Abstract
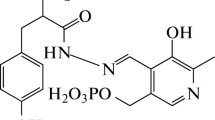
The kinetics and mechanism of condensation of pyridoxal hydrochloride with L-α-asparagine, L‑α- and D-α-aspartic acids are analyzed via UV spectroscopy and polarimetry. It is found that L‑α‑asparagine containing α-NH2 and γ-NH2 groups interacts with pyridoxal via the γ-NH2 group, forming Schiff bases that are resistant to chemical transformations. Rearrangement produces Schiff bases that form the cyclic structure from the amino acid moiety. L-α- and D-α-aspartic acids interacting with pyridoxal via α‑NH2 groups create Schiff bases that form quinoid structures after elimination of α-hydrogen or СО2. Their subsequent hydrolysis results in pyridoxamine, α-ketoacids, and aldehyde acids, respectively. Schemes of the condensation mechanisms of L-α-asparagine, L-α-, D-α-aspartic acids with pyridoxal hydrochloride are proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The stereochemistry of amino acids plays a key role in the structures of human and animal proteins, peptides, and enzymes. The components of these biological objects are L-amino acids. This is due to stereospecific features of the enzyme’s interaction with substrates of a certain configuration. D-series amino acids are found in many peptides produced by microorganisms and are contained in the biopolymers of their cells. The inclusion of D-α-amino acids in the structures of human proteins and enzymes can have undesirable outcomes. Aspartic acid plays the central role in removing nitrogen from organic compounds. The transamination reaction is the initial stage in the catabolism of excess amino acids. In an organism, aspartic acid participates in the removal of nitrogen in the form of urea [1, 2]. In proteins, the side chains of asparagine amino acid and asparagine apparently impart hydrophilic or hydrophobic properties to proteins and help form their structure. This is in addition to participating in many biochemical processes, depending on their physicochemical properties.
There are a number of works in the literature that analyze the kinetics and mechanism of the interaction of pyridoxal-5'-phosphate with arginine, lysine, alanine, aspartic and glutamic acids under different experimental conditions. Schemes of their interaction have been proposed, reaction rate constants have been calculated, thermodynamic characteristics of these reactions have been given, and the reactions have been shown to be controlled by the entropy factor [3–5].
Having found that breast cancer cells cannot invade other parts of body without asparagine (one of the twenty most abundant amino acids), researchers managed to completely stop the growth of its most aggressive forms. The spread of metastases in the bodies of mice stopped completely when biologists blocked the Asns gene responsible for the formation of asparagine molecules, showing that this approach is highly promising in the fight against cancer. Studying the kinetics and mechanism of the interaction between pyridoxal hydrochloride and L-α- and D-α-aspartic acids with one α-NH2 group each and L-asparagine with two α-NH2 and γ-NH2 groups is of great interest.
EXPERIMENTAL
In this work, we used pyridoxal hydrochloride (Ferak Berlin; chemically pure), amino acids and their amides (Reanal, Great Britain). Buffer solutions were prepared using a generally accepted procedure. The reaction kinetics was measured on a SF-26 spectrophotometer and a DigiPol DS automatic saccharimeter. The reaction mixtures were thermostatted in a UH‑8 unit with an accuracy up to ±0.1°С. Weighed portions of pyridoxal hydrochloride, amino acids, and their amides in equimolar amounts were dissolved in water–alcohol buffer solutions and kept at the set temperature for 30 min. The onset of the reaction was considered to be the instant of mixing thermostatted solutions of pyridoxal, amino acids, and their amides. Kinetic measurements were made in thermostatted cuvettes 1.008 mm thick and polarimetric tubes 1.9 dm long. Since the UV spectra of pyridoxal solutions change depending on the pH values of the medium and the solvent, equimolar pyridoxal solutions in the same solvent and with the same рН value were placed in comparison cuvettes. The рН values of the solutions were measured on a universal EV-74 ion meter with an accuracy of ±0.1 рН unit. Rate constants of pyridoxal condensation with L-α-, D-α-aspartic acids and L‑α-asparagine were calculated using computer programs for reversible and irreversible reactions [6]. Initial and final products were identified via elemental analysis, UV and IR spectroscopy, TLC, and LC. IR spectra were measured on a Nicolet Impact 420 IR spectrophotometer. Products of interaction were analyzed on a PLC-20 Cole Parmer liquid chromatograph with a C-185 microcolumn, eluent H2O : CH3CN = 80 : 20%. The structure and atomic charges upon the optimization of geometric and thermodynamic factors were found using the HyperChem MNDO program. Products of the condensation of pyridoxal with L-α- and D-α-aspartic acids were synthesized and identified according to [7–13].
