Abstract
Purpose
To evaluate the advantages of intraprocedural CT during adrenal venous sampling (AVS) to confirm accurate catheterization of the right adrenal vein (RAV).
Materials and Methods
This single-institution study included 106 patients (mean age 52.4 years; range 28–74 years) with primary aldosteronism who performed contrast-enhanced CT (CECT) before AVS following AVS between January 2011 and March 2018. After catheterization of the RAV under fluoroscopic guidance, unenhanced CT images were obtained to confirm catheter position on unified CT angiography system. Catheter repositioning was performed when the catheter was inaccurately positioned. Venography findings were classified into two groups: (1) presumably cannulated in the RAV (presumed RAV group) and (2) obscured visualization of the RAV because of collateral vessels (obscured RAV group). Success rates of AVS were compared using Fisher’s exact test.
Results
The overall success of AVS was achieved in 104 patients (98.1%). Catheter was deviated into the IVC during intraprocedural CT in four patients. Fourteen patients (14.0%) required catheter repositioning by intraprocedural CT images, and accurate catheterization in the RAV was eventually accomplished. The success rate of AVS was significantly higher in the presumed RAV group (90.1% [73/81]) than that in the obscured RAV group (68.4% [13/19]) (p = 0.024). If intraprocedural CT was not acquired during AVS, the success rate of AVS would have been significantly lower (84.9% [90/106]) compared with that use of intraprocedural CT (98.1% [104/106]) (p < 0.001).
Conclusions
Intraprocedural unenhanced CT by referring to the preprocedural CECT before AVS enables the confirmation of accurate catheterization of the RAV.
Level of Evidence
Level 4, case series.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Primary aldosteronism is the most common cause of secondary hypertension; its prevalence in newly diagnosed hypertensive populations is estimated to be approximately 11% [1, 2]. Patients with primary aldosteronism require early targeted treatment because they are at greater risk of experiencing cardiovascular and cerebrovascular events than patients with essential hypertension [3, 4]. It is crucial to diagnose whether hyperaldosteronism derives from the unilateral adrenal gland, because it can be cured by unilateral adrenalectomy or locolesional treatment. Using imaging examinations to detect adrenal adenoma, which potentially produces excessive aldosterone, is not reliable to determine lateralization of aldosterone secretion, because undetectable occult adenomas may cause hyperaldosteronism [5,6,7].
Adrenal venous sampling (AVS) has been thought to be the most promising examination to distinguish unilateral aldosterone-producing adenoma from bilateral hyperaldosteronism [5,6,7]. However, catheterization of the right adrenal vein (RAV) remains technically challenging, and success rates ranged from 74 to 96% even at experienced institutions [5,6,7]. Examination failure in AVS is mainly due to impossibility or inadvertent positioning of the catheter into the RAV [8]. However, venography findings have considerable limitations to confirm accurate catheterization. The RAV is small feature with large anatomical variations that enter the inferior vena cava (IVC) or proximal portion of the accessory hepatic vein in a steep angle [9, 10]. Inappropriate positioning of the catheter tip can lead to a considerable admixture of blood drawn back from the IVC or accessory hepatic vein. Therefore, exact position of the catheter tip is crucial to achieve successful AVS examination.
To achieve higher technical success for AVS, especially for the accurate catheterization of the RAV, we routinely obtain unenhanced CT images at a unified CT angiography system. The use of unenhanced CT images during AVS has advantages over the use of catheter venography alone in avoiding malposition of the catheter by referring the course and direction of the catheter on cross-sectional images. The purpose of this study was to evaluate the advantages of intraprocedural CT during AVS to confirm accurate catheterization of the RAV.
Materials and Methods
Study Population
This retrospective study was approved by the institutional review board with a waiver of written informed consent. A search of our radiological database identified 113 patients with primary aldosteronism who underwent AVS between January 2011 and March 2018. Basically, all AVS procedures were routinely performed at a unified CT angiography system to obtain unenhanced CT images. Inclusion criteria were: (1) Contrast-enhanced CT (CECT) before AVS was obtained to detect anatomical findings of the RAV; (2) intraprocedural CT was performed during AVS. Seven patients were excluded because of the following reasons: CECT before AVS could not be performed (n = 4); AVS was performed at another angiographic suite (n = 2); and data were missing (n = 1). Consequently, the final study population consisted of 106 patients (55 men, 51 women; mean age 52.4 ± 11.1 years; range 28–74 years).
