Abstract
Selective venous sampling (SVS), an invasive radiographic procedure that depends on contrast media, holds a unique role in diagnosing and guiding the treatment of certain types of secondary hypertension, particularly in patients who may be candidates for curative surgery. The adrenal venous sampling (AVS), in particular, is established as the gold standard for localizing and subtyping primary aldosteronism (PA). Throughout decades of clinical practice, AVS could be applied not only to PA but also to other endocrine diseases, such as adrenal Cushing syndrome (ACS) and Pheochromocytomas (PCCs). Notably, the application of AVS in ACS and PCCs remains less recognized compared to PA, with the low success rate of catheterization, the controversy of results interpretation, and the absence of a standardized protocol. Additionally, the AVS procedure necessitates enhancements to boost its success rate, with several helpful but imperfect methods emerging, yet continued exploration remains essential. We also observed renal venous sampling (RVS), an operation akin to AVS in principle, serves as an effective means of diagnosing renin-dependent hypertension, aiding in the identification of precise sources of renin excess and helping the selection of surgical candidates with renin angiotensin aldosterone system (RAAS) abnormal activation. Nonetheless, further basic and clinical research is needed.

Selective venous sampling (SVS) can be used in identifying cases of secondary hypertension that are curable by surgical intervention. Adrenal venous sampling (AVS) and aldosterone measurement for classificatory diagnosis of primary aldosteronism (PA) are established worldwide. While its primary application is for PA, AVS also holds the potential for diagnosing other endocrine disorders, including adrenal Cushing’s syndrome (ACS) and pheochromocytomas (PCCs) through the measurements of cortisol and catecholamine respectively. In addition, renal venous sampling and renin measurement can help to diagnose renovascular hypertension and reninoma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The prevalence of secondary hypertension (SH) has probably been underestimated in the past. The latest guidelines suggest that SH may involve approximately 10% hypertensive cases [1,2,3]. Some SH can be cured by surgery, but in a number of patients, the diagnosis of SH was missed, as well as the opportunity for a long-term cure and better control of hypertension since clinical doctors and patients usually pay more attention to the damage and remedial treatment of target organs caused by hypertension but not the exact disease that caused SH. Selective venous sampling (SVS) is a method to detect the location of lesions by comparing the hormone levels from the blood sampling site and peripheral vein. It is a minimally invasive interventional technique for the diagnosis and evaluation of diseases, utilizing catheters directed to the drainage vein of specific glands for sampling and disease-related hormone analysis [4].
It is well known that adrenal endocrine disorders and renal disease are two main causes of secondary hypertension [5], recognizing them is significant for the diagnosis and treatment of secondary hypertension. Adrenal venous sampling (AVS), recognized as the gold standard for localizing and subtyping primary aldosteronism (PA) [6], was first introduced by Melby et al. in the 1960s [7]. It holds significant guidance for making treatment decisions in patients with confirmed primary aldosteronism who are considering surgery for a long-term cure. Moreover, if analysing adrenal cortex and medullary hormones of blood samples from bilateral adrenal veins and peripheral veins, AVS could also be used for other adrenal gland endocrine disorders that lead to hypertension, such as adrenal Cushing syndrome (ACS) and pheochromocytoma (PCC). For patients with hypercortisolism, AVS confirms the laterality of an adrenal cortisol-producing adenoma or hyperplasia. Although AVS may not be very effective in diagnosing PCCs because of the left-right natural differences and fluctuations in adrenal medulla hormone secretion, it can still be used as an alternative in difficult cases. However, the utilization rate and success rate of AVS are not sufficient, even for PA [8]. Within years of clinical practice, there has been a series of improved methods for AVS, such as CT/CBCT, ACTH stimulation, rapid cortisol assay, altering the catheter approach, special contrast medium, and premedication, all of which can improve the success rate to a certain extent. Scintigraphy can also be used as one alternative method when AVS is not available [9,10,11]. Renal venous sampling (RVS), a technique often confused with AVS, enables the identification of renin-dependent hypertension by measuring renin levels in the renal vein, such as renovascular hypertension [12] and a renin-producing tumor [13, 14].
AVS for PA and other endocrine disorders
1 PA
Why is AVS recommended
The prevalence of PA in patients with newly diagnosed hypertension in China is at least 4% [15]. PA is the most common cause of secondary hypertension and drug-resistant hypertension [16, 17]. Compelling evidence indicates that primary aldosteronism increases the risk of cardiovascular morbidity [18], nephrotoxicity, and mortality [19]; therefore, early diagnosis and accurate treatment are key to preventing hypertension-related cardiovascular events and reversing damage. Aldosterone excess in PA can be caused by bilateral (idiopathic hyperaldosteronism) or unilateral (usually aldosterone-producing adenoma) adrenal diseases, and their treatment is completely different, with the former requiring targeted medication, while the latter is best treated by surgery (unilateral adrenalectomy) [20]. Therefore, it is the last but crucial step of PA diagnosis to perform subtyping examinations.
Imaging techniques are commonly used to locate lesions. Professors recommend that patients diagnosed with PA and seeking a long-term cure through adrenalectomy should first undergo imaging examinations [21]. The presence of a solitary nodule or diffuse, uniform thickening of the adrenal cortex on a CT image can primarily indicate whether the adrenal disease is unilateral or bilateral [22]. Additionally, CT scan is capable of detecting adrenocortical carcinoma [23]. However according to previous studies, it is not reliable to classify PA based only on MRI/CT results [24, 25]. Even as imaging techniques continue to improve, both of them can only be applied morphologically to detect hyperplasia or adenoma, cannot differentiate between functional (hormonal active) and non-functional (hormonal inactive), and do not detect minor lesions [26]. In this case, nonhormonal adenomas misidentified as hormonal sources may result in the removal of normal adrenal glands. Moreover, small unilateral adenomas, which cannot be detected by imaging, often lead to missed radical surgery and the failure of hypertension control. As the current clinical practice guidelines recommend, adrenal vein sampling (AVS) is the gold standard for typing PA, as determining unilateral primary aldosteronism is needed before surgery [20]. In the AVS procedure, plasma cortisol concentration (PCC) and aldosterone concentration (PAC) in the adrenal vein and peripheral venous obtained by catheter were examined to determine which adrenal gland had superior secretion and which type of treatment (curative adrenalectomy or pharmacotherapy) is most appropriate. According to large multicentre studies, when adrenalectomy is performed under AVS guidance, 84% of PA patients can achieve a clinical cure, and 96% can achieve biochemical cure [27, 28] (based on blood pressure, use of antihypertensive drugs, plasma potassium, and aldosterone concentrations, and plasma renin concentrations or activities), which is significantly higher than non-AVS-guided surgery [29, 30], suggesting that AVS is helpful to identify patients who could benefit from surgery. However, some studies have suggested that treating PA, whether guided by CT imaging or AVS, does not show significant differences in clinical benefits or biochemical success, indicating that the evidence supporting the superiority of AVS is limited. [31, 32]. As the gold standard of the classificatory diagnosis of PA, AVS is currently the best but not perfect choice [33]. The limitations of AVS are significant and include the following aspects: (1) “Lack of Morphological Information”: AVS cannot provide any morphological details as what imaging examinations offer. Typically, the identification of the adrenal veins during most AVS procedures relies on prior CT imaging. Additionally, for patients who may require adrenalectomy, preoperative imaging is essential for surgical planning. (2) “Technical and Accessibility Challenges”: AVS is technically demanding, limiting its availability across all medical institutions. Furthermore, it inherently carries the risk of radiation exposure. (3) “Absence of Standardized Protocols”: The lack of unified protocol and data interpretation for AVS can result in varying diagnoses for the same patient undergoing the procedure. This discrepancy could also contribute to the differing conclusions regarding the efficacy of AVS. Dr. Chen suggests that for younger patients with PA who show unilateral adenomas on CT scans, surgical intervention based on CT imaging could be considered a viable option. However, for individuals exhibiting either normal adrenal morphology or bilateral adrenal disease, the treatment approach should be carefully determined through AVS [34].
