Abstract
Amyloid-β (Aβ) accumulation in the brain is a pivotal event in the pathogenesis of Alzheimer’s disease (AD), and its clearance from the brain is impaired in sporadic AD. Previous studies suggest that approximately half of the Aβ produced in the brain is cleared by transport into the periphery. However, the mechanism and pathophysiological significance of peripheral Aβ clearance remain largely unknown. The kidney is thought to be responsible for Aβ clearance, but direct evidence is lacking. In this study, we investigated the impact of unilateral nephrectomy on the dynamic changes in Aβ in the blood and brain in both humans and animals and on behavioural deficits and AD pathologies in animals. Furthermore, the therapeutic effects of the diuretic furosemide on Aβ clearance via the kidney were assessed. We detected Aβ in the kidneys and urine of both humans and animals and found that the Aβ level in the blood of the renal artery was higher than that in the blood of the renal vein. Unilateral nephrectomy increased brain Aβ deposition; aggravated AD pathologies, including Tau hyperphosphorylation, glial activation, neuroinflammation, and neuronal loss; and aggravated cognitive deficits in APP/PS1 mice. In addition, chronic furosemide treatment reduced blood and brain Aβ levels and attenuated AD pathologies and cognitive deficits in APP/PS1 mice. Our findings demonstrate that the kidney physiologically clears Aβ from the blood, suggesting that facilitation of Aβ clearance via the kidney represents a novel potential therapeutic approach for AD.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD), the most common neurodegenerative disease, imposes a heavy and growing burden on patients and the society [1]. Amyloid-β (Aβ) deposition in the brain is the main pathological hallmark of AD [2]. It has been proposed that abnormal Aβ accumulation in the brain plays a pivotal role in disease pathogenesis and causes neurodegeneration, neuroinflammation, impaired neuronal function, and ultimately cognitive decline in AD [3, 4]. Dysfunction of Aβ clearance is regarded as the main reason for Aβ accumulation in sporadic AD, which accounts for 99% of all AD cases [5]. Hence, understanding the mechanism of Aβ clearance from the brain is crucial for the clinical management of AD and the development of therapeutics for AD [6].
Previous studies on Aβ clearance have focused on the brain, including Aβ degradation by proteases and phagocytosis by microglia and astrocytes [7]. However, brain-derived Aβ is also transported to the peripheral blood via the blood--brain barrier (BBB), cerebrospinal fluid (CSF) absorption in arachnoid granulations, and recently identified lymphatic vessels [8,9,10,11]. It has been suggested that approximately 40– 60% of brain-derived Aβ is cleared through transport into the periphery [12,13,14]. However, where and how these brain-derived Aβ peptides are cleared in the periphery remains unclear. Elucidation of Aβ clearance in the periphery will provide novel insight into the pathogenesis of AD and present new opportunities for systemic interventions [8, 15].
The kidney is an important metabolic organ that excretes metabolites and maintains homeostasis. The kidney is thought to be responsible for clearing Aβ from the blood [7]. However, direct evidence of the physiological Aβ clearance ability of the kidney is lacking, and the pathophysiological roles of the kidney in AD pathogenesis remain unknown. In this study, we explored the physiological contribution of the kidney in clearing Aβ from the blood and brain, evaluated the role of impaired kidney-mediated Aβ clearance in AD pathogenesis, and explored the therapeutic potential of kidney-mediated Aβ clearance for AD.
Materials and methods
This study was approved by the Institutional Review Board of Daping Hospital, Third Military Medical University, Chongqing, China. A total of 17 kidney donors who had undergone unilateral nephrectomy surgery over three years and age- and sex-matched controls with normal kidney and cognitive functions (1:3) were enrolled in the study, and written consent was obtained from the participants (Table S1). Aβ levels in human blood and urine were detected with an ultrasensitive single-molecule array (SIMOA) [16]. Kidney puncture specimens from five subjects with normal renal function were obtained. The presence of Aβ in the kidney was detected by immunohistochemistry and immunofluorescence staining.