CECT Before AVS
CECT before AVS was performed using a multidetector-row CT scanner (Aquilion 64 or Aquilion ONE; Toshiba Medical Systems, Otawara, Japan, and SOMATOM Force; Siemens Healthcare, Forchheim, Germany) equipped at our institution. The scanning parameter details are as follows: tube voltage, 120 or 70 kVp; detector configuration, 64 × 0.5 mm or 128 × 0.6 mm; pitch factor, 0.6 or 0.64; reconstruction thickness/interval, 0.5/0.3 mm or 0.6/0.4 mm. Dual-phase CECT images before AVS were obtained through an intravenous 20-gauge catheter in the antecubital vein with a dose of 510–600 mgI/kg of total body weight of iodinated contrast agent. Injection time was fixed at 30 s, and the injection rate was changed according to the patients’ body weights. Contrast-enhanced images were obtained 40 s and 60 s after administration of the contrast agent.
AVS Procedure
All AVS procedures were performed by at least one of the eight interventional radiologists (K.S., T.O., Y.K., E.U., R.T., T.G., H.H., and M.Y.) including three non-experts (R.T., T.G., and H.H.) for this procedure with local anesthesia using a unified CT angiography system (AREX-VC830A and Aquilion 16; Cannon Medical Systems, Otawara, Japan). The technical details of the AVS procedure were almost identical to the previously published paper [9]. Samples were obtained at five points (proximal and distal IVC, proximal and distal to the inferior phrenic vein confluence of the left adrenal vein, and the RAV) before cosyntropin stimulation (defines as baseline venous sampling) and 25–45 min after the initiation of intravenous cosyntropin stimulation (defined as post-stimulation venous sampling).
At the beginning of the AVS procedure, 7-Fr introducer sheath was inserted via the right common femoral vein. A 6.5-Fr N-shaped catheter was inserted into the left adrenal vein, and a 5- or 6.5-Fr catheter specifically designed for the RAV (Adselect series; Hanako Medical, Tokyo, Japan) was inserted into the orifice of the RAV. When performing venography with the catheter to seek the orifice of the RAV, approximately 5 mL of half diluted contrast agent (Omnipaque 350; Daiichi Sankyo, Tokyo, Japan) was loaded in a 10 mL syringe for manual injection. After the catheter was positioned at ostium of the presumed RAV by catheter venography, a 2.2-Fr microcatheter with split tip (Goldcrest microcatheter; Goldcrest Medic, Tokyo, Japan) was coaxially inserted and then intraprocedural CT was performed.
Intraprocedural unenhanced CT images were obtained to confirm accurate catheterization of the RAV after the catheterization of the presumed RAV on fluoroscopic findings. All CT images were obtained with patients’ arms alongside the body without breath-holding using the following parameters: tube voltage, 120 kV; mAs effective tube current–time product, 106.4–239.4 depending on the patients’ physique; beam collimation, 16 × 1.0 mm; rotation time, 0.5 s; helical pitch, 0.94; and slice thickness/intervals, 5.0/5.0 mm; acquisition time, 4–7 s. Automatic exposure control (AEC [Sure Exposure; Cannon Medical Systems]) was used to optimize the dose for each patient. Interventional radiologists and endocrinologist (G.I) interpreted the unenhanced CT images to assess whether the microcatheter was accurately positioned at the orifice of the RAV by referring the direction of the catheter and CECT images before AVS. When the catheter was not accurately positioned into the RAV, catheter manipulation was performed, and unenhanced CT was obtained after catheter repositioning. The time from obtaining catheter venography, table setting, acquisition of topogram and CT images, image interpretation, to venous sampling was 4 min on average. The algorithm for the AVS procedure with use of intraprocedural CT and representative case are provided in Figs. 1 and 2, respectively.