PROCEDURE FOR SYNTHESIZING THE PRODUCT OF INTERACTION BETWEEN PYRIDOXAL HYDROCHLORIDE AND L-ASPARAGINE VIA THE Γ-NH2 GROUP
Synthesis of Sodium 2-(3-Hydroxy-5-hydroxymethyl-2-methyl-4-yl)-pyrimidin-4-oxo-5-carbonate
Pyridoxal hydrochloride (0.132 g) was dissolved in 5 mL of 96% ethanol and 0.085 g of L-α-asparagine were dissolved in 11 mL of ethanol + 3 mL of a 90% water-ethanol acetate buffer solution on heating until their complete dissolution. Both colorless solutions were mixed after cooling, with the mixture becoming bright yellow. New absorption maxima appeared in the UV spectra at 350 and 430 nm. The mixture was poured into a Petri dish and evaporated at room temperature up to precipitation. The course of the reaction was monitored via UV spectrophotometry (absorbance maxima at 350 and 430 nm), TLC and LC (the disappearance of spots and peaks of initial products and appearance of spots and peaks of final products). The yield was 0.157 g (∼79%) when Тmelt > 340° (with charring).
In the IR spectrum (KBr), ν = 3150–3350 cm‒1(NH), 1625 cm–1 (no C=N), and 1570 cm–1 and 1620 cm–1 (amide-1 and amide-2 bands). In the UV spectrum, λmax = 350 nm. Polarimetry gave \([\alpha ]_{{\text{D}}}^{{20}}\) = −0.21° (70% ethanol).
Calculated, %: С 47.2; H 5.25; N 13.77.
Found, %: С 47.4; Н 5.2; N 12.9: C12H16N3O5 ⋅ Na.
RESULTS AND DISCUSSION
The kinetics and mechanism of the interaction between amino acids with different structures (e.g., L‑α-alanine, β-alanine, glycine, arginine, and tryptophan) and pyridoxal were studied via UV spectroscopy. It was found that when colorless solutions of the initial components were mixed, an intense yellow color appeared at λmax 350 and 430 nm. Due to the formation of С=N bonds, Schiff bases formed with high yields, except for products of its condensation with amino acid amides (glutamine and asparagine) (Fig. 1).
We suggest that products of pyridoxal condensation with glutamic and aspartic amino acids (Schiff bases) undergo chemical transformations more rapidly with the formation of final products. This seems to occur via terminal acceptor СООН groups that favor the removal of the α-hydrogen atom in L-α‑ or СО2 in D-α-asparagine amino acids.
It was of special interest to study the kinetics and mechanism of interaction between pyridoxal and L‑α‑asparagine with two nitrogen atoms.
The structures and charges on nitrogen atoms of α‑NH2 (−0.280) and γ-NH2 (−0.338) groups in the L-α-asparagine molecule were calculated using the HyperChem MNDO program. It was found that the probability of their interaction with pyridoxal via the γ-NH2 group and space factors for the formation of Schiff bases resistant to chemical transformations is greater than with the α-NH2 group.
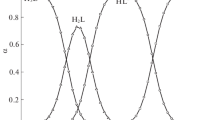
The results presented in Figs. 1 and 2 show considerable differences in the rates and mechanisms of formation of amino alcohols and Schiff bases in the interaction between pyridoxal and L-α-asparagine, compared to its interaction with L-α-aspartic acid. In the interaction between pyridoxal and L-α-asparagine, Schiff bases resistant to chemical transformations formed with a high yield at the stage of the formation of amino alcohols and the subsequent stage of their dehydration, with the absorbance of the amino acid moiety being retained.
