Abstract
Clearance of amyloid-beta (Aβ) from the brain is an important therapeutic strategy for Alzheimer’s disease (AD). Current studies mainly focus on the central approach of Aβ clearance by introducing therapeutic agents into the brain. In a previous study, we found that peripheral tissues and organs play important roles in clearing brain-derived Aβ, suggesting that the peripheral approach of removing Aβ from the blood may also be effective for AD therapy. Here, we investigated whether peritoneal dialysis, a clinically available therapeutic method for chronic kidney disease (CKD), reduces brain Aβ burden and attenuates AD-type pathologies and cognitive impairments. Thirty patients with newly diagnosed CKD were enrolled. The plasma Aβ concentrations of the patients were measured before and after peritoneal dialysis. APP/PS1 mice were subjected to peritoneal dialysis once a day for 1 month from 6 months of age (prevention study) or 9 months of age (treatment study). The Aβ in the interstitial fluid (ISF) was collected using microdialysis. Behavioural performance, long-term potentiation (LTP), Aβ burden and other AD-type pathologies were measured after 1 month of peritoneal dialysis. Peritoneal dialysis significantly reduced plasma Aβ levels in both CKD patients and APP/PS1 mice. Aβ levels in the brain ISF of APP/PS1 mice immediately decreased after reduction of Aβ in the blood during peritoneal dialysis. In both prevention and treatment studies, peritoneal dialysis substantially reduced Aβ deposition, attenuated other AD-type pathologies, including Tau hyperphosphorylation, glial activation, neuroinflammation, neuronal loss, and synaptic dysfunction, and rescued the behavioural deficits of APPswe/PS1 mice. Importantly, the Aβ phagocytosis function of microglia was enhanced in APP/PS1 mice after peritoneal dialysis. Our study suggests that peritoneal dialysis is a promising therapeutic method for AD, and Aβ clearance using a peripheral approach could be a desirable therapeutic strategy for AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease. Extracellular senile plaques comprising amyloid-beta protein (Aβ) are the neuropathological hallmarks of AD [11, 12, 28]. It has been suggested that excessive Aβ production or deficits in Aβ clearance play pivotal or causative roles in AD pathogenesis [37]. AD comprises familial AD and sporadic AD. Familial AD afflicts approximately 1% of total AD patients and is caused by mutations in APP, PS1 or PS2 genes, leading to the overproduction of Aβ, whereas sporadic AD affects over 99% of AD patients in which impaired Aβ clearance in the brain is one of primary contributing factors [29]. Therefore, the enhancement of brain Aβ clearance is one of the most promising strategies for AD prevention and treatment [37].
Current Aβ clearing strategies mainly focus on enhancing brain Aβ clearing capacities by introducing agents into the brain [27]. However, introducing exogenous substance into the brain likely causes adverse effects [17, 23]. For example, in clinical trials of immunotherapy, which target Aβ clearance, the entry of therapeutic antibodies leads to various adverse effects, such as neuroinflammation, vasogenic oedema [35], microhaemorrhage [30], neuronal hyperactivity [5] and the increasing conversion of Aβ fibrils to more toxic Aβ oligomers [23]. Thus, to avoid these adverse effects, using a peripheral approach to clear brain Aβ, which reduces brain-derived Aβ in the blood, would be a safer strategy [24].
In previous studies, we revealed that the physiological Aβ clearance capacity in peripheral organs and tissues plays a significant role in the clearance of brain Aβ [44], suggesting that clearing blood Aβ may be a potential approach to removing brain Aβ. In the clinic, dialysis, including haemodialysis and peritoneal dialysis, is an effective method to remove metabolites and wastes from blood to maintain the homeostasis of the internal environment of the body. Dialysis can also recover the homeostasis of the microenvironment in the brain, as indicated by the effectiveness of dialysis in treating encephalopathies due to liver and kidney failures or toxicosis [32]. Previous clinical studies have suggested that dialysis reduces blood Aβ levels [22, 25, 34, 39]. Thus, we investigated whether peritoneal dialysis can reduce brain Aβ and exert therapeutic benefits to AD in the present study.