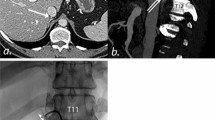
A 56-year-old man with primary aldosteronism. A Contrast-enhanced CT prior to AVS clearly depicts the anatomy of the RAV (arrow). B The catheter venogram demonstrates the right adrenal vein (RAV). C Intraprocedural CT was obtained during AVS to confirm proper catheterization of the RAV referring the direction of the catheter and the CECT images before AVS
Baseline venous sampling through the RAV was performed after confirmation of successful and accurate catheterization on unenhanced CT images. After the baseline venous sampling, 0.25 mg cosyntropin was injected via a peripheral venous line, and post-stimulation venous sampling through the RAV was obtained. During venous sampling, proper catheter positioning was verified and occurrence of catheter malpositioning was carefully checked on fluoroscopy. Success of AVS was defined as successful catheterization and venous sampling from the RAV followed by biochemical success. Biochemical success was defined as satisfying the following established criteria: a selectivity index (ratio between the adrenal veins and the distal IVC plasma cortisol concentrations) ≥ 5 after cosyntropin stimulation [11, 12].
Data Analysis
Two board-certified interventional radiologists (K.M. and K.S., with 8 and 17 years’ experience retrospectively) evaluated AVS procedure by consensus. At first, all clinical reports and intraprocedural CT images were reviewed and recorded whether catheter repositioning was required or not in each procedure. When catheter repositioning caused by inaccurate catheterization of the RAV was ascertained, the name of the vessel was recorded based on the intraprocedural CT images along with corresponding catheter venography where malpositioned catheter was inserted. Then, to clarify the reason why inaccurate catheterization occurred, catheter venography findings both from the RAV and inaccurately inserted vessel were reviewed in all cases. Venography findings were classified into two groups based on catheter venogram: (1) The catheter was presumably cannulated in the RAV (presumed RAV group) and (2) catheterization of the RAV was uncertain because collateral vessels (i.e., right hepatic vein or retroperitoneal vein) obscured certain visualization of the RAV (obscured RAV group). Retroperitoneal vein included renal capsular vein, because they could be differentiated on CT catheter venography but could not be differentiated on catheter venogram alone [13]. Representative images in each group are provided in Figs. 3 and 4.
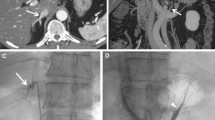
A 42-year-old woman with primary aldosteronism (classified into the “presumed RAV group”). A Contrast-enhanced CT prior to AVS depicts the anatomy of the right adrenal vein (RAV) (arrow). B The catheter venogram demonstrates that the catheter is presumably cannulated in the RAV. C. Intraprocedural CT reveals that the catheter is not accurately positioned into the RAV but into accessory hepatic vein. D The catheter venogram after catheter repositioning demonstrates that the catheter is presumably cannulated in the RAV. E Intraprocedural CT after catheter repositioning reveals accurate catheterization of the RAV with reference to the CT images before AVS
A 40-year-old woman with primary aldosteronism (classified into the “obscured RAV group”). A Contrast-enhanced CT prior to AVS clearly depicts the anatomy of the RAV (arrow). B The first catheter venogram shows that the retroperitoneal vein is visualized and catheterization of the RAV was uncertain. C Intraprocedural CT reveals that the catheter is not accurately positioned into the RAV but into the retroperitoneal vein. D The catheter venogram after catheter repositioning demonstrates that the catheter is presumably cannulated in the RAV, which communicates with retroperitoneal veins. Operator considered that the retroperitoneal veins obscure obvious visualization of the RAV. E Intraprocedural CT after catheter repositioning verifies the accurate and definite catheterization of the RAV
Statistical Analysis
Continuous variables are expressed as the mean ± standard deviation (SD) and categorical variables are presented as counts and proportions. Overall success rate of AVS was calculated from the entire study population. Success rates of AVS between the presumed RAV group and obscured RAV group were separately provided and compared using Fisher’s exact test. Then, the success rate of the entire study population was compared with the success rate that repositioning of the catheter would not have been performed using Fisher’s exact test. For the analysis, we tried to compare two situations in which catheterization of the RAV could have been confirmed by intraprocedural CT or not. Assuming that intraprocedural CT images could not have been acquired during AVS, cases of repositioning of the catheter would have resulted in technical failure.
For all statistical analyses, commercially available software (JMP 13.0; SAS Institute Japan, Tokyo, Japan) was used. A p value < 0.05 was considered as statistically significant.