An important thing is that AVS is not an operation to confirm PA, but a test should be aimed at identifying surgically curable cases of PA [35]. AVS should only be utilized to guide treatment choices in patients who have a confirmed diagnosis of PA and are desiring surgery for a long-term cure. Before performing AVS, it is mandatory to make an unequivocal biochemical diagnosis of PA [6], but the exception is drug-resistant hypertension (RH) patients [36, 37]. Multiple antihypertensive drugs render complicated biochemical indicators and make it difficult to obtain a clear diagnosis of PA. In this case, if unilateral laparoscopic adrenalectomy is performed under the demonstration of a lateralized aldosterone excess by AVS, RH caused by PA can often be detected and resolved. An observational prospective cohort study showed that in RH patients who had at least one clue to PA underwent AVS-guided adrenalectomy, 96% were biochemically cured and 20% patients were clinically cured in that they no longer needed any antihypertensive treatment [38].
Interpretations of results
The current global adoption rate of AVS, as a gold standard diagnostic technique, remains insufficient. A retrospective, multinational, multicentre questionnaire-based survey performed by Japanese specialists containing 4818 PA patients from 16 centers found that the rates of AVS implementation and successful AVS were 66.3% and 89.3%, respectively [8]. The reason for the low performance rate of AVS may be the ideas that AVS is not always necessary, effective results are not easy to obtain, patients’ misconceptions of invasive surgical operations and their complications [39], and last but not least, the standard operating procedures and diagnostic criteria are urgently deficient [36]. Many different protocols are used to perform AVS, and there are currently varying interpretations of results. To address this situation, experts have performed a variety of clinical practices and studies, but there is still no hard evidence of which one should be given preference. According to different analysis procedures, the diagnosis of the same patient may differ, suggesting that some patients may be improperly managed because of a lack of criteria [40].
The direct results obtained from AVS generally are plasma aldosterone concentration and plasma cortisol concentration from the adrenal veins and peripheral vein (often from the inferior vena cava). However, because of the anatomical characteristics and blood flow of the adrenal vein, adrenal venous blood is likely to be contaminated by blood from other tributaries. If only crude aldosterone concentrations were compared, the interpretation of AVS results would be biased because the dilution effects on both sides may be different. Assuming that the same cortisol concentration in the adrenal vein and different cortisol concentrations in the left and right samples reflect different dilution effects, this bias can be corrected by measuring cortisol concentration and several related indices [41]. The evaluation of AVS results comprises the selectivity index (SI), lateralization index (LI), contralateral suppression index (CSI) and relative secretion index (RASI) [42,43,44,45,46]. Each of them has a different meaning and usage (Table 1).
Cortisol is always the most widely used corrective hormone. However, some studies have found that a few patients have aldosterone and cortisol co-secretion lesions [47, 48], which could affect the lateralization index and lead to a misdiagnosis of the subtype. Measurement of the adrenal androgen (androstenedione/DHEA) [49]/metanephrine [50]/normetanephrine [51, 52] levels in the AVS procedure helps to assess selectivity and lateralization, thus making the diagnosis more accurate. In addition, there is a novel “superselective” adrenal venous sampling (ssAVS) method using specialized “microcatheters”, which collects blood samples from adrenal tributary veins to differentiate segmental hyper-functional areas of the adrenal gland. The plasma aldosterone concentration in ssAVS samples does not require other hormones for normalization because there is no or a limited amount of irrelevant venous blood contained in the samples. The ssAVS method allows bilateral PA patients to be treated surgically by bilateral adrenalectomy while sparing lesion-free segments [53, 54].
2 ACS
Cushing syndrome (CS) is a rare disease characterized by long-term elevated cortisol levels, which can also cause hypertension. Adrenal Cushing syndrome (ACS), also known as ACTH-independent Cushing syndrome (AICS), is characterized by low plasma ACTH concentration and excess cortisol produced autonomously by the adrenal glands, accounting for approximately 20% of all CS [55]. The most common cause of ACS is unilateral cortisol-producing adrenal adenoma, but approximately 10% of ACS patients have bilateral adrenal disease [56]. The standard of treatment in adrenal Cushing’s syndrome is resection of the affected gland, unilateral adrenalectomy in the case of an adenoma, or bilateral adrenalectomy in the case of hyperplasia [55]. However, imaging examination, the most commonly used localizing check, does not always correspond to the source of hormonal excess [57]. Among patients with ACS with bilateral adrenal masses in imaging, it is often difficult to determine the source of excess cortisol, which largely leads to inappropriate treatment. If the location of hormone excess is not confirmed before bilateral adrenalectomy, the patients with bilateral images would be treated with lifelong glucocorticoid replacement therapy, then they are prone to adrenal crisis and life risk. Therefore, locating functional adrenal lesions is very important for further treatment. Multiple reports have shown that AVS helped to distinguish unilateral and bilateral lesions in patients with ACS [58,59,60,61,62], and the postoperative pathological results proved the correct clinical diagnosis under AVS. However, the applications of AVS in patients with ACS are less established than in those with PA, and the procedures and results interpretations in different centres vary significantly. Young et al. suggested using epinephrine concentrations to indicate successful catheterization and using the AV/IVC cortisol ratio and cortisol lateralization ratio (CLR) to indicate unilateral excess cortisol secretion [63, 64]. Many studies followed their criteria [57, 61, 62], but the reliability if these criteria still needs to be verified in the future.
3 PCCs
Pheochromocytomas (PCCs) are catecholamine-producing neuroendocrine neoplasms that usually present with secondary hypertension [65]. Excess catecholamine causes chronic or paroxysmal hypertension associated with sweating, headaches, and palpitations and increases cardiovascular morbidity and mortality. Most clinical sequelae and complications of PPCs are related to hypertension [66]. Theoretically, AVS can also be used in the localization diagnosis of pheochromocytoma. Since the last century, it has been used by some scholars to assist in the diagnosis of pheochromocytoma [67]. With advances in imaging techniques, most PCCs have become detectable by computed tomography (CT), and AVS is rarely performed in the diagnosis of PCCs. Moreover, a recent study found a wide range of epinephrine and norepinephrine levels in non-pheochromocytoma patients’ adrenal veins [68], and up to an 83-fold difference between the left and right adrenal veins was previously detected [69], suggesting that AVS may be of limited utility in determining the laterality of disease in patients with PCCs. It should only be used as a supplementary tool in difficult cases [70], such as patients with suspected pheochromocytoma without clear imaging or laboratory features, with bilateral adrenal gland lesions on imaging or accompanied by symptoms of primary aldosterone or hypercortisolism [71], which are more suitable for AVS [72].
4 Advances
AVS is an interventional radiological procedure with a very low rate of complications (e.g., adrenal vein rupture [73], iodine contrast media allergy), but it is currently still underutilized in many countries. The main reasons for its limited use may be due to the technical challenge of catheterization caused by the small diameter and anatomical variations of the adrenal vein [74]. The right adrenal vein is shorter and often enters the inferior vena cava (IVC) directly at an acute angle; thus, failure to diagnose is usually due to failure of catheterization of the right adrenal vein [75]. An affiliated procedure of a study attempted to determine whether RVS with less technical difficulty could be used to replace AVS [76]. However, from both the anatomical view and experimental data, the correlation between renal vein and adrenal vein samples is poor. A series of methods have been proposed and perfected by scholars in this field gradually, both for problems during operation and post-operation (Fig. 1). Given the high success rate of AVS practices, the wide skepticism about AVS might fade in the future. The increase in the use of AVS will lead to an increase in the rate of adrenalectomy, and the cure rate of PA may be improved.