Materials and methods
Subjects
A total of 30 patients newly diagnosed with chronic kidney disease (CKD) were enrolled in the study. The blood was sampled before and immediately after the first time peritoneal dialysis, and the peritoneal dialysis solution was also collected after dialysis. Plasma was separated and stored with the dialysis solution within 2 h after sampling at −80 °C for future analysis. The present study was approved by the Institutional Review Board of Daping Hospital.
Surgery for peritoneal dialysis of mice
The transgenic mouse line expressing APPswe/PSEN1dE9 transgenic mice was obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Female mice were used in the present study to exclude the influence of gender on AD pathologies in the brain [19]. Peritoneal dialysis surgery was performed as previously described [38]. Briefly, the mice were anaesthetized with ketamine (100 mg/kg), xylazine (20 mg/kg), and acepromazine (3 mg/kg). An “open” permanent system was selected. First, tubing was subcutaneously tunnelled from the abdomen to the neck after pre-operative skin preparation. Subsequently, the abdomen was opened and tubing was placed in the peritoneal cavity. Next, the abdomen was closed using a purse–string suture. Post-operation mice were recovered in a warm and clean husbandry area. Prophylactic antibiotic treatment (enrofloxacin 5 mg/kg) was initiated 1 day prior to surgery and continued for 1 week. All animals received analgesic/anti-inflammatory treatment (acetylsalicylic acid, 5 mg/kg) for 2 weeks. For peritoneal dialysis, 2 ml of 2.5% dialysis solution (Baxter Healthcare Corporation, Guangzhou, China; 100 ml of solution contains 2.5 g of dextrose hydrous, 538 mg of sodium chloride, 448 mg of sodium lactate, 18.3 mg of calcium chloride and 5.1 mg of magnesium chloride) at 37 °C was injected into peritoneal cavity via tubing and collected 2 h later under sterile conditions. The recovery efficiency of peritoneal dialysis was approximately 60% (1.2 ml of dialysis solution). All mouse husbandry procedures were approved through the Third Military Medical University Animal Welfare Committee.
Prevention or treatment experiments were conducted. Mice aged 6 months (prevention group, n = 9), upon the initiation of Aβ deposition in the brain, and mice aged 9 months (treatment group, n = 8), when abundant deposits were formed in the brain, were subjected to daily peritoneal dialysis for 30 days. Age-matched AD mice with the same surgical procedures and antibiotic treatment but without peritoneal dialysis were used as controls (prevention control group, n = 7; treatment control group, n = 8).
Microdialysis
The parameters for the microdialysis probes used for in vivo experiments were 220-µm OD membrane which is made of hydrophilic cellulose and does not absorb Aβ (MBR-1-5 brain microdialysis probe: length of membrane was 1 mm, length of cannula was 5 mm, 35 kDa molecular weight cut-off; Bioanalytical Systems, West Lafayette, USA). Guide implantation surgery was performed as previously described [9, 26]. Briefly, a separate group of 9-month-old AD mice (n = 7) was anaesthetized, and the skin was removed to expose the skull. Bore holes (0.75 mm) were made above the left hippocampus (bregma −3.1 mm, 2.4 mm lateral, −0.6 mm relative to dura mater). MBR-5 guide cannulas were stereotactically inserted into the hippocampus (12° angle) and cemented using binary dental cement. The solution circle system, including a CMA 402 Syringe Pump, CMA 120 System for Freely Moving Animals and CMA 142 Microfraction Collector, was connected using fluorinated ethylene propylene (FEP) tubing. As ISF Aβ levels decrease approximately 14% under anaesthetic conditions [31], the mice were kept awake during microdialysis. The constant flow rate was 1 μl/min. Microdialysis samples were collected hourly using a refrigerated fraction collector.