Results
The results of this study are summarized in Table 1 and Fig. 5. Success of accurate catheterization of the RAV and venous sampling from the RAV, which was confirmed by biochemical success was achieved in 104 of 106 patients including four patients in whom CECT before AVS could not detect anatomical findings of the RAV (Fig. 6), yielding overall success rate of AVS as 98.1%. Successful venous sampling from the RAV could not be accomplished in two patients due to the RAV injury during catheter insertion (n = 1) and impossible cannulation into the RAV due to no detection of the RAV on CECT before AVS (n = 1). The RAV drained into the IVC either directly (independent type [n = 92]) or indirectly from the accessory hepatic vein (common-trunk type [n = 14]).
A 63-year-old woman with primary aldosteronism (classified into the “obscured RAV group”). A Contrast-enhanced CT prior to AVS cannot depict the anatomy of the RAV. B The catheter venogram demonstrates development of the retroperitoneal veins and the visualization of the RAV is unclear. C Intraprocedural CT reveals accurate catheterization of the RAV, in which the microcatheter reaches the right adrenal gland
Catheter venography could be obtained in 104 patients who underwent CT images during AVS. On the venography findings, the retroperitoneal vein was visualized in 61 patients (58.7% [61/104]), and among these 61 patients retroperitoneal veins obscured the certain visualization of the RAV in 19 patients (31.1% [19/61]). According to these findings, we classified patients into either the presumed RAV group (n = 85) or obscured RAV group (n = 19). In four (3.8%) of the 104 patients, catheter was deviated into the IVC during intraprocedural CT images because of patient’s rough breathing, and we eventually gave up acquisition of intraprocedural CT but achieved success of AVS. Of the remaining 100 cases, 14 patients (14.0%) required catheter repositioning by intraprocedural CT images. Malpositioned catheters were inserted into the accessory hepatic vein (n = 7) and retroperitoneal vein (n = 7), as confirmed on the intraprocedural CT images. In the presumed RAV group, catheters were accurately inserted into the RAV in 73 patients and the remaining 8 patients were inaccurately catheterized in the accessory hepatic vein (n = 7) and retroperitoneal vein (n = 1). In the obscured RAV group, 13 patients were accurately catheterized in the RAV, whereas inaccurate catheterization into retroperitoneal vein occurred in the remaining six patients. Thus, the success rate of AVS was significantly higher in the presumed RAV group (90.1% [73/81]) than that in the obscured RAV group (68.4% [13/19]) (p = 0.024).
In all 14 patients who underwent catheter repositioning, accurate catheterization into the RAV was eventually accomplished, which was confirmed by intraprocedural CT images followed by biochemical success. If intraprocedural CT images could not have been performed during AVS, success rate of AVS would have been significantly lower (84.9% [90/106]) compared to the procedure with use of intraprocedural CT images (98.1% [104/106]) (p < 0.001). For the additional radiation exposure, mean volume CT dose index and dose length product for each intraprocedural CT examination were 29.5 ± 5.6 mGy (range 12.4–49.9 mGy) and 452.1 ± 122.7 mGycm (range 161.2–868.9 mGycm).
Discussion
The present study evaluated the utility of intraprocedural unenhanced CT images to confirm accurate catheterization of the RAV. This procedure enables inadvertent catheter repositioning and confirms accurate catheterization in the RAV. Additionally, the success rate of AVS with the use of intraprocedural CT in combination with venography guidance was significantly higher than that with the use of venography guidance alone. These findings indicate that intraprocedural unenhanced CT is an effective technique to increase the technical success of AVS. Onozawa et al. [14] also reported the usefulness of intraprocedural CT, and we investigated the utility of combination use of CECT before AVS for the reference images. Furthermore, we examined to clarify the reason why inaccurate catheterization occurred on catheter venography findings from intraprocedural CT images.