Methods for problems during AVS operation and post-operation. Several improvements have been introduced to address the primary challenge encountered during AVS. The primary challenge in AVS procedure is the difficulty in catheterizing the adrenal veins, especially the right adrenal vein. Moreover, although post-operative iodine contrast media allergy is rare, it remains a significant concern. Techniques such as imaging, cosyntropin stimulation, rapid cortisol assays, and the antecubital catheterization approach have been progressively proposed to enhance AVS success rates. For patients with iodine contrast media allergies, alternatives such as CO2 or Gadolinium-based contrast media, along with premedication, have been suggested, though they are not without their flaws. Additionally, when AVS is not feasible or reliable, scintigraphy serves as an alternative diagnostic tool. These advancements, while not perfect, contribute to improving the procedural success rate of AVS to a certain degree
Methods for problems during operation
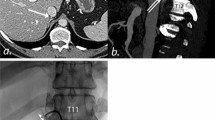
Imaging prior to an AVS procedure allows evaluation of the position and anatomy of the adrenal vein and IVC [77, 78], which can shorten the operation and fluoroscopy time [79]. Moreover, CT or cone-beam CT (CBCT) during the operation can significantly improve the success rate. When the catheter is observed in the right place under fluoroscopic guidance, CT is performed to ensure blood is sampled from the adrenal vein. A study found that intraprocedural CT images detected 14.0% inaccurate catheterization in the right adrenal vein and helped to reposition successfully [80]. Cone-beam CT (CB-CT)-producing images are similar to CT scans, adding to the use of rotational angiography through a C arm that rotates around the patient [81]. A meta-analysis based on data from 809 patients showed that intraoperative CT improved the success rate by 19.8% [82]. A retrospective study by Meyrignac et al. evaluated the contribution of CB-CT to the success rate of AVS. The overall success rate of AVS with CB-CT was 80% vs. 44% without CB-CT (p = 0.00046), with a right-sided selectivity of 88% vs. 49% (p < 0.0001) [9]. However, CT technology significantly increases the amount of additional radiation and should be used with caution. It is preferably just used in situations where experienced operators are unavailable or the patient characteristics make it difficult to carry out the AVS [83].
Stimulation with cosyntropin (a man-made form of adrenocorticotropic hormone, ACTH) bolus or infusion before sample collection can increase the concentration of aldosterone and cortisol collected from the adrenal vein by one to two orders of magnitude, enhancing the PCC gradient between the adrenal vein and the inferior vena cava and increasing the selectivity index values and confidence of successful sampling [84]. According to some research data in recent years, after stimulation with cosyntropin, the success rate of bilateral cannulation could be increased by approximately 20%, while the lateralization index decreased [10, 85, 86]. Some of the aldosterone-producing adenomas would be misidentified as idiopathic hyperaldosteronisms. A retrospective analysis of the largest international registry of 1625 individual AVS studies (AVIS-2 study) suggested that cosyntropin could not improve AVS outcomes since it reduced lateralization rates [73]. There still are studies following the treatment outcomes and pathological assessments of patients exhibiting discordant lateralization results before and after ACTH stimulation. A multicenter retrospective study analyzed the AVS data of 314 patients with PA, both at baseline and ACTH stimulation, who subsequently underwent adrenalectomy. These patients were categorized into three groups based on their AVS outcomes: those with a basal Lateralization Index (LI) ≥ 2 and ACTH-stimulated LI ≥ 4 on the ipsilateral side (classified as Unilateral (U) to U group, n = 245); individuals with a basal LI < 2 and ACTH-stimulated LI ≥ 4 (transitioning from Bilateral (B) to (U) group, n = 15); and patients who demonstrated a basal LI ≥ 2 with an ACTH-stimulated LI < 4 ((U) to (B) group, n = 54). Patients in U to U group had better surgical outcomes than those in U to B group [87]. This study confirmed that the use of AVS does not affect the accuracy of AVS results. Similarly, in the study conducted by Dr. Nicholas [86], a focus was placed on 7 patients who underwent unilateral adrenalectomy, selected based on pre-ACTH lateralization and additional indicators suggestive of aldosterone-producing adenoma. Of these seven, four patients achieved a surgical cure, all sharing the common attribute of exhibiting a LI greater than 2 after ACTH stimulation. However, the remaining three patients obtained limited biochemical improvement after surgery, characterized by a pre-ACTH LI greater than 3.0 and a post-ACTH LI below 2.0. These findings further affirm the utility of ACTH stimulation tests, indicating that they hold value, albeit not absolute, in refining the selection process for surgical candidates in the context of PA. Therefore, the application of ACTH stimulation needs further exploration through more extensive clinical practice and controlled trials. Moreover, a related point is that ACTH stimulation can reduce the time difference of PAC between the first sampling side and the other adrenal vein during sequential sampling [36, 88]. When centers have a low rate of catheterization success and there are no experienced radiologists to perform simultaneous sampling, ACTH-stimulated sequential sampling should be considered.
The rapid cortisol assay is an intraprocedural on-site test, allowing operators to check if the catheter is properly placed before the end of the procedure and perform resampling rather than reintubation. The introduction of the rapid cortisol assay significantly increased the technical success rate [89, 90], decreased the radiation exposure [76], and had no effect on subtype diagnosis [91, 92]. The only disadvantage of this technique is the additional 25–30 min needed during the operation to wait for laboratory results. However, since 2016, the adrenal vein sampling accuracy kit (AAK) has been available [11]. This is a one-time point-of-care cortisol measuring device. It is very simple to use and only requires moving the heparinized sample to the sampling port. Cortisol concentrations were measured during AVS in 2–5 min and were significantly correlated with a conventional assay. Retrospective studies showed that using the AAK device increased cannulation success by approximately 30% compared with no intraoperative cortisol measurement [11, 93], the radiation dose was reduced, and no complications were found. The rapid cortisol assay does not have the side effects of the above two methods and may become an assistive technology that can be routinely used in the AVS process.
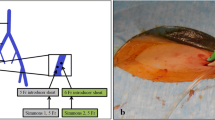
Most AVS procedures are performed via femoral vein access; however, in recent years, some Chinese radiologists and cardiovascular specialists believe that sampling via the antecubital approach can significantly reduce the difficulty of catheterization, with a success rate obviously better than that through the femoral vein (Fig. 2). From an anatomical point, 89% of patients’ right adrenal veins join the inferior vena cava from a caudal direction [94], and AVS via the antecubital approach may be more appropriate. In a retrospective multicentre study involving 7 Chinese medical centers [95], 1226 patients with primary aldosteronism underwent AVS via an antecubital approach. The success rates of bilateral, right, and left sampling were 91.5%, 94.9%, and 95.1%, respectively. Only 0.46% of participants experienced adrenal vein rupture, showing that AVS via an antecubital approach was safe and feasible, with a high rate of successful sampling. A previous study [96] involving 194 patients demonstrated the same conclusion; the rates of successful adrenal sampling via an antecubital approach on the right, left, and bilateral sides were all approximately 90%.