Three-month-old female rabbits were used to investigate the differences in Aβ levels between the renal artery and renal vein. Blood from the renal artery, blood from the renal vein, and urine were collected. APPswe/PSEN1dE9 transgenic mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Female mice were used in our study to eliminate the potential impacts of sex on AD pathologies [17]. To investigate the chronic impacts of Aβ clearance by the kidney on AD pathologies and behavioural performance, 3-month-old AD mice were subjected to unilateral nephrectomy of the left kidney and analysed at 9 months of age. To investigate the acute influence of Aβ clearance by the kidney on the dynamics of Aβ in the blood and brain, 6-month-old AD mice were subjected to ligation of the renal artery, vein, and ureter, and Aβ levels in the blood and interstitial space fluid (ISF) were monitored for 6 h. To evaluate the therapeutic potential of the diuretic drug furosemide on AD, the acute effects of furosemide on Aβ levels in the blood and urine were assessed, and the chronic effects of furosemide on 8-month-old AD mice were examined by intraperitoneally injecting furosemide (40 mg/kg) every 4 days for 1 month. To assess the behavioural performance of the mice, the Y-maze test, open-field test, and Morris water maze were performed as previously described [18]. Aβ burden, neuroinflammation, neurodegeneration, and tau phosphorylation in AD mice were measured by immunohistochemistry, double immunofluorescence staining, and ELISA. Sections of mouse kidneys were stained using immunohistochemistry and double immunofluorescence.
The data are expressed as the mean ± SEM. All analyses were performed using SPSS 20.0 software (Chicago, USA). Comparisons between two groups were made by two-tailed Student’s t-test or paired t-test as deemed appropriate. Comparisons among multiple groups were made by one-way ANOVA followed by the least significant difference (LSD) test. Normality and equal-variance testing were performed for all assays. A p-value < 0.05 was considered statistically significant. All figures were plotted using GraphPad Prism software (San Diego, USA).
Detailed information on the materials and methods is presented in the Supplementary Information.
Results
The kidney physiologically clears Aβ from the blood
We first investigated whether the kidney physiologically clears Aβ from the blood. Aβ accumulation was detected with anti-Aβ antibody 6E10 in the renal tubules (megalin-positive) of both humans and AD mice (Fig. 1A, B, D, and E). Staining of conformational Aβ aggregates was used to differentiate Aβ accumulation from the local expression of amyloid precursor protein (APP) in the kidney. Staining with an antibody specific to Aβ aggregates showed positive signals in the renal tubules of both human and AD mice (Fig. S1A). Aβ peptides were also detected in the kidney homogenates of AD mice but not WT mice in Western blotting analysis (Fig. S1B). These findings imply that there is Aβ accumulation in AD kidney.
A Immunohistochemical staining of the kidneys of NCs with or without anti-Aβ antibody (6E10). B Representative images of Aβ deposition in the kidneys of humans, as detected by immunofluorescence staining with antibodies against megalin (green) and 6E10 (red) and DAPI (blue). The arrows indicate Aβ staining in renal tubules. C Aβ levels in the urine of humans (n = 19). D 6E10 immunostaining in the kidneys of 9-month-old WT and AD mice. E Representative images of Aβ deposition in the kidneys of mice, as detected by immunofluorescence staining with antibodies against megalin (green) and 6E10 (red) and DAPI (blue). F Schematic diagram of kidney blood and urine sample collection from rabbits. G Comparison of Aβ levels in the renal arteries (RAs) and renal veins (RVs) of rabbits. Paired t-test, n = 8 per group. H Aβ levels in the urine of rabbits. Scale bar = 50 µm. *p < 0.05. The error bars represent the SEMs.
Next, Aβ42 but not Aβ40 was detected in human urine by a SIMOA (Fig. 1C). We further examined the differences in Aβ levels between the renal artery and renal vein in rabbits, as the sequence of Aβ in rabbits is identical to that in humans and because it was practical to collect blood from the renal vessels of rabbits. We found that the blood levels of Aβ42 and Aβ40 in the renal vein were 20 and 11% lower than those in the renal artery (Fig. 1F, G), and both Aβ40 and Aβ42 were also detected in the urine (Fig. 1H), suggesting that Aβ in the blood is removed when the blood flows through the kidney. These findings indicate that the kidney physiologically removes Aβ from the blood.
