Abstract
The kinetics and mechanism of the condensation and transaldimination of products of pyridoxal condensation with L-α-, D-α-, and β-alanines are studied via UV spectroscopy and polarimetry. It is found that pyridoxal hydrochloride interacts stereospecifically with L-α- and D-α-alanines, accompanied by the formation of intermediate products with a strictly defined structure that promote the elimination of hydrogen atoms (with L-α-alanine) or CO2 (with D-α-alanine) and the subsequent formation of a quinoid structure whose hydrolysis yields the final products. The reactions of transaldimination between pyridoxalidene-β-alanine, pyridoxalidene-L-α-, pyridoxalidene-D-α-alanines, and L-α- and D-α-alanines are studied. It is shown that transaldimination proceeds in two stages: the addition of L-α- and D-α-amino acids to Schiff bases with the formation of N-acetals of a strictly defined structure, and the cleavage of one of the amino acids with the formation of new Schiff bases. Schemes are proposed for the mechanisms of the studied reactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Pyridoxal (PL) and pyridoxal-5'-phosphate (PLP) are coenzymes participating in a great many enzymatic processes [1, 2]. A wide variety of chemical transformations of amino acids occur under the action of these coenzymes (e.g., elimination, racemization, transamination, decarboxylation, cleavage of side chains of amino acids, and transaldimination).
A great many works have been devoted to studying these biochemical processes under the effects of PLP-dependent enzymes. Tremendous difficulties arise in studying these reactions, however, due to the complexity of enzymatic systems and the rapid and sometimes hard-to-follow processes. Model compounds containing coenzymes or structural analogs of them are therefore used to confirm proposed schemes of the biochemical transformations of amines and amino acids.
Amino acids other than glycine are optically active compounds (i.e., ones with chiral centers). The overwhelming majority of amino acids have one chiral center, with the exception of isoleucine, threonine, and 4-hydroxyproline, which have two. The number of stereoisomers is determined by formula 2n, where n is the number of chiral centers. Only L amino acids are proteinogenic, due to the stereospecificity of the enzymes interaction with substrates.
D-series α-amino acids are not used to build animal proteins. D-α-amino acids are found in many peptides produced by microorganisms, and are part of the biopolymers of their cells.
Introducing D-α-amino acids into the structure of proteins and enzymes causes negative biochemical processes that can ultimately lead to a fatal outcome.
One way of incorporating D-α-amino acids into the structure of proteins of the human body is the transaldimination of proteins containing lysine under the action of pyridoxal or pyridoxal-5'-phosphate coenzymes.
Studying the condensation and transaldimination of amino acids in enzymatic systems presents enormous difficulties, due to the rapid and often hard-to-follow biochemical process. Model compounds—products of the condensation of coenzymes with L-α-amino acids and D-α-amino acids—are therefore used to solve this important problem.
The aim of this work was to study the kinetics and mechanism of interaction between pyridoxal hydrochloride and L-α-, D-α-, and β-alanines, along with the transaldimination of products of the condensation of pyridoxal with these amino acids.
EXPERIMENTAL
L-α- and D-α-alanines produced by Reanal (Hungary) and chemically pure pyridoxal hydrochloride from Ferak (Germany) were used as the initial reagents. Schiff bases were synthesized and identified according to [3].
The kinetics of transaldimination was measured in thermostatted cuvettes and polarimetric tubes. Thermostatting was done using a U-4 thermostat with an accuracy of ±0.1°C. The moment when the solutions were mixed was taken as the start of the reaction. Products of the reaction were identified via elemental analysis, UV and IR spectroscopy, and polarimetry [3]. The rate constants of condensation and transaldimination reactions were calculated using second-order equations for reversible and irreversible reactions [4].
RESULTS AND DISCUSSION
Our earlier studies showed that when pyridoxal interacts with L-α- and D-α-alanines, a new chiral center appears in the amino alcohol with positive signs (+) when pyridoxal interacts with L-α-alanine and negative signs (−) when pyridoxal interacts with D-α-alanine, the absolute values of which grow over time [5]. These results can be explained by additions of the NH2 groups of amino acids being not perpendicular to the plane of the carbonyl group of pyridoxal, but along its plane with the formation of an amino alcohol and the emergence of chiral centers. Rotational isomerism apparently follows with the formation of intermediate and final products by optimizing their energy and geometric parameters (an increase in the absolute value of the specific rotation over time). The question then arises of why L-α- and D-α-alanines form amino alcohols with different signs of the specific rotation when interacting with pyridoxal, and why their absolute values grow over time.