AVS via femoral vein access (A) and AVS via antecubital approach (B). Conventional AVS is typically performed via femoral vein access. However, the antecubital approach, which involves navigating an angiocatheter through the forearm vein, superior vena cava, right atrium, and inferior vena cava into the adrenal vein, presents a viable alternative or complementary method. This method is particularly beneficial for patients whose right adrenal veins enter the inferior vena cava from a caudal direction [94]
Methods for problems post-operation
AVS is a procedure that often relies on iodine contrast media (ICM) to confirm catheter position, especially in the right adrenal vein. This becomes a challenge for patients with allergies to ICM or those with severe renal dysfunction, yet who require AVS for therapeutic decision-making. Studies have highlighted that approximately 2.6–4% [97, 98] of patients seeking AVS have documented allergic reactions to these contrast dyes. Consequently, alternative strategies have been explored to accommodate these patients.
A noteworthy alternative, as demonstrated in recent research, is the use of carbon dioxide (CO2) as a contrast medium. In one institution, 18 patients with an allergy history to ICM underwent AVS using carbon dioxide venography combined with intraprocedural CT, successfully identifying the left and right adrenal veins [99]. Additionally, Gadolinium-based contrast media (GBCM) have been identified as another viable option [100] for performing AVS with a high success rate and safety. However it is not recommended for individuals with compromised kidney function [101]. Dr. Younes and colleagues [98] have offered another valuable approach for patients with ICM allergies, showcasing the feasibility of using a small dose of dexamethasone and a nonselective antihistamine as premedication. These advancements enhance the safety and effectiveness of AVS, making it accessible to a wider range of patients while minimizing the risk of adverse reactions.
A potential second-line tool
When patients present contraindications to AVS due to reasons such as contrast allergies, or when AVS results are either unattainable or inconclusive, scintigraphy emerges as a helpful and non-invasive alternative for lateralization of PA [102, 103]. Unlike traditional anatomical radiological imaging techniques, scintigraphic imaging of the adrenal glands utilizing specific radiopharmaceuticals offers the unique advantage of correlating functional activity with anatomical anomalies. This approach merges the characteristics of both AVS and CT. Scintigraphy, encompassing both adrenomedullary and adrenocortical applications, has been proven effective in distinguishing between benign and malignant lesions as well as differentiating unilateral lesions from bilateral hyperplasia [104].
Introduced in 1977 [105], adrenocortical scintigraphy using 131I-NP-59 has demonstrated its utility in diagnosing and subtyping primary aldosteronism and Cushing’s syndrome [106, 107]. Furthermore, adrenomedullary scintigraphy based on 131I or 123I-labeled metaiodobenzylguanidine (MIBG) uptake has been established as a method for the localization and treatment of pheochromocytoma and paraganglioma [108, 109]. However, a notable limitation of scintigraphy is its sensitivity dependency on the size of the adenoma, with difficulty in detecting adenomas smaller than 1.5 cm in diameter [20], leading to its diminished use in many countries.
Recent years have seen a resurgence of interest in adrenal cortical scintigraphy, thanks, in part, to advancements in single photon emission computed tomography (SPECT). Studies confirm that the accuracy of NP-59 SPECT in diagnosing primary aldosteronism is comparable with that provided by CT/MRI and AVS [103, 110], suggesting that scintigraphy remains a viable second-line diagnostic tool when AVS is not feasible or its results are questionable.
Renal venous sampling (RVS) and renin-dependent hypertension
Renal venous sampling (RVS) collecting blood samples from renal veins and peripheral veins and analysing renin in them might be helpful in detecting renin-secreting disease. Although RVS has not been fully popularized in hypertension diagnosis, some cases and studies have proven that RVS has a unique role in the diagnosis and evaluation of accurate treatment of some cases of renin-dependent hypertension [12, 111].
Renovascular hypertension (RVHT)
Renovascular disease is a common cause of hypertension, accounting for 1% to 5% of all cases of hypertension in the general population and 5.4% of secondary hypertension cases in young adults, and 90% of renovascular hypertension is caused by atherosclerotic renal artery stenosis [112]. Renal artery stenosis induces renal hypoperfusion, which activates the renin-angiotensin-aldosterone system (RAAS), subsequently leading to an increase in blood pressure [113]. Catheter angiography and digital subtraction angiography are the gold standard for the diagnosis of renal artery stenosis [114]. Non-invasive imaging methods such as ultrasonography (US), scintigraphy, computed tomography angiography (CTA), and magnetic resonance angiography (MRA) have become increasingly utilized in medical diagnostics [115, 116]. A number of researches conducted in this field support that these imaging techniques can accurately detect renal artery stenosis [117].
However, Dr. Kannan et al. conducted a review of previous studies and discovered that revascularization procedures guided only by radiographic image information of renal artery stenosis have not yielded the expected outcomes [118]. This discovery emphasizes the importance of basing treatment decisions on physiological criteria, not just on radiographic evidence. The reason for this approach is that renovascular hypertension—triggered by renal artery stenosis via the renin-angiotensin-aldosterone system (RAAS)—is fundamentally different from hypertension with atherosclerotic renal artery stenosis [119]. Surgical interventions like renal revascularization, particularly for renal artery stenosis that develops as a secondary consequence of long-standing hypertension, may not be effective [120].
Patients with renovascular hypertension often present with both clear imaging evidence of renal artery stenosis and biochemical markers indicative of RAAS activation. The presence of renal artery stenosis identified through angiography in patients with hypertension does not necessarily confirm it as the causative factor for their high blood pressure. Renal vein sampling (RVS) and renin measurements provide a more physiologically grounded method for differentiating the ischemic effects of renovascular disease, offering a logical advantage over imaging or peripheral venous renin measurements alone [111, 121].
A case report [12] showed a patient with high systolic pressure greater than 200 mmHg, hypokalaemia, and elevated plasma renin activity (PRA). Occlusion of the left renal artery and atrophy of the left kidney were shown in CT image. Then, RVS was performed, indicating that the PRA of the left vein was 3.2 times higher than that of the right vein, proving that endocrine hyperfunction and hypertension were caused by renal artery stenosis and an atrophic kidney. Subsequent surgery for narrowed arteries made blood pressure and potassium normalized. It is well known that patients with renal vascular disease without proper treatment often develop renal atrophy. A previous study of renal atrophy with hypertension found that nephrectomy was effective for blood pressure control in patients with a bilateral renin ratio >1.5 [122]. Thus, RVS plays a unique role in accurately identifying patients with “true” renovascular hypertension who are likely to benefit from surgical intervention.
Reninoma
Reninoma (juxtaglomerular cell tumour, JCT) is a rare, renin-secreting tumour of the kidney that can cause hypertension and hypokalaemia and is usually diagnosed in adolescents and young adults. Once correctly diagnosed, reninomas can be cured by surgical resection, with the majority of the patients becoming and remaining normotensive. Thus, for young patients with hypertension combined with high plasma renin activity, it is important to recognize reninoma. Renal ultrasound, CT and MRI are common tests for diagnosis, but they often fail to detect small lesions, resulting in delayed diagnosis and therapy, which can lead to the preventable loss of nephrons [123]. Because the level of renin in the affected side kidney is much higher than that in the opposite kidney, renin sampling from both renal veins and the peripheral vein can help to identify the site of excessive renin production. In previous cases, patients underwent RVS to confirm the diagnosis of reninomas. After partial nephrectomy, hypertension, hyperreninemia, and hypokalaemia were completely resolved, and histopathology postoperation also confirmed the diagnosis [13, 14, 123,124,125]. In some cases where diagnosis is difficult, the location of the lesion can be identified more precisely by segmental RVS from each renal vein [126, 127].
