grade) with 100 μL of phosphorus trichloride (99%) in 1.5–2.0 mL of chloroform (cp grade) in the presence of 450 μL of N,N-dimethylaniline used without purification. The reagents were mixed in a 5-mL vessel in the following order: N,N-dimethylaniline was added to a solution of alcohol in chloroform, and phosphorus trichloride was added dropwise under constant stirring; this was accompanied by heating of the reaction mixture and changing the color to yellow-brown. After cooling, the reaction mixture was stratified; the upper (colored) layer contained a significant portion of dimethylaniline hydrochloride and PCl3 hydrolysis products. The lower (transparent) layer of the reaction mixture was used for analysis by gas chromatography–mass spectrometry; it was diluted 25–50 times with chloroform.
To characterize 2-ethoxy- and 2-propoxy-1,3,2-dioxaphospholanes, 100 μL of ethylene glycol was added to the reaction mixture. The amounts of all other reagents remained the same .
Gas chromatography–mass spectrometry. The reaction mixtures were analyzed using a Shimadzu QP 2010 SE chromatograph–mass spectrometer with electron ionization. The temperature of the interface and of the ion source was 200°C; for the reaction mixtures containing C7–C9 alcohols, the temperature was increased to 250°C. We used an RTX-5 M column 30 m in length and 0.32 mm in internal diameter; the thickness of the stationary phase film was 0.25 μm. The following analysis mode was used: temperature programming from 50 to 200°C (for reaction mixtures containing C7–C9 alcohols, the upper limit was increased to 250°C) at a rate of 5 K/min; injector temperature 180°C; detector temperature 200°C (250°C); carrier gas helium at a volumetric rate of 1.84 mL min–1 (linear velocity 49 cm s–1); flow splitting at 1 : 10; sample volume 0.5 μL. To determine retention indices, mixtures of reference n-alkanes C8–C12, C14, C15, and C18–C24 in hexane were added to the samples.
Processing of results. To calculate the linear-logarithmic retention indices, we used the Microsoft Office Excel 2016 software and the QBasic program. In this system, the indices are calculated by the retention times of three reference components rather than two of them. Such an approach allows one to calculate the retention indices using different triads of reference components with the subsequent averaging of the results. Dialkyl phosphonates were identified in the reaction mixtures according to their previously recorded mass spectra and retention indices [4]; phosphorus-free components were identified using the database [2].
RESULTS AND DISCUSSION
General characteristics of the composition of reaction mixtures. For the mass spectrometric characterization of trialkyl phosphites, they were obtained by a known reaction (from phosphorus trichloride and the corresponding alcohols) and identified directly in the composition of the reaction mixtures without preparative isolation. Such an approach is convenient for practical purposes, but it implies that, in order to eliminate possible uncertainties, not only the reaction must be sufficiently specific, but also all components of such mixtures must be uniquely identified. We used a similar approach earlier, in particular, to characterize dialkyl phosphonates [4].
As was noted in the introduction, the interaction of aliphatic alcohols ROH with PCl3 in inert nonpolar solvents (chloroform) in the absence of bases leads to the formation of only the corresponding dialkyl phosphonates (RO)2PHO; trialkyl phosphites (RO)3P are entirely absent in the reaction mixtures [4]. Their occurrence becomes possible in carrying out the reaction in the presence of weak bases; we used stoichiometric (calculated for the resulting HCl) amounts of N,N-dimethylaniline (pKa 5.1 ± 0.1). Despite this, some amounts of dialkyl phosphonates are detected in the reaction mixtures, for the identification of which prerecorded EI mass spectra and retention indices were used [4]. Dialkyl phosphonates and trialkyl phosphites are most often the main components of such mixtures.
The analytical parameters and the results of identification of the components of 13 reaction mixtures of PCl3 with C2–C9 alcohols are summarized in Table 1, including their retention indices on a column with a nonpolar stationary phase RTX-5 MS (according to the classification adopted in [2], this phase is referred to as semi-standard type because of the presence of 5% of the phenyl groups) and EI mass spectra.