To clarify this issue, we considered the structures of products of the interaction between pyridoxal and L‑α- and D-α-alanines with optimization of their geometric and energy parameters in the Hyper Chem program. The structure of these products was considered by placing a fragment of the pyridine ring ~90° from the eye of the observer (a combination of its carbon atoms in ortho- and meta-positions). Analysis of the considered structures showed that the OH group lies approximately in the same plane as the pyridine ring (the conventional left side). Due to the nonlinearity of its structure, the CH2OH group protrudes beyond the plane of the pyridine ring (the conventional right side). The CH2OH group in aminoalcohols is on the left, and the amino acid fragment is on the right of the plane of the pyridine ring in the condensation of D-α-alanine with pyridoxal. The opposite picture is observed for aminoalcohols in the condensation of L-α-alanine with pyridoxal (the CH2OH group on the right, and the amino acid fragment on the left). The same arrangement is observed for Schiff bases: the >C=N–R group on the left for the D-α-alanine fragment, and on the right for the L-α-alanine fragment.
A number of assumptions can be made, based on our consideration of the structures of the intermediate products of the condensation of pyridoxal with L-α- and D-α-alanines and their final products (Schiff bases).
1. The carbonyl group in pyridoxal is at an angle of 90° relative to the plane of the pyridine ring.
2. Amino acids are added to pyridoxal stereospecifically along the plane of the carbonyl group with the formation of amino alcohols and the emergence of chiral centers (optical activity) in them.
3. Rotational isomerism then occurs stereospecifically by optimizing the geometric and energy parameters of amino alcohols and Schiff bases, altering the angle of rotation via the rotation of amino alcohol fragments at the chiral center, relative to the plane of the pyridine ring with the formation of R (D) or S (L) isomers.
The results in [6–12] on the interaction between pyridoxal and structurally different amino acids and amines showed that
1. β- and ε-amino acids (β-alanine and lysine) interact with pyridoxal to form Schiff bases that are resistant to further chemical transformations.
2. L-\(\alpha ~\) amino acids interacting with pyridoxal form Schiff bases, which (after the elimination of α-hydrogen) are rearranged into a quinoid structure, followed by hydrolysis and the formation of keto acids and pyridoxamine as final products.
3. Schiff bases with D-α-amino acids are decarboxylated with subsequent rearrangement into a quinoid structure with the formation of pyridoxal and ethylamine after hydrolysis.
It is known from the literature [13] that new Schiff bases apparently form via the addition of an amino group not to the >C=C group of PLP-dependent enzymes, but to the C=NH+ group through transaldimination. The addition to the product of condensation of pyridoxal with L-lysine is followed by the elimination of the ε-amino group of the lysine molecule. Indirect proof of this assumed mechanism is the hypothesis proposed in [13] that the addition to HC=NH+ group proceeds much faster than addition to the HC=O group. According to the authors of [13] this is confirmed, by the disappearance of positive circular dichroism after the substrate is added to the coenzyme. Circular dichroism reappears after the transformation of the substrate.
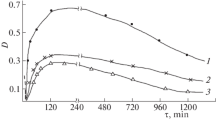
When pyridoxalidene-β-alanine interacts with L‑α-alanine at λ = 430 nm, the optical density of the mixture solutions falls sharply at the stage of addition. It then grows over time upon the elimination of β-alanine. When pyridoxalidene-β-alanine interacts with D-α-alanine, the optical density of the mixture of solutions at the same wavelength grows sharply over time at the first stage, and then rises slowly to a certain constant value at the second stage (Fig. 1).
Analysis of the kinetic curves allowed us to assume that at the first stage (a rapid rise or drop in the optical density of mixtures of solutions), NH2 groups of amino acids are added to the Schiff base with the formation of an intermediate product (aminal). At the second stage, β-amino acid is cleaved with the formation of the final product (pyridoxalidene-L-α-alanine or pyridoxalidene-D-α-alanine). The question arises as to why there is a drop in the optical density of the mixture of solutions as a result of the interaction between pyridoxalidene-β-alanine and L-α-alanine at the first stage. In contrast, there is a sharp increase in the optical density of the mixture of solutions during the interaction between pyridoxalidene-β-alanine and D-α-alanine, though the same intermediate product (aminal) forms at the first stage, according to literature data and our experimental data. To clarify this issue, we studied the kinetics and mechanism of transaldimination by means of polarimetry. A chiral center should arise at the stage of adding L-α-alanine or D‑α-alanine to a Schiff base. The data shown in Fig. 2 show that the reaction between pyridoxalidene-β-alanine to L-α-alanine at the first stage very quickly forms a product with a positive specific rotation. The opposite picture is observed when pyridoxalidene-β-alanine interacts with D-α-alanine. An intermediate product (aminal) with a negative specific rotation forms very quickly.