The recovery rate of Aβ in microdialysis was estimated following the previously described protocols [2]. In brief, 1 ml of Tris-buffered saline (TBS) extract of brains from 10-month-old AD mice was used as the external medium of microdialysis. Flow rate of dialysis solution was randomly changed over six different values (0.1, 0.2, 0.3, 0.5, 1 and 2 μl/min). The concentration of Aβ40 and Aβ42 in TBS extracts and microdialysis fractions was measured with ELISA. The effects of flow rate on recovery rate was calculated as per the interpolated zero flow method [18]. The Aβ recovery rates from external media with different dilutions of brain TBS extracts (1:1, 1:2, 1:4, 1:9) at the same flow rate (1 μl/min) were also calculated, to confirm the recovery rate estimated from interpolated zero flow method.
Brain sampling
The brains were sampled and weighed. The left hemispheres were fixed in 4% paraformaldehyde (pH 7.4) for 24 h, followed by incubation with 30% sucrose for 24 h. The right hemispheres were snap frozen in liquid nitrogen and stored at −80 °C for future biochemical analysis. For the animals of the treatment group, a part of the fresh right hemisphere containing the hippocampus, which corresponds to the brain region from bregma −1.5 to −2.5 mm, was dissected coronally for future Golgi staining.
AD-type pathology and quantification
Brain sections were cut coronally at a thickness of 35 µm and stored at 4 °C in phosphate-buffered saline (PBS) containing 0.1% sodium azide. A series of five equally spaced brain sections (~1.3 mm apart) were used for each type of stain. Congo red staining was used for compact Aβ plaques, and total Aβ plaques containing both compact and diffuse Aβ plaques were visualized using antibody 6E10 immunohistochemistry as previously described [45, 46]. The apoptosis of neuronal cells was detected using NeuN and Caspase-3 double immunofluorescence staining. Neuronal loss and neurite degeneration were detected using NeuN and microtubule-associated protein (MAP)-2 double immunofluorescence staining. Immunohistochemistry of anti-CD45 antibody detecting activated microglia, and anti-glial fibrillary acidic protein (GFAP) antibody detecting astrocytes were used to visualize astrocytosis and microgliosis. The area fraction and/or density of positive staining and the number of cells were quantified using ImageJ software.
Double immunofluorescence staining of Iba-1 and 6E10 was performed to verify the phagocytic ability of microglia. Orthogonal function of confocal microscope (ZEISS LSM 880, Germany) was used for three-dimensional construction to measure co-localization of Iba1 and Aβ staining. A series of three sections containing CA1 region were used for the staining. The part of CA1 containing the axons, not the cell body, were imaged for the quantification. The diameter of Iba1+ cell bodies was quantified using ImageJ software. Percentage of microglia co-localized with Aβ was quantified by an investigator who was blinded to group information. In this co-localization study, diameter of at least 10 μm was used to judge the positive Aβ staining following a previous study [16].
Golgi staining was performed as per the manufacturer’s protocols (FD rapid Golgi Stain kit, Fdneurotech, Columbia, MD, USA). The numerical density of spines was assessed in CA1 pyramidal neurons. Spines were counted in one 50-μm segment per cell, located in the middle of one of the secondary dendrites that protrude from the apical dendrite. The number of dendritic spines was estimated at high magnification (1000×) in a blinded manner.
ELISA assays
Frozen brains were homogenized in liquid nitrogen and extracted with TBS, 2% sodium dodecyl sulfonate (SDS), and 70% formic acid (FA) solutions as previously described [20]. The omentum was extracted with RIPA buffer, which contains 50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, with supplement of sodium orthovanadate, sodium fluoride and ethylenediaminetetraacetic acid (EDTA). The levels of Aβ40 and Aβ42 in brain and omentum extracts, plasma, peritoneal dialysis solution and microdialysis fluid were measured using ELISA kits (Invitrogen, cat no. KHB3481 which detects human Aβ1-40, and cat no. KHB3544 which detects human Aβ1-42). The concentrations of the pro-inflammatory cytokines IL-6, IL-1β, IFN-γ, TNF-α and anti-inflammatory cytokines IL-4, IL-10 were quantitatively measured using ELISA kits (eBioscience, Vienna, Austria). All experiments were performed per the manufacturer’s instructions.