The number of the detected components of the reaction mixtures of alcohols ROH with PCl3, as in the case of dialkyl phosphonates [4], exceeds the number of expected products. The reason for this was the presence of ethanol in the chloroform used as a solvent, which led to the formation of mixed dialkyl phosphonates (C2H5O)2PHO, (RO)(C2H5O)PHO, and (RO)2PHO and trialkyl phosphites (C2H5O)3P, (RO)(C2H5O)2P, (RO)2(C2H5O), and (RO)3P. Ethanol additives are necessary to prevent the formation of phosgene during the storage of chloroform [17]. As in [4], in order to characterize the series with a larger number of homologues more fully, we did not exclude such products from consideration.
Among the other (expected) components of the reaction mixtures of CnH2n + 1OH with PCl3, we note the initial (unreacted) alcohols, traces of N-methylaniline (impurity in N,N-dimethylaniline), chloroalkanes RCl, acetals Cn – 1H2n – 1CH(OCnH2n + 1)2, alkyl alkanoates Cn – 1H2n – 1CO2CnH2n + 1, and, in some cases, traces of trialkyl phosphates (CnH2n + 1O)3PO. In addition, a number of unexpected heterocyclic 2-alkoxy-1,3,2-dioxaphospholanes deserve special comment.
The patterns of fragmentation of trialkyl phosphites (RO)3P under EI mass spectrometry, as well as dialkyl phosphonates [4] and other aliphatic esters of various phosphorus acids, are similar. The peaks of molecular ions in the EI mass spectra are not recorded except for the simplest homologues (R = CH3 and C2H5). The first stage of their fragmentation is the cleavage of the alkenyl radical CnH2n – 1 with the largest number of carbon atoms (n > m) as a result of the so-called double hydrogen rearrangement [18], followed by a double loss of olefin fragments (CmH2m):
This results in the formation of [HP(OH)3]+ ions, characteristic of trialkyl phosphates, with m/z 83, which with the further elimination of H2O yield [HPO(OH)]+ ions with m/z 65. The composition of such ions (absence of carbon) explains the anomalously low intensity of their isotopic peaks (less than 1%). Such ions are characteristic of dialkyl phosphonates, but there they formed in two rather than three stages. Similar sequences of fragmentation, leading to similar ions, determine the mass spectrometric features of the esters of other phosphorus acids, including phosphates ([P(OH)4]+ ions, m/z 99), thiophosphates ([P(SH)(OH)3]+ ions, m/z 115), dithiophosphates ([P(SH)2(OH)2]+ ions, m/z 131), chlorophosphates ([PCl(OH)3]+ ions, m/z 117), dichlorophosphates ([PCl2(OH)2]+ ions, m/z 135), methyl phosphonates ([P(CH3)(OH)3]+ ions, m/z 97), methyl fluorophosphonates ([PF(CH3)(OH)2]+ ions, m/z 99), and others. The assignment of the main signals of the mass spectra is indicated in Table 1.
The predominant formation of [HP(OH)3] ions with m/z 83 determines a significant similarity of the mass spectra of dialkyl phosphonates and trialkyl phosphites. The mass spectra of dipropyl phosphonate (C3H7O)2PHO and tripropyl phosphite (C3H7O)3P, differing in positions and intensities of only weak signals, are compared (Fig. 1). In such cases, to eliminate possible identification errors, it is necessary to use gas chromatographic retention indices in addition to mass spectra.
Chromatographic and gas chromatography–mass spectrometry parameters of trialkyl phosphites. According to the definition of the main systems of retention indices, a change in the composition of molecules by the homologous difference CH2 should lead to a change in the retention index values of approximately 100 index units. This pattern is satisfactorily observed in the case of dialkyl phosphonates: the differences in the retention indices of the homologues (Cn + 1H2n + 3O)2PHO and (CnH2n + 1O)2PHO are 185–210 index units. However, for trialkyl phosphites, the corresponding differences are noticeably less than 300 and are 237 (Et – Me), 256 (Pr – Et), 263 (Bu – Pr), 269 (C5H11 – Bu), 2 × 278 (C7H15 – C5H11), and 275 (C8H17 – C7H15), that is, vary in the range of 237–278. The same anomaly is observed for trialkyl phosphates, namely, 201 (Et – Me), 247 (Pr – Et), 276 (Bu – Pr), 240 (C5H11 – Bu), etc. [2]; that is, the range is 201–276. A discussion of the reasons for its occurrence is beyond the scope of this work, and we have limited ourselves to its mention.