Conclusion
We reviewed clinical research investigating the applications and protocols of selective venous sampling (SVS) in diagnosing subtypes and guiding treatment strategies for complex cases, especially those lacking definitive imaging or laboratory markers. Among the SVS techniques, adrenal vein sampling (AVS) is particularly noteworthy. As the gold standard for classificatory diagnosis of primary aldosteronism (PA), it could be applied not only to PA but also to other endocrine diseases associated with secondary hypertension, such as adrenal Cushing syndrome (ACS) and Pheochromocytomas (PCCs). Despite its potential, AVS’s application and recognition in managing ACS and PCCs lag behind its established role in PA, with the low success rate of catheterization, the controversy of results interpretation, and the absence of a standardized protocol.
Moreover, the AVS technique for PA necessitates refinements to enhance its procedural success. Intraprocedural cortisol testing (CCF) has shown promise in improving catheterization success rates in AVS for PA. The deployment of imaging methods and ACTH stimulation may benefit centers with low AVS success rates in PA diagnosis. The standard protocol for results interpretation and catheterization via the antecubital approach need further investigation. The use of carbon dioxide (CO2) or Gadolinium-based contrast media (GBCM), alongside with premedication should be recommended for patients with allergies to iodine contrast media (ICM) to reduce adverse events. Finally, when AVS is not a viable option, scintigraphy emerges as a potential diagnostic alternative.
Renal venous sampling (RVS), which is similar to AVS in principle and procedure, emerges as a diagnostic tool for identifying renin-dependent hypertension, such as renovascular hypertension and reninoma. Although not routinely recommended as a prevalent tool, evidence supports RVS’s efficacy in pinpointing the source of excess hormone and identifying optimal surgical candidates among hypertensive patients with renin angiotensin aldosterone system (RAAS) abnormal activation.
In summary, increasing the use of SVS can help to improve the diagnosis and cure rate of SH. Experts should adopt it more flexibly and fearlessly so that the technology becomes more mature, used more frequently, applied to more diseases, less technically difficult, and accepted by more patients, as it should be. Making high-quality SVS more widely available is better for our profession and, more importantly, for patients.
References
Carey RM, Whelton PK. Prevention, detection, evaluation, and management of high blood pressure in adults: Synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension Guideline. Ann Intern Med. 2018;168:351–8.
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481.
Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334–57.
England RW, Geer EB, Deipolyi AR. Role of venous sampling in the diagnosis of endocrine disorders. J Clin Med. 2018;7:114.
Tziomalos K. Secondary hypertension: novel insights. Curr Hypertens Rev. 2020;16:11.
Rossi GP. Primary Aldosteronism: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:2799–811.
Melby JC, Spark RF, Dale SL, Egdahl RH, Kahn PC. Diagnosis and localization of aldosterone-producing adenomas by adrenal-vein catheterization. N. Engl J Med. 1967;277:1050–6.
Ohno Y, Naruse M, Beuschlein F, Schreiner F, Parasiliti-Caprino M, Deinum J, et al. Adrenal venous sampling-guided adrenalectomy rates in primary aldosteronism: Results of an International Cohort (AVSTAT). J Clin Endocrinol Metab. 2021;106:e1400–e1407.
Meyrignac O, Arcis É, Delchier MC, Mokrane FZ, Darcourt J, Rousseau H, et al. Impact of cone beam - CT on adrenal vein sampling in primary aldosteronism. Eur J Radiol. 2020;124:108792.
Sung TY, Alobuia WM, Tyagi MV, Ghosh C, Kebebew E. Adrenal vein sampling to distinguish between unilateral and bilateral primary hyperaldosteronism: To ACTH stimulate or not? J Clin Med. 2020;9:1447.
Yoneda T, Karashima S, Kometani M, Usukura M, Demura M, Sanada J, et al. Impact of new quick gold nanoparticle-based cortisol assay during adrenal vein sampling for primary aldosteronism. J Clin Endocrinol Metab. 2016;101:2554–61.
Maruyama K, Chinda J, Kabara M, Nakagawa N, Fujino T, Takeuchi T, et al. Successful percutaneous transluminal angioplasty for the treatment of renovascular hypertension with an atrophic kidney. Heart vessels. 2015;30:274–9.
Wolley M, Gordon RD, Stowasser M. Reninoma: the importance of renal vein renin ratios for lateralisation and diagnosis. Am J Nephrol. 2014;39:16–19.
Wang B, Ding L, Xu S, Fan Y, Wang J, Zhao X, et al. A case of atypical reninoma with mild hypertension and normal plasma renin activity but elevated plasma renin concentration. BMC Endocr Disord. 2022;22:71.
Xu Z, Yang J, Hu J, Song Y, He W, Luo T, et al. Primary Aldosteronism in patients in China with recently detected hypertension. J Am Coll Cardiol. 2020;75:1913–22.
Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126–48.
Bioletto F, Bollati M, Lopez C, Arata S, Procopio M, Ponzetto F, et al. Primary Aldosteronism and resistant hypertension: a pathophysiological insight. Int J Mol Sci. 2022;23:4803.
Parasiliti-Caprino M, Lopez C, Prencipe N, Lucatello B, Settanni F, Giraudo G, et al. Prevalence of primary aldosteronism and association with cardiovascular complications in patients with resistant and refractory hypertension. J Hypertens. 2020;38:1841–8.
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018;72:658–66.
Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101:1889–916.
Mulatero P, Monticone S, Deinum J, Amar L, Prejbisz A, Zennaro MC, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the Working Group on Endocrine Hypertension of The European Society of Hypertension. J Hypertens. 2020;38:1919–28.
Delivanis DA, Vassiliadi DA, Tsagarakis S. Adrenal imaging in patients with endocrine hypertension. Endocrinol Metab Clin North Am. 2019;48:667–80.
Stowasser M, Gordon RD. Primary Aldosteronism: Changing definitions and new concepts of physiology and pathophysiology both inside and outside the kidney. Physiol Rev. 2016;96:1327–84.
Ladurner R, Sommerey S, Buechner S, Dietz A, Degenhart C, Hallfeldt K, et al. Accuracy of adrenal imaging and adrenal venous sampling in diagnosing unilateral primary aldosteronism. Eur J Clin Investig. 2017;47:372–7.
Kaur R, Young S. Discordant imaging: adrenal vein sampling in almost half of patients with primary aldosteronism and a unilateral adrenal adenoma. Intern Med J. 2023;53:1409–14.
Loberg C, Antoch G, Stegbauer J, Dringenberg T, Steuwe A, Fürst G, et al. Update: Selective adrenal venous sampling (AVS) - Indication, technique, and significance. RoFo : Fortschr auf dem Geb der Rontgenstrahlen und der Nuklearmedizin. 2021;193:658–66.
Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. The lancet Diabetes &. endocrinology 2017;5:689–99.
Rossi GP, Rossitto G, Amar L, Azizi M, Riester A, Reincke M, et al. Clinical outcomes of 1625 patients with primary aldosteronism subtyped with adrenal vein sampling. Hypertension. 2019;74:800–8.
Williams TA, Burrello J, Sechi LA, Fardella CE, Matrozova J, Adolf C, et al. Computed tomography and adrenal venous sampling in the diagnosis of unilateral primary aldosteronism. Hypertension. 2018;72:641–9.
Arjani S, Bostonian TJ, Prasath V, Quinn PL, Chokshi RJ. Cost-effectiveness of adrenal vein sampling- vs computed tomography-guided adrenalectomy for unilateral adrenaloma in primary aldosteronism. J Endocrinol Investig. 2022;45:1899–908.
Dekkers T, Prejbisz A, Kool LJS, Groenewoud H, Velema M, Spiering W, et al. Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial. lancet Diabetes Endocrinol 2016;4:739–46.
Deinum J, Prejbisz A, Lenders JWM, van der Wilt GJ. Adrenal vein sampling is the preferred method to select patients with primary aldosteronism for adrenalectomy: con side of the argument. Hypertension. 2018;71:10–14.