Western blotting
Western blotting was used to analyse the levels of molecules or enzymes involving Aβ metabolism, phosphorylated Tau, and synapse-related proteins. Proteins in the animal brain homogenate were extracted using RIPA buffer. The samples were loaded onto SDS-PAGE (4–10% acrylamide) gels. The separated proteins were transferred to nitrocellulose membranes. The blots were probed with the following antibodies: anti-APP C-terminal antibody (Cat. no. 171610, Millipore, Billerica, MA, USA) to detect C-terminal fragment (CTF)-α (CTF-α) and CTF-β, anti-Aβ antibody (Cat. no. 6E10, Sigma, St. Louis, MO, USA) was used to detect Aβ, full-length APP (APPfl), secreted APP (sAPP)-α (sAPPα), anti-sAPP antibody (Cat. no. 22C11, Millipore) to detect sAPPα and sAPPβ; anti-β-secretase (BACE)-1 antibody (Cat. no. ab108394, Abcam, Cambridge, UK); anti-insulin-degrading enzyme (IDE) antibody (Cat. no. 3862, Epitomics, California, USA); anti-neprilysin (NEP) antibody (Cat. no. AB5458, Millipore); anti-receptor for advanced glycation end products (RAGE, Cat. no. AB9714, Millipore); anti-low-density lipoprotein receptor-related protein 1 (LRP-1) (Cat. no. 5A6, Calbiochem, LaJolla, CA); anti-phosphorylated-Tau antibodies, including anti-PS396 (Cat. no. 11102, Signalway, Maryland, USA) and anti-PS199 (Cat. no. ab81268, Abcam); anti-total tau (Cat. no. MAB361, tau-5; Abcam); anti-Synaptophysin (Cat. no. ab108990, Abcam); anti-Synapsin-1 (Cat. no. AB1543, Millipore); anti-PSD95 (Cat. no. MAB1599, Millipore); anti-PSD93 (Cat. no. Jan-74, Epitomics); anti-β-actin (Sigma). The membranes were incubated with IRDye 800 CW secondary antibodies (LiCOR) and scanned using the Odyssey fluorescent scanner. The band density was normalized to β-actin for analysis.
Behavioural tests
Mice from both prevention and treatment studies, as well as the age- and gender-matched wild-type mice (n = 9 for prevention controls, n = 8 for treatment controls), were subjected to behavioural tests. Y-maze and open-field tests were performed following as previously described [20]. In a spontaneous alternation test, the mice were allowed to move freely through a Y-maze during a 5-min session. Alternation was defined as successive entries into the three arms on overlapping triplet sets. The percentage of alternation was calculated as the total number of alternation × 100/(total number of arm entries − 2). A novel arm exploration test was also performed in the Y-maze. One arm was blocked (defined as the novel arm), and the mice were allowed to explore the other two arms (home arm and familiar arm) for 5 min. After a 2-h interval, the mice were allowed to freely explore all three arms for 5 min. The number of novel arm entries and time spent in the novel arm were recorded. In the open-field test, the mice were placed in the centre of the open-field apparatus for 3 min. Rearing, grooming, defecation, and urination were recorded per mouse. The paths were tracked using a computer tracking system (Limelight, ActiMetrics, Wilmette, IL, USA) and the distance travelled was recorded.
Electrophysiology
A separate group of AD mice treated with peritoneal dialysis for 1 month from 9 to 10 months of age (n = 8), age-matched AD mice without peritoneal dialysis (n = 7) and wild-type mice (n = 8) were subjected to electrophysiology tests as described previously [7, 43]. In brief, the mice were deeply anaesthetized with 30% chloral hydrate (3 ml/kg, i.p.) and transcardially perfused with N-methyl-d-glucamine (NMDG) artificial cerebral spinal fluid (ACSF) prior to decapitation. The brains were rapidly sampled from the skull and placed for sectioning in ice-cold cutting solution (NMDG ACSF) aerated with 95% O2 and 5% CO2. Acute coronal hippocampal slices (400-μm thick) were sectioned from the middle third of the hippocampus using a vibratome (VT1000S, Leica Microsystems, Bannockburn, IL, USA) in cutting solution. The slices were incubated in oxygenated HEPES ACSF for 1 h at 30 °C. Subsequently, the slices were gently transferred into a recording chamber filled with normal ACSF. The fEPSPs evoked through the stimulation of the Schaffer collateral/commissural pathways were recorded in the hippocampus using pipettes (1–2 MΩ) filled with ACSF. Test fEPSPs were evoked at a frequency of 0.033 Hz and at a stimulus intensity adjusted to approximately 50% of the intensity that elicited the maximal response. After a 20-min stable baseline, long-term potentiation (LTP) was induced by high-frequency stimulation (HFS, 100 pulses at 100 Hz). All recordings were conducted at room temperature (approximately 25 °C) using a Multiclamp EPC 10 amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany).