Acute kidney ligation increases blood and ISF Aβ levels in AD mice
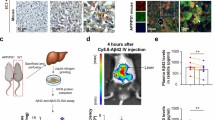
Next, we examined the impact of the kidney on the dynamic changes in Aβ clearance in the blood and brain ISF. The left kidneys of 6-month-old AD mice were ligated, and Aβ levels in the blood and ISF were monitored using microdialysis. The levels of Aβ40 and Aβ42 in the blood increased rapidly after kidney ligation (Fig. 2A, B). Importantly, the levels of Aβ40 and Aβ42 in the ISF were also elevated in parallel with the increase in Aβ in the blood. Aβ levels in the ISF were correlated with those in the blood (Fig. 2C). These results suggest that the kidney plays a critical role in regulating the dynamics of Aβ levels in the blood and brain.
A, B Levels of Aβ40 and Aβ42 in the plasma and ISF of AD mice before and after kidney ligation. Unpaired t-test, n = 4 per group. C Correlation between the changes in Aβ levels in the ISF and the blood of AD mice from both groups at 6 h after kidney ligation. KL: kidney ligation. Pearson’s correlation, n = 8 per group. *p < 0.05, **p < 0.01, ***p < 0.001. The error bars represent the SEMs.
Unilateral nephrectomy aggravates brain Aβ burden
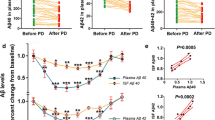
We tested the chronic effects of nephrectomy on Aβ levels in the blood and brain. First, we found that kidney donors with unilateral nephrectomy had higher blood Aβ levels and lower urine Aβ levels than age- and sex-matched cognitively normal controls (CNs) (Fig. 3A, B). Both kidney donors and CNs had normal levels of creatinine (kidney donors vs. CNs, 63.75 vs. 62.01 μmol/L, p = 0.698) in the blood (Table S1), suggesting that the general kidney functions of the kidney donors were normal.
A Schematic diagram of the unilateral nephrectomy donor. B Aβ levels in the plasma and urine of kidney donors and cognitively normal controls. Paired t-test, n = 17 per group. C Schematic diagram of unilateral nephrectomy in AD mice. D Aβ levels in the blood of nephrectomized mice and sham mice. Unpaired t-test, n = 8 per group. E, F Immunostaining and quantification of Aβ plaques stained with 6E10 and Congo red in the neocortices and hippocampi of 9-month-old nephrectomized mice and sham mice. Unpaired t-test, n = 8 per group. G Comparison of Aβ40 and Aβ42 levels in the TBS, SDS, and FA fractions of brain homogenates between nephrectomized mice and sham mice. Unpaired t-test, n = 7 per group. KD kidney donors, CN cognitively normal control, Uni-Nx unilateral nephrectomy. *p < 0.05, **p < 0.01, ***p < 0.001, ns denotes nonsignificant. Scale bar = 1 mm. The error bars represent the SEMs.
Next, we found that 6 months after unilateral nephrectomy (Fig. 3C), the levels of both Aβ40 and Aβ42 in the blood of nephrectomized mice were higher than those in the blood of controls (Fig. 3D). There were no differences in blood creatine levels between the two groups (Fig. S2A). Compared with the control mice, AD mice that underwent nephrectomy had higher levels of Aβ in the Tris-based buffer solution (TBS), sodium dodecyl sulfate (SDS), and formic acid (FA) fractions of brain homogenates and a higher area fraction and number of both compact plaques (Congo red-positive) and total plaques (6E10 antibody-immunopositive) in the neocortex and hippocampus (Fig. 3E–G).