Catheter venography is primarily used to check the accurate catheterization of the RAV, and various venography findings have been reported [8]. However, a small accessory hepatic vein sometimes mimics the RAV, which may increase the incidence of inaccurate catheterization [13, 15]. Substantially developed retroperitoneal veins may obscure the precise visualization of the RAV. In the present study, we tried to clarify the cause of inaccurate catheterization based on catheter venography. The development of the retroperitoneal veins obscured the RAV in 18% of the patients (19/104), and considerable visualization of developed retroperitoneal veins was main cause of inaccurate catheterization in 32% of these patients. Meanwhile, even when the catheter was presumed to be cannulated into the RAV, the catheter was inaccurately cannulated in 10% of the cases, which was mostly catheterized in the accessory hepatic vein.
In our study, the biochemical success of AVS accomplished in all patients who achieved technical success, and finally 98% overall success rate of AVS was achieved. This substantial success rate was mainly because the use of intraprocedural CT in combination with catheter venography confirmed the accurate position of the catheter tip. Although the exact position of the catheter tip cannot be confirmed through catheter venography alone, the unified CT angiography system can readily acquire cross-sectional images without transport of patients from the angiography suite to the CT scanner. Recently, CECT has been able to visualize the precise anatomy of the RAV in 93–98% of the patients prior to AVS [9, 10]. Intraprocedural CT could confirm the precise position and direction of the catheter with high confidence by referring to the CECT before AVS, and the catheter position could be adjusted if inadvertent catheter positioning was confirmed. Consequently, the success rate would have improved from 85 to 98% by adding to the intraprocedural CT during AVS in our study, which concurs with previous paper [14].
The advantage of intraprocedural CT during AVS is that it keeps overall success rate to avoid inadvertent catheterization and biochemical failure, even when CECT before AVS could not detect the anatomical finding of the RAV or non-experts performed AVS procedure. Additionally, intraprocedural CT may be helpful for non-experts to accelerate their learning curve for AVS procedure by confirming whether the cannulated vessels were the RAV or not. On the other hand, routine use of intraprocedural CT during AVS would increase radiation exposure and procedure time. Moreover, four patients (4%) were exposed to redundant radiation exposure because of examination failure caused by catheter deviation. In experienced institutions, overall success rate of AVS achieved more than 90% only with use of catheter venography [9]. Therefore, intraprocedural CT may be conducted only for complicated cases that catheterization of the RAV cannot be confirmed by catheter venography alone or CECT before AVS cannot detect precise anatomical findings of the RAV, which warrant further evaluation.
Several techniques have been reported to improve technical success rate of AVS, particularly in confirming accurate catheterization in the RAV. Intraprocedural cortisol assay has contributed to a high success rate of AVS, which increased from 73 to 97% after incorporating rapid cortisol assay [16, 17]. However, it requires specific equipment and a substantial increase of overall examination time ranging from 20 min to more than 1 h. Another technique is a C-arm CT catheter venography which attempts to verify accurate catheterization into the RAV with manual injection of the contrast agent through the inserted catheter [13]. C-arm CT catheter venography detected catheter malpositioning in 10% of the cases and accomplished 95% technical success rate. However, this technique risks such as RAV injury, dislodgement of the catheter caused by blind injection of contrast agent, and radiation exposure for the operator. Additionally, examination variability based on operators’ experience may affect visualization of the RAV, although gentle manual injection of the 25% diluted contrast agent achieved high technical success without any complication in the previous study [13].
C-arm CT can be used as an alternative method to confirm catheterization of the RAV instead of unified CT angiography system. C-arm CT had generally several drawbacks compared with unified CT angiography system, such as image degradation caused by several artifacts, longer breath-hold, necessity to raise arms above the head [18]. The unified CT angiography system could keep image quality even by the patient’s arms alongside the body without breath-holding as our study did. The recent C-arm CT system has addressed limitations, including image degradation caused by several artifacts and longer acquisition time, and future studies are warranted for the confirmation of the RAV catheterization by using C-arm CT system [19, 20]. Importantly, unified CT angiography system has drawbacks such as higher radiation exposure to the patients as well as more expensive equipment [18, 19].
Some limitations must be considered. This study was conducted by retrospective design at a single institution, and AVS procedure was performed by several interventional radiologists. Second, direct comparison between AVS with and without intraprocedural CT, including overall radiation exposure and procedure time, could not be performed because unenhanced CT images were routinely obtained at our institution. Alternatively, we evaluated additional radiation exposure and examination time by obtaining intraprocedural CT. Future comparative study may be needed to elucidate whether intraprocedural CT would decrease or increase the amount of radiation overall by limiting the number of technical failures, in which the procedure would have to be repeated. Finally, the clinical impact of accurate diagnosis to distinguish unilateral from bilateral hyperaldosteronism has not been determined, which warrants further studies to clarify the clinical utility for introducing intraprocedural CT to AVS.