Statistical analysis
All data represent the mean ± SEM. Statistical analysis included two-tailed Student’s t test and paired t test for the comparison of two groups, and one-way ANOVA and Tukey’s test for the comparison of multiple groups when required. Normality and equal-variance testing was performed for all assays. P < 0.05 was considered significant. All analyses were completed using SPSS software, version 19.0.
Results
Peritoneal dialysis decreases plasma Aβ in both human and mice
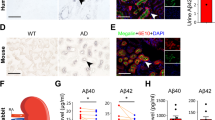
Compared with plasma Aβ levels before peritoneal dialysis, plasma Aβ levels after peritoneal dialysis significantly decreased (Aβ40: 141.35 ± 76.15 pg/ml vs. 181.61 ± 109.14 pg/ml, P = 0.0014; Aβ42: 35.78 ± 6.88 pg/ml vs. 46.53 ± 8.33 pg/ml, P < 0.0001) in patients with CKD (Fig. 1a–c). The average levels of Aβ40 in the recovered dialysis solution was 37.16 ± 9.90 pg/ml and Aβ42 in the recovered dialysis solution was 28.50 ± 2.83 pg/ml. An estimated 131.33 ng of Aβ was extracted into the dialysis solution each time, calculated by multiplying the Aβ concentration in recovered dialysis solution with the volume, and this value is approximately 2% of the total Aβ in the normal human brain (7760 ng/brain) [33].
Peritoneal dialysis reduces Aβ in blood and interstitial fluid (ISF) of brain. a–c The alteration of plasma Aβ after peritoneal dialysis in patients with CKD, the green points represent the Aβ levels before peritoneal dialysis, and the red points represent Aβ levels after peritoneal dialysis (n = 30, paired t test). d Variation diagram of Aβ levels in plasma and brain ISF of mice after peritoneal dialysis. e Correlation between the changes of Aβ levels in plasma and ISF (Pearson’s correlation). n = 7. PD peritoneal dialysis. One-way ANOVA, versus Aβ levels at hour 0, *P < 0.05, **P < 0.01, ***P < 0.001. Error bar SEM
In mice, the plasma Aβ40 and Aβ42 levels also significantly decreased after peritoneal dialysis, the level of Aβ40 in the recovered dialysis solution was 117.72 ± 100.99 pg/ml and the level of Aβ42 in the recovered dialysis solution was 83.26 ± 45.69 pg/ml. An estimated 0.24 ng of Aβ was removed each time, which is approximately 0.07% of total Aβ (360 ng) in the brain of AD mice.
Dynamic changes of Aβ in blood and ISF during peritoneal dialysis
We further investigated the dynamic interaction between blood Aβ levels and brain ISF Aβ levels. Aβ levels in microdialysis fluid were used to estimate ISF Aβ levels. The recovery rates of Aβ in microdialysis solution at flow rate of 1 μl/min were estimated as 18.22% for Aβ40, 15.99% for Aβ42 and 16.98% for total Aβ with interpolated zero flow method, respectively (Supplemental Fig. 1a–c), which were further confirmed with different dilutions of microdialysis external medium (Supplemental Fig. 1d–f). Correspondingly, the Aβ concentration in ISF of 9-month-old AD mice before microdialysis was estimated as 798.63 pg/ml for Aβ40, 597.55 pg/ml for Aβ42 and 1396.17 pg/ml for total Aβ (Supplemental Fig. 6).