Furthermore, we investigated the changes in Aβ metabolism in the brain after nephrectomy. Compared with control mice, AD mice subjected to nephrectomy had higher levels of CTF-α, CTF-β, Aβ, BACE1, and the Aβ-degradation enzyme neprilysin (NEP) in brain homogenates. There were no differences in the levels of APPfl, sAPPα/β, ADAM10, Aβ-degradation enzyme insulin-degrading enzyme (IDE), or Aβ transporters on the blood--brain barrier (BBB), including low-density lipoprotein receptor-related protein 1 (LRP1) and receptor for advanced glycation end products (RAGE), between the two groups (Fig. S3A, B). These results suggest there were changes in the Aβ metabolism in the brain after nephrectomy, such as increased APP processing via both the amyloidogenic and nonamyloidogenic pathways and elevated Aβ degradation.
Unilateral nephrectomy aggravates neuroinflammation and neurodegeneration
Compared with the control mice, AD mice subjected to nephrectomy had higher levels of microgliosis (Iba1 staining) and astrogliosis (GFAP staining) in the neocortex and hippocampus (Fig. S4A, B), as well as higher levels of pro-inflammatory cytokines, including TNF-α and IFN-γ, and lower levels of the anti-inflammatory cytokine IL-10 in brain homogenates (Fig. S4C). There were no differences in the levels of these pro-inflammatory and anti-inflammatory cytokines in the blood between the two groups (Fig. S2B). In addition, the blood levels of klotho, an anti-ageing protein mainly generated by the kidney, were not changed after unilateral nephrectomy (Fig. S2C).
Compared with sham mice, AD mice subjected to nephrectomy had higher levels of neuronal apoptosis (cleaved Caspase-3 staining) and neuronal loss (MAP-2 staining) in the hippocampus (Fig. S4D, E). Nephrectomized AD mice also had higher levels of tau phosphorylation in the brain, as reflected by the elevated levels of phosphorylated Thr231 and Ser199, relative to sham mice (Fig. S4F). The pS9-Gsk3β level was also increased after nephrectomy, and no differences were detected in the levels of total Tau (Tau5) and Gsk3β between the two groups (Fig. S4G), suggesting that the elevation of phosphorylated tau was due to the increased phosphorylation of tau, not the increased expression of tau protein.
These findings indicate that unilateral nephrectomy aggravates neuroinflammation, neurodegeneration, and tau phosphorylation in the brains of AD mice.
Unilateral nephrectomy aggravates cognitive impairments
Age- and sex-matched wild-type mice performed better than AD mice subjected to nephrectomy and non-nephrectomized AD mice in the novel arm test, open-field test, and Morris water maze test. Compared with sham AD mice, AD mice subjected to nephrectomy spent less time in the novel arm and exhibited a lower percentage of spontaneous alternations in the spontaneous alternation test (Fig. 4A, B). Moreover, AD mice subjected to nephrectomy exhibited less rearing and travelled a shorter distance in the open-field test than sham AD mice (Fig. 4C, D). AD mice that underwent nephrectomy also performed worse in the Morris water maze than sham AD mice, as reflected by a longer distance travelled and a longer escape latency in the platform trial and less time spent in the target quadrant in the probe trial than sham mice and wild-type mice (Fig. 4E, F). No differences were observed in swimming speed among the three groups. These results indicate that unilateral nephrectomy aggravates cognitive impairments in AD mice.
A, B Novel arm entries, time spent in the novel arm, and percentage of alternations in the Y-maze test. C, D Rearing and tracing graphs of wild-type, nephrectomized mice, and sham mice in the open-field test. E, F Latency from day 1 to day 5, time spent in the platform quadrant and distance traveled in the target quadrant by wild-type, nephrectomized, and sham mice in the Morris water maze. WT wild-type, Uni-Nx unilateral nephrectomy. One-way ANOVA, n = 8 per group. *p < 0.05, **p < 0.01, ****p < 0.0001, ns denotes nonsignificant. The error bars represent the SEMs.
Effects of furosemide treatment on Aβ levels and cognition
To evaluate the therapeutic potential of enhancing Aβ clearance via the kidney for AD, AD mice were treated with furosemide, which is a diuretic. In the acute test, the levels of both Aβ40 and Aβ42 in the blood of -month-old AD mice were significantly reduced after peritoneal injection of furosemide, suggesting that furosemide is able to promote Aβ clearance via the kidney (Fig. 5A).