Changes in specific rotation over time upon interaction between a 0.01 M mixture of (1) a pyridoxalidene-β-alanine solution with (2) L-α-alanine and (3) D-α-alanine at the stage of the formation of N-acetals (90% alcohol–water buffer solution, pH 6.7; T = 20°C). A DigiPol DS automatic saccharimeter (United States) was used; specific rotation of (4) L-α-alanine and (5) D-α-alanine.
Critical analysis of kinetic curves via UV spectroscopy and polarimetry, along with using the Hyper Chem program to study the structure of intermediate (aminals) and final products, showed that.
1. In Schiff bases and (pyridoxalidene-β-alanine) and in the final products, the amine group is rotated by 90° relative to the plane of the pyridine ring.
2. An amino acid attaches to a Schiff base stereospecifically along the plane \( {>} {\text{C}}{=} {\text{NH}}{-} \) groups, due to steric hindrance (OH and CH2OH groups being in the ortho-position) with the formation of aminal (\( {-} {\text{CH}}{<} \begin{array}{*{20}{c}} {{\text{NHR}}} \\ {{\text{NHR}}{\kern 1pt} '} \end{array}\)) in strictly defined positions of the amino acid fragments in space. There is then a very rapid change in the optical density of the mixture of solutions (a sharp increase or drop) with the emergence of chiral centers.
3. Rotational isomerism apparently occurs due to optimization of the geometric and energy parameters with elimination of one of the components in the N‑aminals (Hyper Chem program).
Due to the high instability of intermediate products, attempts to isolate aminal failed even when conducting reactions at low temperatures. The question arises: Why do aminals with different signs of specific rotation and changes in their absolute values over time form during interaction between pyridoxalidene-β-alanine with L-α- and D-α-alanines? To explain this complex issue, we examined the structures of aminals and Schiff bases using the Hyper Chem program, allowing for the optimization of their geometric and energy parameters. As noted earlier, \( {>} {\text{C}}{ = } \mathop {{\text{NHR}}}\limits^ + \) and \( {>} {\text{C}}{ < } \begin{array}{*{20}{c}} {\mathop {{\text{N}}{{{\text{H}}}_{{\text{2}}}}{\text{R}}}\limits^ + } \\ {{\text{NHR'}}} \end{array}\) groups are rotated ~90° from the eye of the observer relative to the plane of the pyridine ring, so the structure of intermediate and final products was considered by combining the carbon atoms of the pyridine ring in the ortho- and meta-positions. Analysis of the considered structures showed that in the ortho-position, the OH group lies approximately in the same plane of the pyridine ring (the conventional left side). At the same time, the CH2OH group protrudes beyond the plane of the pyridine ring (the conventional right side), due to its nonlinearity.
The D-α-amino acid fragment in the N-aminals and Schiff bases is on the left in these structures, while the β-amino acid fragment is on the right. When pyridoxalidene-β-alanine interacts with L-α- and D-α-alanines, N-aminals thus form as intermediates with a different arrangement of amino acid fragments in space, relative to the plane of the pyridine fragment, accompanied by the formation of two configurations R (D +) and S (L−).
One of the fragments is then cleaved in N-aminals with simultaneous rotational isomerism, accompanied by the formation of the final product with the optimum energy and geometric parameters. These experimental data seem to explain the relationship between the specific rotation and its sign, and the structure of intermediate and final products detected by UV spectroscopy.
Our study of the kinetics and mechanism of the transaldemiration of products of condensation of L‑α-alanine and D-α-alanine with pyridoxal by means of UV spectrophotometry showed that the reaction proceeds through two kinetically distinguishable stages: (1) the addition of amino acids to the Schiff base with the formation of an intermediate product (N-aminal) and (2) the cleavage of one enantiomers of amino acids with the formation of a new Schiff base. This is shown by the kinetic curves of the change in the optical density of a mixture of reacting solutions over time. According to the data in Fig. 3, there is in both cases a sharp drop in the optical density of the mixture of solutions at the first stage (addition). At the second stage, there is an increase in optical density, due to the formation of a new Schiff base.