We observed that the levels of Aβ40 and Aβ42 in blood decreased after initiating peritoneal dialysis, and reached the lowest levels at 3–4 h after beginning dialysis, and gradually increased and returned to baseline levels at 5 h after ending dialysis. Interestingly, the changes of the Aβ40 and Aβ42 levels in microdialysis fluid paralleled those in the blood (Fig. 1d). The regression analysis showed that the Aβ levels in microdialysis were positively correlated with those in the blood (Fig. 1e). The ratio of the changes of Aβ concentrations in ISF to blood was 1:3. These results suggest that the changes of Aβ levels between the brain ISF and blood are dynamically correlated in AD mice.
Peritoneal dialysis reduces brain Aβ burden
We investigated whether long-term peritoneal dialysis reduces brain Aβ. In a prevention study, compared with control mice, mice treated with peritoneal dialysis had a significantly lower area fraction of both compact plaques stained with Congo red and total plaques stained with 6E10 in neocortex, and lower area fraction of total plaques in the hippocampus (Supplemental Fig. 2a, b). The levels of Aβ40 and Aβ42 in the brain homogenates were also significantly reduced in mice treated with peritoneal dialysis relative to control mice (Supplemental Fig. 2c).
We next investigated whether peritoneal dialysis is effective in reducing brain Aβ after abundant deposition of Aβ. In a treatment study, peritoneal dialysis-treated mice had significantly less Congo red and 6E10-positive Aβ plaques in the neocortex and hippocampus than control mice (Fig. 2a, b). The levels of Aβ in the brain homogenates were also significantly reduced by 21.44% for Aβ40, 19.49% for Aβ42 and 20.44% for total Aβ in peritoneal dialysis-treated mice compared with control mice (Fig. 2c).
Peritoneal dialysis reduces brain amyloid burden of AD mice. a, b Representative images of Congo red and 6E10 immunohistochemical staining in the neocortex and hippocampus of 10-month-old controls and 10-month-old peritoneal dialysis-treated mice. Insets show the representative morphology at a higher magnification. Scale bars 500 µm. c Comparison of Aβ40, Aβ42 and Aβ40 + 42 levels measured with ELISA in TBS, 2% SDS and 70% FA fractions of brain extracts between peritoneal dialysis-treated mice and controls. d Western blotting and quantitative analysis for APP and its metabolites in brain homogenates. e Western blotting and quantitative analysis for BACE1, Aβ-degrading enzymes and Aβ-transporting receptors in the blood–brain barrier (BBB). Con control, PD peritoneal dialysis. n = 8 per group, two-tailed t test, *P < 0.05, **P < 0.01, ***P < 0.001, n.s. non-significant. Error bar SEM
We next investigated the potential mechanisms underlying the reduction of Aβ deposition after peritoneal dialysis. There were no significant differences in APP and its metabolites, including APPfl, CTF-α, CTF-β sAPPα, sAPPα/β, BACE-1, and Aβ-degrading enzymes IDE and NEP, between the peritoneal dialysis treatment group and the control (Fig. 2d, e; Supplemental Fig. 7), suggesting that decreased Aβ deposition after peritoneal dialysis may not reflect a reduction in Aβ production and enhancement of Aβ degradation. Levels of Aβ transport receptors across the blood–brain barrier (BBB) (LRP-1 and RAGE) were also measured. Interestingly, higher levels of LRP-1 and lower levels of RAGE in homogenates were detected in peritoneal dialysis-treated mice compared with control mice (Fig. 2e), suggesting that peritoneal dialysis enhances the receptor-mediated efflux of Aβ through the BBB. In addition, Aβ levels in the omentum of the treated mice were approximately 3.20-fold higher than those in control mice, suggesting that Aβ entrapment in the omentum also contributed to the reduction of brain Aβ (Supplemental Fig. 4a–c).