A Aβ levels in the blood of AD mice before and after acute furosemide treatment. Paired t-test, n = 8 per group. B Aβ levels in the blood of AD mice before and after chronic furosemide treatment. Unpaired t-test, n = 8 per group. C, D Performance of wild-type and AD mice treated with or without furosemide in the Morris water maze. E Rearing by wild-type and AD mice treated with or without furosemide in the open-field test. F, G Percentage of alternations and novel arm entries in the Y-maze test. One-way ANOVA, n = 8 per group. H and I. Immunostaining and quantification of Aβ plaques stained with 6E10 and Congo red in the neocortices and hippocampi of 9-month-old AD mice treated with or without furosemide. Unpaired t-test, n = 8 per group. Scale bar = 1 mm. WT wild-type, Furo furosemide, Ctr control mice, Inj injection. *p < 0.05, **p < 0.01, ****p < 0.0001. The error bars represent the SEMs.
Next, 8-month-old AD mice were treated with furosemide for 1 month. Chronic furosemide treatment lowered Aβ40 and Aβ42 levels in the blood at different time points (Fig. 5B). Importantly, AD mice treated with furosemide performed better in the Morris water maze, open-field test, and Y maze test than control AD mice (Fig. 5C, D). As expected, the cognitive functions of control AD mice were impaired compared with those of wild-type mice. Notably, the impairment of learning in the Y-maze was also alleviated by furosemide treatment (Fig. 5F, G). The improvement in behavioural performance was in accordance with the attenuation of pathological changes, as the number of both total Aβ plaques and compact plaques was reduced in the neocortices and hippocampi of AD mice after chronic furosemide treatment (Fig. 5H, I). These findings suggest that enhancement of Aβ clearance via the kidney has therapeutic potential to alleviate AD-type pathologies and rescue cognitive impairments in AD mice.
Compared with the control mice, AD mice treated with furosemide had lower levels of astrogliosis (GFAP staining) in the neocortex and hippocampus (Fig. S5A). The furosemide treatment group also had lower levels of neuronal apoptosis (cleaved Caspase-3 staining) and neuronal loss (MAP-2 staining) in the hippocampus than the control group (Fig. S5B, C). Hence, furosemide treatment alleviates astrogliosis and neurodegeneration in the brains of AD mice.
Discussion
Previous studies revealed that approximately half of the Aβ produced in the brain is cleared through transport into the periphery; however, it remains unclear where in the periphery these Aβ peptides produced by the brain are cleared [7]. The kidney is thought to be the main organ for Aβ clearance; however, its functions in Aβ clearance and contributions to the removal of accumulated Aβ in the brain remain largely unknown.
Whether Aβ can be detected in human urine remains unclear [19]. In the present study, we not only detected Aβ in the urine but also observed Aβ deposition in the kidneys of both humans and AD mice. Most importantly, Aβ levels in the renal vein were much lower than those in the renal artery, suggesting that Aβ in the blood is cleared when it circulates through the kidney. Our evidence clearly demonstrates that the kidney physiologically clears Aβ from the blood. Furthermore, we found that Aβ levels in the ISF and blood increased immediately after unilateral kidney ligation, indicating that the kidney physiologically regulates Aβ levels in both the blood and brain. Our findings suggest that there is a regulatory brain--blood--kidney axis of Aβ homeostasis.
However, the mechanism of renal Aβ excretion remains to be elucidated. An interesting finding of our study is that Aβ accumulates in renal tubular epithelial cells. It remains unknown whether this accumulated Aβ in the renal tubules is derived from the blood for excretion into the urine or is absorbed from the urine. Previous studies suggested that protein uptake in the renal tubule is mainly mediated by two receptors, megalin and cubilin, which are expressed in renal tubular epithelial cells [20]. Megalin (also known as LRP-2) has been suggested to transport Aβ from the brain to the blood across the BBB [21]. In our study, Aβ accumulated in megalin-positive renal tubular epithelial cells in both humans and AD mice, suggesting that megalin may absorb Aβ from the urine. In addition, the Aβ-degrading enzyme NEP was highly expressed in the kidney, and Aβ might also be degraded in the kidney. Our findings support the potential role of the kidney in physiological Aβ clearance. However, the specific mechanism of Aβ clearance via the kidney, as well as its pathophysiological significance in the pathogenesis of AD, needs to be investigated in the future [22].