Rossi GP, Funder JW. Adrenal vein sampling is the preferred method to select patients with primary aldosteronism for adrenalectomy: pro side of the argument. Hypertension. 2018;71:5–9.
Fang C, Dai J, Zhao J, Huang X, He W, Xu J, et al. Surgery based on computed tomography images might be feasible for primary aldosteronism patients with visible unilateral adenoma. J Clin Hypertens. 2023;25:1001–8.
Rossi GP, Maiolino G, Seccia TM. Adrenal venous sampling: where do we stand? Endocrinol Metab Clin North Am. 2019;48:843–58.
Rossi GP. Update in adrenal venous sampling for primary aldosteronism. Curr Opin Endocrinol Diabetes Obes. 2018;25:160–71.
Lenzini L, Pintus G, Rossitto G, Seccia TM, Rossi GP. Primary aldosteronism and drug resistant hypertension: A “Chicken-Egg” story. Exp Clin Endocrinol Diabetes 2023;131:409–17.
Torresan F, Rossitto G, Bisogni V, Lerco S, Maiolino G, Cesari M, et al. Resolution of drug-resistant hypertension by adrenal vein sampling-guided adrenalectomy: a proof-of-concept study. Clin Sci. 2020;134:1265–78.
Araujo-Castro M, Paja Fano M, González Boillos M, Pla Peris B, Pascual-Corrales E, García Cano AM, et al. Adrenal venous sampling in primary aldosteronism: Experience of a Spanish multicentric study (Results from the SPAIN-ALDO Register). Endocrine. 2022;78:363–72.
Lethielleux G, Amar L, Raynaud A, Plouin PF, Steichen O. Influence of diagnostic criteria on the interpretation of adrenal vein sampling. Hypertension. 2015;65:849–54.
Steichen O, Amar L. Diagnostic criteria for adrenal venous sampling. Curr Opin Endocrinol Diabetes Obes 2016;23:218–24.
Monticone S, Viola A, Rossato D, Veglio F, Reincke M, Gomez-Sanchez C, et al. Adrenal vein sampling in primary aldosteronism: towards a standardised protocol. Lancet Diabetes Endocrinol 2015;3:296–303.
Bardet S, Chamontin B, Douillard C, Pagny JY, Hernigou A, Joffre F, et al. SFE/SFHTA/AFCE consensus on primary aldosteronism, part 4: Subtype diagnosis. Annales d’Endocrinologie. 2016;77:208–13.
Mulatero P, Sechi LA, Williams TA, Lenders JWM, Reincke M, Satoh F, et al. Subtype diagnosis, treatment, complications and outcomes of primary aldosteronism and future direction of research: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertension. 2020;38:1929–36.
Wu VC, Hu YH, Er LK, Yen RF, Chang CH, Chang YL, et al. Case detection and diagnosis of primary aldosteronism - The consensus of Taiwan Society of Aldosteronism. J Formos Med Assoc. 2017;116:993–1005.
Rossi GP, Bagordo D, Amar L, Azizi M, Riester A, Reincke M, et al. Unilaterally Selective Adrenal Vein Sampling for Identification of Surgically Curable Primary Aldosteronism. Hypertension (Dallas, Tex : 1979). 2023 (e-pub ahead of print 2023/06/15; https://doi.org/10.1161/hypertensionaha.123.21247).
Arlt W, Lang K, Sitch AJ, Dietz AS, Rhayem Y, Bancos I, et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI insight. 2017;2:e93136.
Kline GA, So B, Campbell DJT, Chin A, Harvey A, Venos E, et al. Apparent failed and discordant adrenal vein sampling: A potential confounding role of cortisol cosecretion? Clin Endocrinol. 2022;96:123–31.
Li H, Zhang X, Shen S, Zhang Y, Zhang W, Feng W, et al. Adrenal androgen measurement for assessing the selectivity of adrenal venous sampling in primary aldosteronism. Steroids. 2018;134:16–21.
Buffolo F, Pieroni J, Ponzetto F, Forestiero V, Rossato D, Fonio P, et al. Prevalence of Cortisol cosecretion in patients with primary aldosteronism: role of Metanephrine in adrenal vein sampling. J Clin Endocrinol Metab. 2023;108:e720–e25.
Liu W, Zhang J, Yang Y, Jin Y, Li Z, You L, et al. Application of Metanephrine and Normetanephrine in evaluating the selectivity of adrenal vein sampling. Horm Metab Res. 2022;54:162–7.
Ceolotto G, Antonelli G, Caroccia B, Battistel M, Barbiero G, Plebani M, et al. Comparison of Cortisol, Androstenedione and Metanephrines to assess selectivity and lateralization of adrenal vein sampling in primary aldosteronism. J Clin Med. 2021;10:4755.
Makita K, Nishimoto K, Kiriyama-Kitamoto K, Karashima S, Seki T, Yasuda M, et al. A novel method: super-selective adrenal venous sampling. J Vis Exp. 2017 e-pub ahead of print 2017/10/11; https://doi.org/10.3791/55716.
Noda Y, Goshima S, Nagata S, Kawada H, Tanahashi Y, Kato T, et al. Utility of microcatheter in adrenal venous sampling for primary aldosteronism. Br J Radiol. 2020;93:20190636.
Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet. 2015;386:913–27.
Lacroix A, Bourdeau I. Bilateral adrenal Cushing’s syndrome: macronodular adrenal hyperplasia and primary pigmented nodular adrenocortical disease. Endocrinol Metab Clin North Am. 2005;34:441–58.
Ueland G, Methlie P, Jøssang DE, Sagen JV, Viste K, Thordarson HB, et al. Adrenal venous sampling for assessment of autonomous cortisol secretion. J Clin Endocrinol Metab. 2018;103:4553–60.
An X, Chen T, Mo D, Shen S, Zhang D, Zhang T, et al. Role of adrenal venous sampling in the differential diagnosis and treatment protocol of ACTH-independent Cushing’s syndrome with bilateral adrenal lesions. Endocrine. 2023;81:562–72.
Papakokkinou E, Jakobsson H, Sakinis A, Muth A, Wängberg B, Ehn O, et al. Adrenal venous sampling in patients with ACTH-independent hypercortisolism. Endocrine. 2019;66:338–48.
Seki T, Yasuda A, Kitajima N, Oki M, Takagi A, Nakamura N, et al. Adrenal venous sampling is useful for a definitive diagnosis in Cushing’s Syndrome with bilateral adrenal tumors. Tokai J Exp Clin Med. 2015;40:149–56.
Wei J, Li S, Liu Q, Zhu Y, Wu N, Tang Y, et al. ACTH-independent Cushing’s syndrome with bilateral cortisol-secreting adrenal adenomas: a case report and review of literatures. BMC Endocr Disord. 2018;18:22.
Zhou Q, Liu X, Zhang H, Zhao Z, Li Q, He H, et al. Adrenal artery ablation for the treatment of hypercortisolism based on adrenal venous sampling: a potential therapeutic strategy. Diabetes Metab Syndr Obes: targets Ther 2020;13:3519–25.
Johnson PC, Thompson SM, Adamo D, Fleming CJ, Bancos I, McKenzie TJ, et al. Adrenal venous sampling for lateralization of cortisol hypersecretion in patients with bilateral adrenal masses. Clin Endocrinol. 2023;98:177–89.
Young WF Jr, du Plessis H, Thompson GB, Grant CS, Farley DR, Richards ML, et al. The clinical conundrum of corticotropin-independent autonomous cortisol secretion in patients with bilateral adrenal masses. World J Surg. 2008;32:856–62.