Change in the specific angles of rotation in mixtures of 0.04 М solutions of pyridoxal hydrochloride with (1) L‑α-asparagine, (2) D-α-aspartic acid, and (3) L‑α-aspartic acid at the stages of amino alcohol formation and dehydration, and their chemical transformations. Specific angles of rotation of initial amino acids: (1 ') L-α-asparagine, (2 ') D-α-aspartic acid, and (3 ') L‑α-aspartic acid (70% water–alcohol buffer solution; рН 6.45; Т = 20°С).
When solutions of a mixture of pyridoxal hydrochloride with L-α-asparagine were kept for a long period of time, the intensities of UV absorption in the spectra near 430 nm fell very slowly (almost to zero over time), relative to the absorption of Schiff bases formed in the initial period of time (Fig. 2). The isolation and identification of final products via elemental analysis, polarimetry, IR spectroscopy, and LC allowed the formation of cyclic forms of the amino acid moiety during the nucleophilic attack of the С=N bond in the Schiff base by the nitrogen lone pair of the α-NH2 group. The very low rate of the cyclization stage of the Schiff base is likely explained by high steric factors of the amino acid moiety.
Our study of the structures of initial, intermediate, and final condensation products of L-α-asparagine with pyridoxal via the γ-NH2 group by means of the HyperChem MNDO program showed that the ОН group in amino alcohols and the azomethine moiety in Schiff bases and its cyclic fragment were perpendicular to the plane of the pyridoxal pyridine ring and turned at ∼90° relative to it









We may assume that the structures with the pyrimidine ring and resembling the modified structure of uracil contained in RNA could be one of the factors impeding the spread of breast cancer metastases to other organs.
Along with calculations of rate constants, the interaction between pyridoxal hydrochloride and L-α- and D-α-aspartic acids showed that in the condensation of pyridoxal with L-α-aspartic acid at the stage of amino alcohol dehydration (k = 0.089 × 10−2 min−1), the α‑hydrogen atom of the amino acid moiety in Schiff bases is at the position favoring its removal and the Schiff base’s transition into a quinoid structure, according to Danathan [1]. Subsequent hydrolysis of the latter results in the formation of pyridoxamine and keto-butanedioic acid. In the interaction between pyridoxal and D-α-aspartic acid (k = 0.781 × 10−2 min−1) a Schiff base forms in which the amino acid moiety is turned 180° relative to the plane of the pyridine ring. As a result, a Schiff base forms with the preferential removal of СО2 and the formation of a quinoid structure whose hydrolysis produces pyridoxamine and 3-oxopropanoic acid. This is supported by kinetic data on the condensation of pyridoxal with L-α- and D‑α‑aspartic acids, obtained via polarimetry (Fig. 2).
The results from our polarimetric measurements show that at the first stage of condensation, the absolute values of specific angles of rotation of a mixture of pyridoxal+L-α-aspartic acid solutions (3) fall rapidly as they grow for pyridoxal+D-α-aspartic acid mixture (2). The absolute values of the specific angles of these mixtures the fall gradually over time. In both cases, new chiral centers with different signs of the specific angles of rotation appear at the stage of amino alcohol formation. According to outdated views, if nucleophilic reagents attack the plane of the carbonyl group from both sides with equal probability, the values and signs of specific rotation angles do not change because racemates in this case form. This contradiction can be explained only by assuming that the nucleophilic attack of the carbonyl group occurs along its plane with the formation of an intermediate product: an amino alcohol with a rapid change in the absorbance of a mixture of solutions (Fig. 1) or their specific angles of rotation (Fig. 2) over time. Then there is a slow stage: the rotational isomerism of the resulting amino alcohol moieties caused by optimization of their energy and geometric parameters promoting the removal of the α-hydrogen atom or the СО2 group with the formation of quinoid structures whose hydrolysis results in final products. This assumption is confirmed by data on the structures of amino alcohols and Schiff bases, obtained using the HyperChem MNDO program with allowance for their geometric and energy parameters. The results from these studies showed that the products of pyridoxal condensation (amino alcohols and Schiff bases) with L-α- and D‑α-aspartic acids differ from one another in the arrangement of amino acid moieties relative to the pyridoxal ring planes. The pyridine moiety of Schiff bases and intermediate products (amino alcohols) has a planar structure. The pyridine ОН group is approximately in the same plane as that of the pyridine ring. Because of its nonlinearity, the СН2ОН group is out of the pyridine ring plane; i.e., it can serve as a label in considering the stereochemistry of the pyridoxal carbonyl group, the ОН group of amino alcohols, and the azomethine moieties in Schiff bases. The results from HyperChem MNDO studies of the structures with regard to the optimization of geometric and energy parameters show that in pyridoxal, amino alcohols, and Schiff bases (products of pyridoxal condensation with L-α- and D-α-aspartic acids via the α-NH2 group), the carbonyl group and azomethine moieties are turned at ∼90° and are on one side relative to the label. In amino alcohols, the ОН group and the amino acid moiety are on different sides, relative to the label.