Trialkyl phosphites, in contrast to dialkyl phosphonates, do not contain a four-coordinated phosphorus atom, which is a chiral center in asymmetric dialkyl phosphonates. Therefore, diastereomers with identical mass spectra only exist when there are more than two chiral centers in the alkyl substituents, for example, in bis(1-methylheptyl)ethyl phosphite. The difference in the retention indices of diastereomers, in this case, is 7 index units, which is comparable, for example, with its value for (1-methylheptyl)ethyl phosphonate (11 index units), but it is smaller than that for bis(1-methylheptyl) phosphonate (three diastereomers, 22–24 index units) [4].
The special value of using gas chromatographic retention parameters for mass spectrometric identification is the ability to calculate and use the combined gas chromatography–mass spectrometric parameters. For this purpose, the mass spectrometric and chromatographic characteristics should be presented on the same scale, for example, as homologous increments of retention indices [19, 20] as
where x = int (M/14), it is a function denoting the integer part of a number (equivalent to expression M = 14x + y), y is the number of the homologous group of the compound, y ≡ M(mod14) [19].
The values of iRI for n-alkanes are zero; for isoalkanes, iRI < 0; and for compounds with significant retention parameters (with the same values of x), iRI > 0. The values of iRI together with the retention indices for trialkyl phosphites are given in Table 2.
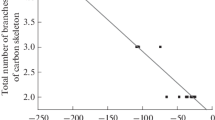
The values of the homologous increments of the retention indices depend both on the chemical origin of the analytes (the homologous series) and on the total number of branches of the carbon skeleton of homologous molecules. Continuing a comparison of trialkyl phosphites and previously characterized dialkyl phosphonates [4], it should be noted that there are significant differences in their respective iRI values (Table 3).
The values of the standard deviations of iRI draw attention to themselves, because they are significantly (on average, six times) higher for trialkyl phosphites than for dialkyl phosphonates, which somewhat affects the information content and uniqueness of the results obtained with their use. In addition, such a comparison confirms the fundamentally different gas chromatographic behavior of trialkyl phosphites: at their molecular weights being equal to those for dialkyl phosphonates (both homologous series belong to the homologous group of y = 12), their retention indices are 250–300 units lower. Such significantly decreased values indicate a low chromatographic polarity [21] of trialkyl phosphites, which is less than, for example, the polarity of trialkyl amines (iRI ± sRI = –137 ± 65 index units), which follows from their comparison (Table 4).
According to this criterion, trialkyl phosphites are second only to volatile tetraalkoxysilanes (the estimate of iRI ± sRI, according to the data for the four simplest homologues, is –558 ± 164 index units).
The absence of the peaks of molecular ions in the EI mass spectra of trialkyl phosphites makes it impossible to calculate the values of iRI directly. This approach does not prevent a comparison of the chromatographic polarity of analytes and the solution of such a practically important task as the estimation of their molecular weights from chromatographic data. By substituting expression M = 14x + y, more precisely, x = (M – y)/14, into Eq. (1) and solving the obtained equation for M, we obtain the following equation:
Since trialkyl phosphites belong to the homologous group with y = 12, the general Eq. (2) for compounds of this series can be converted into
To characterize the possibilities of estimating the molecular weights of three-n-alkyl phosphites, we use the average value of iRI ± sRI = –235 ± 52 and some data of Table 2. Based on the standard deviation sRI, the expected accuracy of molecular weight estimates is 0.14 × 52 ≈ 7 Da. The results for the five homologues (Table 3) show that the directly calculated values of M differ from the true ones by 1–17 Da (on average, by the expected ±7 Da). However, in obtaining such estimates, it is necessary to take into account the specific additional rounding of the data, not to the nearest integer number, but to the nearest value M, comparable to 12 by modulo 14, or, in the symbolism of the theory of residues, M ≡ 12(mod14). If this condition is met, most of the molecular weight estimates of trialkyl phosphites are correct, and only in some cases, the molecular weights of the previous or subsequent homologues are mistakenly recognized (Table 5).