In conclusion, intraprocedural unenhanced CT images on unified CT angiography system enable the confirmation of accurate catheterization of the RAV by referring to the CECT before AVS. This simple and safe technique contributes to increase technical success of AVS by preventing inadvertent catheterization and biochemical failure.
References
Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–300.
Douma S, Petidis K, Doumas M, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet. 2008;371:1921–6.
Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62:331–6.
Mulatero P, Monticone S, Bertello C, et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98:4826–33.
Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151:329–37.
Nishikawa T, Omura M, Satoh F, et al. Guidelines for the diagnosis and treatment of primary aldosteronism—the Japan Endocrine Society 2009. Endocr J. 2011;58:711–21.
Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment—an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–916.
Daunt N. Adrenal vein sampling: how to make it quick, easy, and successful. Radiographics. 2005;25(Suppl 1):S143–58.
Omura K, Ota H, Takahashi Y, et al. Anatomical variations of the right adrenal vein: concordance between multidetector computed tomography and catheter venography. Hypertension. 2017;69:428–34.
Morita S, Nishina Y, Yamazaki H, Sonoyama Y, Ichihara A, Sakai S. Dual adrenal venous phase contrast-enhanced MDCT for visualization of right adrenal veins in patients with primary aldosteronism. Eur Radiol. 2016;26:2073–7.
Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136:1227–35.
Satoh F, Abe T, Tanemoto M, et al. Localization of aldosterone-producing adrenocortical adenomas: significance of adrenal venous sampling. Hypertens Res. 2007;30:1083–95.
Park SI, Rhee Y, Lim JS, et al. Right adrenal venography findings correlated with C-arm CT for selection during C-arm CT-assisted adrenal vein sampling in primary aldosteronism. Cardiovasc Interv Radiol. 2014;37:1469–75.
Onozawa S, Murata S, Tajima H, et al. Evaluation of right adrenal vein cannulation by computed tomography angiography in 140 consecutive patients undergoing adrenal venous sampling. Eur J Endocrinol. 2014;170:601–8.
Chang CC, Lee BC, Chang YC, Wu VC, Huang KH, Liu KL. Comparison of C-arm computed tomography and on-site quick cortisol assay for adrenal venous sampling: a retrospective study of 178 patients. Eur Radiol. 2017;27:5006–14.
Auchus RJ, Michaelis C, Wians FHJ, et al. Rapid cortisol assays improve the success rate of adrenal vein sampling for primary aldosteronism. Ann Surg. 2009;249:318–21.
Reardon MA, Angle JF, Abi-Jaoudeh N, et al. Intraprocedural cortisol levels in the evaluation of proper catheter placement in adrenal venous sampling. J Vasc Interv Radiol. 2011;22:1575–80.
Tanaka T, Arai Y, Inaba Y, et al. Current role of hybrid CT/angiography system compared with C-arm cone beam CT for interventional oncology. Br J Radiol. 2014;87:20140126.
Bai M, Liu B, Mu H, Liu X, Jiang Y. The comparison of radiation dose between C-arm flat-detector CT (DynaCT) and multi-slice CT (MSCT): a phantom study. Eur J Radiol. 2012;81:3577–80.
Loffroy R, Lin M, Rao P, et al. Comparing the detectability of hepatocellular carcinoma by C-arm dual-phase cone-beam computed tomography during hepatic arteriography with conventional contrast-enhanced magnetic resonance imaging. Cardiovasc Intervent Radiol. 2012;35:97–104.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in the study.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
This retrospective study design was approved by our institutional review board. For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Maruyama, K., Sofue, K., Okada, T. et al. Advantages of Intraprocedural Unenhanced CT During Adrenal Venous Sampling to Confirm Accurate Catheterization of the Right Adrenal Vein. Cardiovasc Intervent Radiol 42, 542–551 (2019). https://doi.org/10.1007/s00270-018-2135-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-018-2135-5