After the removal of the α-hydrogen atom in the L‑asparagine moiety or СО2 in the D-asparagine moiety, the products of the interaction between L-α- and D-α-aspartic acids and pyridoxal almost completely lose their absorbance. The rates of each stage depend on the structures of the initial, intermediate, and final products; the рН of the medium and the solvent; the temperature; and steric and thermodynamic factors. An analysis of the literature and our experimental data show that as the basicity of NH2 groups rises, so do the rates of attachment and the stages of amino alcohol formation, while the rates of amino alcohol dehydration fall. The rate of Schiff base formation generally depends on the ratio of the rate constants of these two stages.
CONCLUSIONS
Pyridoxal hydrochloride interacts with L-α-asparagine via the γ-NH2 group, forming a condensation product with the cyclic structure of the amino acid moiety while L-α- and D-α-aspartic acids interact with it via α-NH2 groups with the formation of pyridoxamine, keto, and aldehyde acids, respectively, as final products.
REFERENCES
D. E. Metzler, Biochemistry: The Chemical Reactions of Living Cells (Academic, New York, 1977), Vol. 2.
V. I. Ivanov and M. Ya. Karpeisky, Adv. Enzymol. 32, 21 (1969).
K. J. Laidler, Chemical Kinetics (McGraw-Hill, New York, 1965).
F. V. Pishchugin and I. T. Tuleberdiev, Russ. J. Gen. Chem. 75, 1465 (2005).
V. G. Badelin, E. A. Venediktov, and V. P. Barannikov, Khim. Khim. Tekhnol. 50 (12), 34 (2007).
F. V. Pishchugin and I. T. Tuleberdiev, Russ. J. Gen. Chem. 78, 1225 (2008).
E. A. Venediktov, V. P. Barannikov, and V. G. Badelin, Khim. Khim. Tekhnol. 52 (1), 36 (2009).
F. V. Pishchugin and I. T. Tuleberdiev, Russ. J. Gen. Chem. 79, 117 (2009).
F. V. Pishchugin and I. T. Tuleberdiev, Russ. J. Gen. Chem. 80, 1836 (2010).
V. P. Barannikov, V. G. Badelin, E. A. Venediktov, I. N. Mezhevoi, and S. S. Guseinov, Russ. J. Phys. Chem. A 85, 16 (2011).
F. V. Pishchugin and I. T. Tuleberdiev, Russ. J. Gen. Chem. 82, 1267 (2012).
F. V. Pishchugin and I. T. Tuleberdiev, Russ. J. Gen. Chem. 84, 1362 (2014).
F. V. Pishchugin and I. T. Tuleberdiev, Russ. J. Phys. Chem. A 91, 1851 (2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Chernikova
Rights and permissions
About this article
Cite this article
Pishchugin, F.V., Tuleberdiev, I.T. Interaction between Pyridoxal Hydrochloride and L-α-Asparagine in Comparison to L-α- and D-α-Aspartic Acids. Russ. J. Phys. Chem. 95, 49–54 (2021). https://doi.org/10.1134/S0036024421010222
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421010222