Farrugia FA, Charalampopoulos A. Pheochromocytoma. Endocr Regul. 2019;53:191–212.
Pappachan JM, Tun NN, Arunagirinathan G, Sodi R, Hanna FWF. Pheochromocytomas and hypertension. Curr Hypertens Rep. 2018;20:3.
Newbould EC, Ross GA, Dacie JE, Bouloux PM, Besser GM, Grossman A. The use of venous catheterization in the diagnosis and localization of bilateral phaeochromocytomas. Clin Endocrinol. 1991;35:55–9.
DeLozier OM, Dream S, Findling JW, Rilling W, Kidambi S, Magill SB, et al. Wide variability in catecholamine levels from adrenal venous sampling in primary Aldosteronism. J Surg Res. 2022;277:1–6.
Freel EM, Stanson AW, Thompson GB, Grant CS, Farley DR, Richards ML, et al. Adrenal venous sampling for catecholamines: a normal value study. J Clin Endocrinol Metab. 2010;95:1328–32.
Sze CWC, O’Toole SM, Tirador RK, Akker SA, Matson M, Perry L, et al. Adrenal Vein Catecholamine levels and ratios: reference intervals derived from patients with primary Aldosteronism. Horm Metab Res. 2017;49:418–23.
Leader N, Ushinsky A, Malone CD. Adrenal vein sampling for ACTH-producing pheochromocytomas. Radiol Case Rep. 2021;16:2672–5.
Guan X, Li J, Liu Q, Wang X, Liu D. [The clinical value of adrenal venous sampling in the qualitative and topical diagnosis of pheochromocytoma (report of 4 cases)]. J Minim Invasive Urol. 2022;11:367–71.
Rossitto G, Amar L, Azizi M, Riester A, Reincke M, Degenhart C, et al. Subtyping of primary Aldosteronism in the AVIS-2 Study: Assessment of selectivity and lateralization. J Clin Endocrinol Metab. 2020;105:dgz017.
So CB, Leung AA, Chin A, Kline GA. Adrenal venous sampling in primary aldosteronism: lessons from over 600 single-operator procedures. Clin Radiol. 2022;77:e170–e9.
Cesmebasi A, Du Plessis M, Iannatuono M, Shah S, Tubbs RS, Loukas M. A review of the anatomy and clinical significance of adrenal veins. Clin Anat. 2014;27:1253–63.
Augustin AM, Dalla Torre G, Fuss CT, Fassnacht M, Bley TA, Kickuth R. Reduction of radiation exposure in adrenal vein sampling: impact of the rapid cortisol assay. RoFo : Fortschr auf dem Geb der Rontgenstrahlen und der Nuklearmedizin. 2021;193:1392–402.
Lee I, Lau KK. Image fusion-augmented angiography improves right adrenal vein cannulation success rate in adrenal vein sampling. Am J Roentgenol. 2021;217:945–6.
Wang Y, Chen X, Lu G, Su Y, Yang L, Shi G, et al. Improving the visualization of the adrenal veins using virtual monoenergetic images from dual-energy computed tomography before adrenal venous sampling. Tomogr (Ann Arbor, Mich). 2023;9:485–96.
He X, Sueyoshi E, Tasaki Y, Miyazaki S, Murakami T, Nagayama H, et al. Benefits of adrenal venous sampling with preoperative four-dimensional CT imaging. Acta Radiol. 2023;64:1280–9.
Maruyama K, Sofue K, Okada T, Koide Y, Ueshima E, Iguchi G, et al. Advantages of intraprocedural unenhanced CT during adrenal venous sampling to confirm accurate catheterization of the right adrenal vein. Cardiovasc Intervent Radiol. 2019;42:542–51.
Plank C, Wolf F, Langenberger H, Loewe C, Schoder M, Lammer J. Adrenal venous sampling using Dyna-CT-a practical guide. Eur J Radiol. 2012;81:2304–7.
Hafezi-Nejad N, Gullotti DM, Bailey CR, Lessne ML, Holly BP. Does intraprocedural CT improve the success rate of adrenal venous sampling? A systematic review and meta-analysis of data from 809 patients. Cardiovasc Intervent Radiol. 2022;45:29–40.
Cai R, Hu C, Li HY. Cone-beam computed tomography is not a mandatory procedure in adrenal venous sampling for primary hyperaldosteronism. BMC Med imaging. 2022;22:189.
Liu W, Zhang J, Yang Y, Jin Y, Li Z, You L, et al. Effect of Adrenocorticotropic hormone stimulation during simultaneous bilateral adrenal vein sampling in primary Aldosteronism. Horm Metab Res. 2021;53:364–70.
El Ghorayeb N, Mazzuco TL, Bourdeau I, Mailhot JP, Zhu PS, Thérasse E, et al. Basal and post-ACTH Aldosterone and its ratios are useful during adrenal vein sampling in primary Aldosteronism. J Clin Endocrinol Metab. 2016;101:1826–35.
Chee NYN, Abdul-Wahab A, Libianto R, Gwini SM, Doery JCG, Choy KW, et al. Utility of adrenocorticotropic hormone in adrenal vein sampling despite the occurrence of discordant lateralization. Clin Endocrinol. 2020;93:394–403.
Kobayashi H, Nakamura Y, Abe M, Kurihara I, Itoh H, Ichijo T, et al. Effect of cosyntropin during adrenal venous sampling on subtype of primary aldosteronism: analysis of surgical outcome. Eur J Endocrinol. 2020;182:265–73.
Rossitto G, Battistel M, Barbiero G, Bisogni V, Maiolino G, Diego M, et al. The subtyping of primary aldosteronism by adrenal vein sampling: sequential blood sampling causes factitious lateralization. J Hypertens. 2018;36:335–43.
Chang CC, Lee BC, Chang YC, Wu VC, Huang KH, Liu KL. Comparison of C-arm computed tomography and on-site quick cortisol assay for adrenal venous sampling: A retrospective study of 178 patients. Eur Radiol. 2017;27:5006–14.
Betz MJ, Degenhart C, Fischer E, Pallauf A, Brand V, Linsenmaier U, et al. Adrenal vein sampling using rapid cortisol assays in primary aldosteronism is useful in centers with low success rates. Eur J Endocrinol. 2011;165:301–6.
Kometani M, Yoneda T, Karashima S, Takeda Y, Tsuiki M, Yasoda A, et al. Effect of intraprocedural cortisol measurement on ACTH-stimulated adrenal vein sampling in primary Aldosteronism. J Endocr Soc. 2022;6:bvac104.
Rossi E, Regolisti G, Perazzoli F, Negro A, Grasselli C, Santi R, et al. Intraprocedural cortisol measurement increases adrenal vein sampling success rate in primary aldosteronism. Am J Hypertens. 2011;24:1280–5.
Page MM, Taranto M, Ramsay D, van Schie G, Glendenning P, Gillett MJ, et al. Improved technical success and radiation safety of adrenal vein sampling using rapid, semi-quantitative point-of-care cortisol measurement. Ann Clin Biochem. 2018;55:588–92.
Matsuura T, Takase K, Ota H, Yamada T, Sato A, Satoh F, et al. Radiologic anatomy of the right adrenal vein: preliminary experience with MDCT. AJR. Am J Roentgenol. 2008;191:402–8.
Dong H, Huang J, Zhang Y, Dong Y, Liu M, Yan Z, et al. Adrenal venous sampling via an antecubital approach in primary aldosteronism: A multicenter study. J Clin Endocrinol Metab. 2023(e-pub ahead of print 2023/07/19; https://doi.org/10.1210/clinem/dgad433).