With this assumed mechanism of transaldimination, a chiral center should arise at the stage of the addition of an amino acid to a Schiff base. Our polarimetric study of the kinetics and mechanism of the transaldimination of alanine enantiomers showed that when the solutions were mixed at the initial moment, there is a sharp drop in the positive specific rotation as they interact. The specific rotation becomes negative at the stage of L-α-alanine elimination and the formation of pyridoxalidene-D-α-alanine. The opposite picture is observed when pyridoxalidene-D-α-alanine interacts with L-α-alanine (Fig. 4). At the first stage, there is a sharp drop in the negative specific rotation at the initial moment, and the specific rotation changes slowly from negative to positive at the stage of deamination and the formation of a new Schiff base.
Our study of the kinetics and mechanism of interaction between pyridoxalidene-L-α- and pyridoxalidene-D-α-alanines and D-α- and L-α-alanines, respectively, using the Hyper Chem program showed that as a result of the addition of these amino acids to Schiff bases, they form as intermediates products (N‑aminals) with different arrangements of the amine fragments in them relative to the plane of the pyridine ring. In all likelihood, these compounds have different signs and values of the specific rotation. Similar dependences of the structures of intermediate products (aminals) and new Schiff bases are observed in the reactions of transaldimination with L-α-alanine and D-α-alanine under the action of pyridoxal. The mechanism of transaldimination can be presented as

CONCLUSIONS
Our results from kinetic and structural studies allow a number of important conclusions for biochemistry and the solving of enzymatic problems.
1. In pyridoxal, the carbonyl group expands through 90°, relative to the plane of the pyridine fragment.
2. In products of interaction between pyridoxal with L-α- and D-α-amino acids, amine fragments in Schiff bases are rotated ~90°, relative to the plane of the pyridine fragment.
3. During the condensation of pyridoxal with amino acids and the transaldimination of Schiff bases with amino acids, the reagent attacks \( {-} {\text{HC}}{ = } {\text{NR}}\) links along the planes of their location in space.
4. The structure of the resulting intermediate products (aminoalcohols and N-aminals) and their optical properties (values and signs of specific rotation) are determined by the different arrangement of OH groups and amine fragments, relative to the plane of the pyridine ring.
REFERENCES
D. E. Metzler, Biochemistry: The Chemical Reactions of Living Cells (Academic, New York, 1977), Vol. 2.
V. I. Ivanov and M. Ya. Karpeisky, Adv. Enzymol. 32, 21 (1969).
F. V. Pishchugin and I. T. Tuleberdiev, Izv. KR. NAN, No. 2, 96 (2012).
K. J. Laidler, Chemical Kinetics, 3rd ed. (Prentice Hall, Englewood Cliffs, 1987).
F. V. Pishchugin and I. T. Tuleberdiev, Russ. J. Gen. Chem. 79, 117 (2009). https://doi.org/10.1134/S1070363209010174
V. G. Badelin, E. A. Venediktov, and V. P. Barannikov, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol. 50 (12), 34 (2007).
E. A. Venediktov, V. P. Barannikov, and V. G. Badelin, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol. 52 (1), 36 (2009).
V. P. Barannikov, V. G. Badelin, E. A. Venediktov, I. N. Mezhevoi, and S. S. Guseinov, Russ. J. Phys. Chem. A 85, 16 (2011).https://doi.org/10.1134/S003602441101002X
F. V. Pishchugin and I. T. Tuleberdiev, Russ. J. Gen. Chem. 80, 1836 (2010). https://doi.org/10.1134/S1070363210090203
F. V. Pishchugin and I. T. Tuleberdiev, Russ. J. Gen. Chem. 82, 1267 (2012). https://doi.org/10.1134/S1070363212070146
F. V. Pishchugin and I. T. Tuleberdiev, Russ. J. Gen. Chem. 84, 1362 (2014). https://doi.org/10.1134/S1070363214070196
F. V. Pishchugin and I. T. Tuleberdiev, Russ. J. Phys. Chem. A 91, 1851 (2017). https://doi.org/10.7868/S0044453717100326
E. H. Gordes and W. P. Jencks, Biochemistry 1, 773 (1972).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pishchugin, F.V., Tuleberdiev, I.T. Kinetics and Mechanism of the Interaction and Transaldimination of Products of Condensation of Pyridoxal Hydrochloride with L-α-, D-α-, and β-Alanines. Russ. J. Phys. Chem. 95, 1336–1341 (2021). https://doi.org/10.1134/S0036024421070207
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421070207