If branched alkyl fragments are present in the molecule, then it is necessary to use other average values of homologous increments instead of iRI ± sRI = –235 ± 52. In interpreting the results of chromatography–mass spectrometry analysis of unknown compounds, identifying possible branching of the carbon skeleton is equivalent to the need to test several alternative hypotheses, which is a conventional approach in mass chromatography and gas chromatography–mass spectrometry.
Identification of 2-alkoxy-1,3,2-dioxaphospholans in the reaction mixtures. As was noted above, the characterization of organic compounds of previously insufficiently studied classes without their isolation from reaction mixtures is possible, if all components of such mixtures (including impurities) are unambiguously identified, which is necessary to eliminate potential uncertainties in the determination of their structures. This condition makes it necessary to identify even the “unexpected” components found in the composition of the reaction mixtures of aliphatic alcohols with PCl3. These are compounds with similar mass spectra and retention indices 896 (R = Et), 1087 (Bu), 1187 (C5H11), 1147 [CH2CH(CH3)C2H5], 1390 (C7H15), 1492 (C8H17), 1595 (C9H19), and 1314 (cyclo-C6H13). The peak of molecular ions (M = 136) is reliably recorded only for R = Et, and the most intense signals in the spectra are characterized by the values of m/z 109, 108, and 91. There are no compounds with such mass spectrometric indicators in the database [2].
With an increase in the alkyl fragments of aliphatic alcohols by the homologous difference of CH2, the retention indices of the considered components are 104 (Pr – Et), 97 (Bu – Pr), 100 (C5H11 – Bu), 2 × 102 (C7H15 – C5H11), 102 (C8H17 – C7H15), and 103 (C9H19 – C8H17), that is, vary in a narrow range of 96–103 around the mean value of 100 ± 3, which corresponds to the presence of only one alkyl group in the molecule. The structure, which does not contradict all the above characteristics, is provided by the products of the interaction of PCl3 with one molecule of the aliphatic alcohol and with one molecule of ethylene glycol, that is, cyclic 2-alkoxy-1,3,2-dioxaphospholanes (alkyl ethylene phosphites),

The appearance of ethylene glycol in the reaction mixtures is evidently due to the presence of its traces in the N,N-dimethylaniline preparation used (accidental impurity). The products of its interaction with PCl3 could be neglected if it were not for the above-mentioned need to identify all the components found in the reaction mixtures.
The structure of 1,3,2-dioxaphospholanes explains the appearance of the main signals of fragment ions, namely, with m/z 109 [M–CnH2n – 1], 108 [M–CnH2n], and 91 [M–CnH2n + 1O]. The average value of the homologous increment of their retention indices is –8 ± 4; a significant increase compared with acyclic trialkyl phosphites confirms the presence of a cycle in the molecule. It should be noted that database [2] presents the mass spectrum [(m/z)100 = 91] and the retention index (RI = 881) of 2-chloro-1,3,2-dioxaphospholane (M = 136). The value of iRI calculated from these data is 19, which is consistent with the range of values of this parameter for 2-alkoxy-1,3,2-dioxaphospholanes.
Note that there is another way to prove the presence of 1,3,2-dioxaphospholanes by gas chromatography–mass spectrometry in combination with the features of the reactions of their formation. If PCl3 is reacted with ethylene glycol in the absence of a base, the main expected product of the Michaelis–Arbuzov reaction should be the corresponding cyclic ethylene phosphonate, namely, 1,3,2-dioxaphospholane-2-oxide (M = 108), which has no substituents in position 2. This component was found in the reaction mixture of PCl3 and ethylene glycol (Table 6).
In addition, the interaction of two simple alcohols (ethanol and 1-propanol) with PCl3 was carried out in the presence of ethylene glycol. Components with the mass spectra and retention indices identical to those given in Table 1 and corresponding to 2-ethoxy- and 2-propoxy-1,3,2-dioxaphospholane were found among the products.
CONCLUSIONS
Thus, a joint consideration of mass spectra and gas chromatographic retention indices enable us to identify homologues of a series of trialkyl phosphites that have not been described in sufficient detail to date, directly (without their preparative isolation) in the reaction mixtures of aliphatic alcohols with phosphorus trichloride and reliably distinguish them from the corresponding dialkyl phosphonates that belong to the same homologous group with y = 12. The use of such combined gas chromatography–mass spectrometry parameters as homologous increments of retention indices is informative in determining the molecular weights of trialkyl phosphites, which do not give molecular ion signals in the EI mass spectra. Based on the same parameters, we concluded that trialkyl phosphites exhibit significantly lower chromatographic polarity compared to dialkyl phosphonates.