Jiang X, Dong H, Peng M, Che W, Zou Y, Song L, et al. A novel method of adrenal venous sampling via an Antecubital approach. Cardiovasc Intervent Radiol. 2017;40:388–93.
Battistel M, Ceolotto G, Barbiero G, Rossitto G, Rossi GP. Adrenal venous sampling in dye-allergic primary aldosteronism patients: prevalence, pitfalls and a possible solution. J Hypertens. 2018;36:1942–4.
Younes N, Therasse E, Bourdeau I, Lacroix A. Successful adrenal vein sampling using dexamethasone premedication in patients with iodine contrast media allergy. J Endocr Soc. 2022;6:bvac093.
Kamada H, Seiji K, Oguro S, Ota H, Yanagaki S, Omata K, et al. Utility of carbon dioxide venography and intraprocedural CT for adrenal venous sampling in patients with an allergy to iodinated contrast media. J Vasc Intervent Radiol 2023;34:1963–9.
Yoshida Y, Nagai S, Shibuta K, Miyamoto S, Maruno M, Takaji R, et al. Adrenal vein sampling with gadolinium contrast medium in a patient with florid primary aldosteronism and iodine allergy. J Endocr Soc. 2022;6:bvac007.
Woolen SA, Shankar PR, Gagnier JJ, MacEachern MP, Singer L, Davenport MS. Risk of Nephrogenic Systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a Group II Gadolinium-based contrast agent: a systematic review and meta-analysis. JAMA Intern Med. 2020;180:223–30.
Chan CK, Chang YY, Tsai YC, Chen ZW, Wu CY, Huang WC, et al. Taiwan mini-frontier of primary aldosteronism: Updating treatment and comorbidities detection. J Formos Med Assoc. 2021;120:1811–20.
Di Martino M, García Sanz I, Muñoz de Nova JL, Marín Campos C, Martínez Martín M, Domínguez Gadea L. NP-59 test for preoperative localization of primary hyperaldosteronism. Langenbeck’s. Arch Surg. 2017;402:303–8.
Gross MD, Shapiro B, Francis IR, Glazer GM, Bree RL, Arcomano MA, et al. Scintigraphic evaluation of clinically silent adrenal masses. J Nucl Med. 1994;35:1145–52.
Sarkar SD, Cohen EL, Beierwaltes WH, Ice RD, Cooper R, Gold EN. A new and superior adrenal imaging agent, 131I-6beta-iodomethyl-19-nor-cholesterol (NP-59): evaluation in humans. J Clin Endocrinol Metab. 1977;45:353–62.
Saiga A, Yokota H, Nagano H, Sawada K, Kubota Y, Wada T, et al. 131I-6β-iodomethyl-19-norcholesterol adrenal scintigraphy as an alternative to adrenal venous sampling in differentiating aldosterone-producing adenoma from bilateral idiopathic hyperaldosteronism. Nucl Med Commun. 2020;41:1226–33.
Okada Y, Matsushita S, Yamaguchi K. Investigation of Cushing’s and subclinical Cushing’s syndromes using adrenocortical scintigraphy. Nucl Med Commun. 2021;42:619–24.
Anyfanti P, Mastrogiannis Κ, Lazaridis Α, Tasios Κ, Vasilakou D, Kyriazidou Α, et al. Clinical presentation and diagnostic evaluation of pheochromocytoma: case series and literature review. Clin Exp Hypertens. 2023;45:2132012.
Zhang X, Wakabayashi H, Hiromasa T, Kayano D, Kinuya S. Recent advances in radiopharmaceutical Theranostics of Pheochromocytoma and Paraganglioma. Semin Nucl Med. 2023;53:503–16.
Sato T, Matsutomo N, Yamamoto T, Fukami M, Kono T. Evaluation of standardized uptake value on (131)I-6β-iodomethyl-19-norcholesterol scintigraphy for diagnosis of primary aldosteronism and correspondence with adrenal venous sampling. Ann Nucl Med. 2023;37:89–98.
Covic A, Gusbeth-Tatomir P. The role of the renin-angiotensin-aldosterone system in renal artery stenosis, renovascular hypertension, and ischemic nephropathy: diagnostic implications. Prog Cardiovasc Dis. 2009;52:204–8.
Herrmann SM, Textor SC. Renovascular hypertension. Endocrinol Metab Clin North Am. 2019;48:765–78.
Gavras H, Brunner HR, Thurston H, Laragh JH. Reciprocation of renin dependency with sodium volume dependency in renal hypertension. Science. 1975;188:1316–7.
Mannemuddhu SS, Ojeda JC, Yadav A. Renovascular hypertension. Prim Care. 2020;47:631–44.
Tullus K, Brennan E, Hamilton G, Lord R, McLaren CA, Marks SD, et al. Renovascular hypertension in children. Lancet. 2008;371:1453–63.
Bhattad PB, Jain V. Renal Artery Stenosis as etiology of recurrent flash pulmonary edema and role of imaging in timely diagnosis and management. Cureus. 2020;12:e7609.
Garovic VD, Textor SC. Renovascular hypertension and ischemic nephropathy. Circulation. 2005;112:1362–74.
Balamuthusamy S, Kannan A, Thajudeen B, Ottley D, Jalandhara N. Mild renal artery stenosis can induce renovascular hypertension and is associated with elevated renal vein renin secretion. Semin Dial. 2015;28:293–8.
Bavishi C, de Leeuw PW, Messerli FH. Atherosclerotic renal artery stenosis and hypertension: pragmatism, pitfalls, and perspectives. Am J Med. 2016;129:635.e635–635.e614.
Saad A, Herrmann SM, Crane J, Glockner JF, McKusick MA, Misra S, et al. Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circ Cardiovasc intervent. 2013;6:428–35.
Marks LS, Maxwell MH. Renal vein renin: value and limitations in the prediction of operative results. Urol Clin North Am. 1975;2:311–25.
Marboeuf P, Delsart P, Hurt C, Villers A, Hossein-Foucher C, Beregi JP, et al. [Management of renal atrophy in hypertensive patients: experience in. Lille] Presse Med (Paris, Fr : 1983) 2010;39:e67–76.
Trnka P, Orellana L, Walsh M, Pool L, Borzi P. Reninoma: an uncommon cause of Renin-mediated hypertension. Front Pediatr. 2014;2:89.
Mondschein R, Kwan E, Abou-Seif C, Rajarubendra N. Accurate lesion localisation facilitates nephron sparing surgery in reninoma patients: case report and discussion. Urol Case Rep. 2022;43:102069.
Wong L, Hsu TH, Perlroth MG, Hofmann LV, Haynes CM, Katznelson L. Reninoma: case report and literature review. J Hypertens. 2008;26:368–73.
Osawa S, Hosokawa Y, Soda T, Yasuda T, Kaneto H, Kitamura T, et al. Juxtaglomerular cell tumor that was preoperatively diagnosed using selective renal venous sampling. Intern Med. 2013;52:1937–42.
Koriyama N, Kakei M, Yaekura K, Nakazaki M, Morimitsu S, Hamada H, et al. A case of renal juxtaglomerular cell tumor: usefulness of segmental sampling to prove autonomic secretion of the tumor. Am J Med Sci. 1999;318:194–7.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (Grant nos. ZR2021MH039, ZR2015HL006, 2012YD18051), Clinical Science and Technology Innovation Program (Grant no. 202019089), and Major Project of Shandong University (Grant no. qlyxjy-201830).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, C., Zheng, F., Zhang, X. et al. Selective venous sampling for secondary hypertension. Hypertens Res 47, 1766–1778 (2024). https://doi.org/10.1038/s41440-024-01699-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-024-01699-3
- Springer Nature Singapore Pte Ltd.