Peritoneal dialysis attenuates neuroinflammation and enhances Aβ phagocytosis by microglia
Compared with control mice, peritoneal dialysis-treated mice had a lower area fraction of activated astrocytosis (GFAP positive) and microgliosis (CD45 positive) in both the neocortex and hippocampus. Peritoneal dialysis also significantly reduced levels of pro-inflammatory cytokines, including TNF-α, IFN-γ and IL-6, and increased levels of anti-inflammatory cytokine IL-10, in brain homogenates (Fig. 3a, b, d). Interestingly, there were more microglial cells containing intercellular Aβ in the CA1 of the hippocampus of peritoneal dialysis-treated mice compared with control mice (Fig. 3c). These findings suggest that peritoneal dialysis attenuates neuroinflammation and enhances Aβ phagocytosis by microglia in the brain.
Peritoneal dialysis attenuates neuroinflammation and enhances Aβ phagocytosis by microglia in the brain of AD mice. a Immunostaining and quantification of activated microgliosis and b astrocytosis (Scale bar 500 µm). c Representative images and three-dimensional reconstruction of microglia and Aβ plaques in the CA1 region of the hippocampus stained with anti-Iba1 and anti-Aβ (6E10) immunofluorescence, and quantification of the diameter of Iba1-positive microglia cell bodies and percentage of Aβ-positive microglia (indicated with white cycle) in 10-month-old peritoneal dialysis-treated mice and control mice. Insets show the morphology of microglia marked with asterisk (*) at a higher magnification (Scale bars 50 µm). d Comparison of TNF-α, IFN-γ, IL-1β, IL-4, IL-6 and IL-10 levels in the brain homogenates of 10-month-old peritoneal dialysis-treated mice and control mice. Con control, PD peritoneal dialysis. n = 8 per group, two-tailed t test, *P < 0.05, **P < 0.01, ***P < 0.001, n.s. non-significant. Error bar SEM
Peritoneal dialysis alleviates neurodegeneration in the brain
The levels of phosphorylated Tau (PS396) were significantly reduced in the brains of mice after peritoneal dialysis (Fig. 4g, h). Compared with control mice, the neuronal apoptosis was also significantly reduced in the peritoneal dialysis-treated mice compared with the control mice, as detected by caspase-3 staining in the hippocampus (Fig. 4a–d). The levels of synapse-associated protein expression, including PSD93, PSD95, synapsin-1, and synaptophysin, in the brain homogenates, and the number of dendritic spines detected via Golgi staining in the hippocampus were increased in the peritoneal dialysis-treated mice compared with control mice (Fig. 4e, f). These data suggest that neurodegeneration was attenuated in the brains of AD mice after peritoneal dialysis.
Peritoneal dialysis alleviates neuronal degeneration and loss in the hippocampus of 10-month-old AD mice. a Representative images of the neurons and dendrites in the CA1 region of the hippocampus stained with anti-NeuN and anti-MAP-2 immunofluorescence in peritoneal dialysis-treated and age-matched control mice (Scale bars 100 µm). Comparison of the area fractions of NeuN, MAP-2 b and caspase-3 c staining between peritoneal dialysis-treated and control mice. d Representative images of neuronal apoptosis in the CA3 region of the hippocampus as illustrated with activated caspase-3 immunofluorescence (Scale bars 100 µm). e Western blotting and quantitative analysis of synapse-associated proteins, including PSD93, PSD95, synapsin1 (SYN-1), and synaptophysin (Synap), in the brain homogenates of peritoneal dialysis-treated and control mice. f Representative photomicrograph and quantification of dendritic spine intensity of basal segment of CA1 hippocampal neurons, n = 8 per group (Scale bar 10 μm). g PS396 immunohistochemical staining in the hippocampus of peritoneal dialysis-treated and control mice. Insets show the representative morphology at a higher magnification (Scale bars 100 μm). Comparison of the PS396-positive area fraction in the hippocampus of peritoneal dialysis-treated and control mice. h Western blot and quantification for phosphorylated Tau at multiple sites, including PS396-Tau, PS199-Tau, and total Tau (Tau5), in brain homogenates. Con control, PD peritoneal dialysis. n = 8 per group, two-tailed t test, *P < 0.05, **P < 0.01, ***P < 0.001, n.s. non-significant. Error bar SEM
Peritoneal dialysis rescues LTP and cognition impairment
AD mice treated with peritoneal dialysis (9–10 mon) and their age-matched controls were subjected to cognitive tests. In the open-field test, a higher number of rearing and a longer distance travelled were observed for peritoneal dialysis-treated mice compared with the controls. However, no difference in the number of grooming behaviours was observed between the two groups (Fig. 5e, f). In the Y-maze test, peritoneal dialysis-treated mice had better performance than controls, reflected by a higher spontaneous alternation percentage, increased number of total entries into the three arms in alternation tests, and increased number of entries and time spent in the novel arm (Fig. 5a–d, Supplemental Fig. 3). These results indicate that peritoneal dialysis can prevent or halt cognitive decline in AD mice.