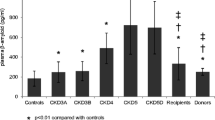
Whether a reduction in the Aβ clearance function of the kidney contributes to the pathogenesis of AD remains unknown. Our previous study found that chronic kidney disease (CKD) is associated with increased blood levels of Aβ [23]. Aβ deposition has been detected in the brains of patients with CKD [24]. In addition, subjects with unilateral nephrectomy (donors for kidney transplantation) show higher plasma Aβ levels than cognitively normal controls [25], suggesting that kidney dysfunction might lead to Aβ accumulation in the brain. In addition, renal function decreases with ageing [26]. Since ageing is consistently one of the most important pathogenic factors of AD, dysfunction of Aβ clearance by the kidney may play a critical role in the progression of AD [22]. To test this hypothesis, we generated a chronic unilateral nephrectomy model in AD mice. We found that Aβ levels in the brains of AD mice increased by approximately 20% 6 months after unilateral nephrectomy and that this change was accompanied by the aggravation of neuroinflammation, neurodegeneration, and cognitive deficits. Unilateral nephrectomy did not increase the blood levels of inflammatory cytokines, suggesting that peripheral inflammation is unlikely to be the main reason for the increased brain Aβ burden. The blood levels of klotho, an anti-ageing protein mainly generated by the kidney in the periphery [27], were not changed after unilateral nephrectomy. These findings further support the critical role of the kidney in clearing Aβ and maintaining homeostasis of the brain environment. Given that kidney function declines with age and that kidney dysfunction is prevalent in elderly people with high levels of amyloidosis [28], it is probable that dysfunction of Aβ clearance via the kidney may participate in the pathogenesis of AD [8, 22].
Clearance of Aβ from the brain is a major therapeutic strategy for AD [7]. The traditional therapeutic approach is to enhance Aβ clearance mechanisms in the brain, such as by introducing reagents into the brain or enhancing local Aβ degradation and phagocytosis [7]. This approach needs to overcome the obstacle of the BBB and is likely associated with potential adverse effects [29, 30]. Previously, we found that enhancing physiological Aβ clearance in the periphery is able to reduce brain Aβ deposition [14, 31]. Indeed, peritoneal dialysis can effectively reduce brain Aβ deposition and attenuate AD-type pathologies and cognitive deficits in AD mice [31], and haemodialysis is able to reduce Aβ plaques in the brains of CKD patients [24]. A recent clinical trial suggested that plasma exchange with albumin replacement can slow cognitive and functional decline in AD [32]. Although these methods of removing Aβ from the blood show therapeutic benefits for AD, they are not desirable for long-term use, as AD is a chronic disease [33]. In this regard, our findings show that chronic use of the diuretic furosemide can reduce the Aβ burden and attenuate neurodegeneration and cognitive deficits, suggesting that improving renal Aβ clearance might be a promising and favourable strategy for the prevention and treatment of AD. A previous clinical study demonstrated that the use of diuretics is associated with a reduced risk of AD [34], indicating that enhanced kidney-mediated Aβ excretion and clearance may ameliorate AD pathology in the brain and showing the therapeutic potential of targeting the kidney for AD.
In conclusion, our study revealed that the kidney has a physiological Aβ clearance function and plays critical roles in regulating Aβ levels in the blood and brain. Dysfunction of Aβ clearance via the kidney may be involved in the development of AD. Our findings suggest that AD might be a systemic disorder and that enhancement of Aβ clearance via the kidney represents a novel therapeutic approach for AD [15].
References
Alzheimer’s Association . 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020. https://doi.org/10.1002/alz.12068.
Braak H, Del, Trecidi K. Neuroanatomy and pathology of sporadic Alzheimer’s disease. Adv Anat Embryol Cell Biol. 2015;215:1–162.
Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–5.
Beyreuther K, Masters CL. Amyloid precursor protein (APP) and beta A4 amyloid in the etiology of Alzheimer’s disease: precursor-product relationships in the derangement of neuronal function. Brain Pathol. 1991;1:241–51.
Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774.
Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid-beta targeting therapies for Alzheimer disease. Nat Rev Neurol. 2019;15:73–88.
Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11:457–70.
Wang J, Gu BJ, Masters CL, Wang YJ. A systemic view of Alzheimer disease - insights from amyloid-beta metabolism beyond the brain. Nat Rev Neurol. 2017;13:612–23.
Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2009;8:16–30.
Sweeney MD, Zlokovic BV. A lymphatic waste-disposal system implicated in Alzheimer’s disease. Nature. 2018;560:172–4.
Cheng Y, Wang YJ. Meningeal lymphatic vessels: a drain of the brain involved in neurodegeneration? Neurosci Bull. 2020;36:557–60.
Qosa H, Abuasal BS, Romero IA, Weksler B, Couraud PO, Keller JN, et al. Differences in amyloid-beta clearance across mouse and human blood-brain barrier models: kinetic analysis and mechanistic modeling. Neuropharmacology. 2014;79:668–78.
Yuede CM, Lee H, Restivo JL, Davis TA, Hettinger JC, Wallace CE, et al. Rapid in vivo measurement of beta-amyloid reveals biphasic clearance kinetics in an Alzheimer’s mouse model. J Exp Med. 2016;213:677–85.
Xiang Y, Bu XL, Liu YH, Zhu C, Shen LL, Jiao SS, et al. Physiological amyloid-beta clearance in the periphery and its therapeutic potential for Alzheimer’s disease. Acta Neuropathol. 2015;130:487–99.
Sun BL, Chen Y, Fan DY, Zhu C, Zeng F, Wang YJ. Critical thinking on amyloid-beta-targeted therapy: challenges and perspectives. Sci China Life sci. 2020. https://doi.org/10.1007/s11427-020-1810-y.
Verberk IMW, Slot RE, Verfaillie SCJ, Heijst H, Prins ND, van Berckel BNM, et al. Plasma amyloid as prescreener for the earliest Alzheimer pathological changes. Ann Neurol. 2018;84:648–58.
Jiao SS, Bu XL, Liu YH, Zhu C, Wang QH, Shen LL, et al. Sex dimorphism profile of Alzheimer’s disease-type pathologies in an APP/PS1 mouse model. Neurotox Res. 2016;29:256–66.
Jiao SS, Yao XQ, Liu YH, Wang QH, Zeng F, Lu JJ, et al. Edaravone alleviates Alzheimer’s disease-type pathologies and cognitive deficits. Proc Natl Acad Sci USA. 2015;112:5225–30.
Ghiso J, Calero M, Matsubara E, Governale S, Chuba J, Beavis R, et al. Alzheimer’s soluble amyloid beta is a normal component of human urine. FEBS Lett. 1997;408:105–8.
Nielsen R, Christensen EI, Birn H. Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease. Kidney Int. 2016;89:58–67.
Dietrich M, Antequera D, Pascual C, Castro N, Bolos M, Carro E. Alzheimer’s disease-like impaired cognition in endothelial-specific megalin-null mice. J Alzheimer’s Dis. 2014;39:711–7.
Stanciu GD, Ababei DC, Bild V, Bild W, Paduraru L, Gutu MM, et al. Renal Contributions in the pathophysiology and neuropathological substrates shared by chronic kidney disease and Alzheimer’s disease. Brain Sci. 2020;10:563.
Liu YH, Xiang Y, Wang YR, Jiao SS, Wang QH, Bu XL, et al. Association between serum amyloid-beta and renal functions: implications for roles of kidney in amyloid-beta clearance. Mol Neurobiol. 2015;52:115–9.
Sakai K, Senda T, Hata R, Kuroda M, Hasegawa M, Kato M, et al. Patients that have undergone hemodialysis exhibit lower amyloid deposition in the brain: evidence supporting a therapeutic strategy for Alzheimer’s disease by removal of blood amyloid. J Alzheimers Dis. 2016;51:997–1002.