REFERENCES
Zenkevich, I.G. and Fakhretdinova, L.N., J. Anal. Chem., 2016, vol. 71, no. 12, p. 1204.
The NIST 17 Mass Spectral Library (NIST17/2017/EPA/NIH). Software/Data Version (NIST17); NIST Standard Reference Database, Number 69, June 2017. National Institute of Standards and Technology, Gaithersburg, MD, 2017. http://webbook.nist.gov. Accessed March 2018.
Zenkevich, I.G. and Nosova, V.E., Analitika Kontrol’, 2016, vol. 20, no. 4, p. 307.
Zenkevich, I.G. and Nosova, V.E., J. Anal. Chem., 2018, vol. 73, no. 12, p. 1162.
Sparkman, O.D., Penton, Z., and Kitson, F.G., Gas Chromatography and Mass Spectrometry: A Practical Guide, New York: Academic, 2011.
Kostiainen, O., Screening of chemicals related to the chemical weapon convention, in Encyclopedia of Analytical Chemistry, Meyers, R.A., Ed., Chichester: Wiley, 2000, p. 963.
Tsunoda, N., J. Mass Spectrom. Soc. Jpn., 2005, vol. 53, no. 3, p. 157.
Mesilaakso, M., Chemical Weapons Convention, Chemical Analysis: Sample Collection, Preparation and Analytical Methods, Chichester: Wiley, 2005.
Terentyev, A.G., Morozik, Yu.N., Rybal’chenko, I.V., et al., J. Anal. Chem., 2016, vol. 71, no. 13, p. 1266.
Morozik, Yu.V., Dudkin, A.V., Rybal’chenko, I.V., et al., J. Anal. Chem., 2018, vol. 73, no. 13, p. 1253.
Dudkin, A.V., Morozik, Yu.I., Rybal’chenko, I.V., et al., J. Anal. Chem., 2018, vol. 73, no. 13, p. 1275.
Zhokhov, A.K., Belousov, E.B., Fomenko, P.V., et al., Zh. Anal. Khim., 2017, vol. 72, no. 6, p. 530.
Bhattacharya, A.K. and Thyagarahan, G., Chem. Rev., 1981, vol. 81, no. 4, p. 415.
Rajeshwaran, G.G., Nandakumar, M., Sureshbabu, R., et al., Org. Lett., 2011, vol. 13, no. 6, p. 1270.
Ford-Moore, A.H. and Perry, B.H., Org. Synth., 1951, vol. 31, p. 111; Collect. 1963, vol. 4, p. 4.
Huyser, E.S. and Dieter, J.A., Org. Chem., 1968, vol. 33, no. 11, p. 4205.
Turk, E., Chem. Eng. News, 1998, vol. 76, no. 9, p. 6.
Hamming, M.C. and Foster, N.G., Interpretation of Mass Spectra of Organic Compounds, New York: Academic, 1979.
Zenkevich, I.G. and Ioffe, B.V., Interpretatsiya mass-spektrov organicheskikh soedinenii (Interpretation of the Mass Spectra of Organic Compounds), Leningrad: Khimia, 1986.
Zenkevich, I.G., Russ. J. Gen. Chem., 2017, vol. 87, no. 4, p. 795.
Héberger, K. and Zenkevich, I.G., J. Chromatogr. A, 2010, vol. 1217, p. 2895.
ACKNOWLEDGMENTS
The study is carried out using the equipment of the Resource Center in the field of “Chemistry” at the Institute of Chemistry, St. Petersburg State University. The authors are grateful to all colleagues working at the Center for their assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by O. Zhukova
Rights and permissions
About this article
Cite this article
Zenkevich, I.G., Nosova, V.E. Features of the Identification of Trialkyl Phosphites in Reaction Mixtures and Their Characterization by Gas Chromatography–Mass Spectrometry. J Anal Chem 74, 1305–1319 (2019). https://doi.org/10.1134/S1061934819130124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934819130124