Gronewold J, Klafki HW, Baldelli E, Kaltwasser B, Seidel UK, Todica O, et al. Factors responsible for plasma beta-amyloid accumulation in chronic kidney disease. Mol Neurobiol. 2016;53:3136–45.
O’Sullivan ED, Hughes J, Ferenbach DA. Renal aging: causes and consequences. J Am Soc Nephrology. 2017;28:407–20.
Kuro OM. The Klotho proteins in health and disease. Nat Rev Nephrol. 2019;15:27–44.
Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–38.
Liu YH, Giunta B, Zhou HD, Tan J, Wang YJ. Immunotherapy for Alzheimer’s disease: the challenge of adverse effects. Nat Rev Neurol. 2012;8:465–9.
Iijima-Ando K, Hearn SA, Granger L, Shenton C, Gatt A, Chiang HC, et al. Overexpression of neprilysin reduces Alzheimer amyloid-beta42 (Abeta42)-induced neuron loss and intraneuronal Abeta42 deposits but causes a reduction in cAMP-responsive element-binding protein-mediated transcription, age-dependent axon pathology, and premature death in Drosophila. J Biol Chem. 2008;283:19066–76.
Jin WS, Shen LL, Bu XL, Zhang WW, Chen SH, Huang ZL, et al. Peritoneal dialysis reduces amyloid-beta plasma levels in humans and attenuates Alzheimer-associated phenotypes in an APP/PS1 mouse model. Acta Neuropathol. 2017;134:207–20.
Boada M, Lopez OL, Olazaran J, Nunez L, Pfeffer M, Paricio M, et al. A randomized, controlled clinical trial of plasma exchange with albumin replacement for Alzheimer’s disease: primary results of the AMBAR Study. Alzheimers Dement. 2020;16:1412–25.
Ding XL, Lei P. Plasma replacement therapy for Alzheimer’s disease. Neurosci Bull. 2020;36:89–90.
Chuang YF, Breitner JCS, Chiu YL, Khachaturian A, Hayden K, Corcoran C, et al. Use of diuretics is associated with reduced risk of Alzheimer’s disease: the Cache County Study. Neurobiol Aging. 2014;35:2429–35.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (91749206, 81930028, 81625007, and 31921003).
Author information
Authors and Affiliations
Contributions
Y.J.W., J.W., and H.W.Z. conceived and designed the project, D.Y.T., X.L.H., Y.Y.S., G.H.Z., and S.H.C. conducted patient enrolment, assessment, and sample treatment, D.Y.T., Y.C., Z.Q.Z., C.Y.H., Q.G.P, M.Z.T., Y.R.W., H.L.S., P.Y.S., Z.Y.Y., D.Y.F., X.L.B., J.W., and C.R.T. conducted animal and in vitro experiments, D.Y.T., Y.C., and C.R.T. analysed data, D.Y.T. and Y.J.W. wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tian, DY., Cheng, Y., Zhuang, ZQ. et al. Physiological clearance of amyloid-beta by the kidney and its therapeutic potential for Alzheimer’s disease. Mol Psychiatry 26, 6074–6082 (2021). https://doi.org/10.1038/s41380-021-01073-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01073-6
- Springer Nature Limited
This article is cited by
-
Risk factors for cognitive decline in non-demented elders with amyloid-beta positivity
Alzheimer's Research & Therapy (2024)
-
Trajectory of brain-derived amyloid beta in Alzheimer’s disease: where is it coming from and where is it going?
Translational Neurodegeneration (2024)
-
Heart rate and breathing effects on attention and memory (HeartBEAM): study protocol for a randomized controlled trial in older adults
Trials (2024)
-
Modulating heart rate oscillation affects plasma amyloid beta and tau levels in younger and older adults
Scientific Reports (2023)
-
Advances in Molecular Psychiatry – March 2023: mitochondrial function, stress, neuroinflammation – bipolar disorder, psychosis, and Alzheimer’s disease
Molecular Psychiatry (2023)