Peritoneal dialysis improves behavioural performances and long-term potentiation (LTP) of 10-month-old AD mice. a, b Percentage of alternation and number of entries. c, d Novel arm entry and time spent in the novel arm in the Y-maze test. e, f Distance travelled and number of rearing in the open-field test. g Representative tracing graphs of open-field test. n = 8 per group. h Hippocampus CA1 long-term potentiation (LTP) in peritoneal dialysis-treated mice. Con control, PD peritoneal dialysis, WT wild type. n = 8 for WT, n = 8 for PD, n = 7 for Con, one-way ANOVA, *P < 0.05, **P < 0.01, ***P < 0.001, n.s. non-significant. Error bar SEM
High-frequency stimulation (HFS) induced reliable LTP, the cellular mechanism underlying learning and memory, in wild-type mice, whereas the strength of LTP was significantly decreased in AD mice compared with wild-type mice, suggesting that the plasticity of the synapse is impaired in the brains of AD mice. Notably, a higher LTP was observed in peritoneal dialysis-treated mice compared with control mice, indicating that peritoneal dialysis can substantially rescue impaired hippocampal LTP induction in AD mice (Fig. 5h).
Discussion
In the present study, peritoneal dialysis effectively decreased blood Aβ and brain ISF-soluble Aβ levels. After 1 month of peritoneal dialysis, brain Aβ burden, neuroinflammation, neurodegeneration and cognitive deficits in AD mice were significantly alleviated, suggesting that peritoneal dialysis might enhance the Aβ efflux from brain to blood, attenuate the brain inflammatory microenvironment, and improve the phagocytosis of Aβ by microglia.
Previous anti-Aβ therapeutic strategies have primarily focused on reducing Aβ production, inhibiting Aβ deposition and facilitating Aβ clearance, all of which depend on Aβ clearing agents entering the brain through the BBB. The BBB restricts the entry of peripheral blood proteins into the brain to maintain the homeostasis of the brain internal environment [13]. Thus, the entry of therapeutic agents into the brain may disrupt the brain internal environment and induce various adverse effects. Numerous studies have shown that the Aβ antibodies used in AD clinical trials cause neuroinflammation, vasogenic oedema, microhaemorrhage and neuronal hyperactivity [5, 23], likely reflecting the entry of antibodies into the brain. Therefore, clearing blood Aβ and accelerating the efflux of Aβ from the brain into the periphery would be a better strategy for clearing brain Aβ [23, 24]. Previous studies have tested the therapeutic efficacy of clearing blood Aβ for AD. For example, brain Aβ could be significantly reduced by increasing Aβ degradation in the liver via the oral administration of Withania somnifera extracts [36], and the peripheral expression of the Aβ-degrading enzyme neprilysin was also effective in clearing blood and brain Aβ [14], despite conflicting findings suggest that the peripheral injection of neprilysin only reduces blood Aβ but not brain Aβ [15, 41]. In the present study, peritoneal dialysis effectively decreased both blood Aβ and blood Aβ burden in CKD patients and in AD mice, indicating that enhancing the clearance of Aβ from the periphery is a promising approach for reducing brain Aβ burden.
The most important prerequisite to peripheral Aβ clearance is the efflux transport of Aβ across the BBB. Previous studies have shown that BBB transporters were changed in AD in a pattern that decreases Aβ efflux and/or increases Aβ influx across the BBB and contributes to AD pathogenesis [6, 8, 10], and a contrasting view has been suggested in a recent study which indicates that there is not the lack of widespread disruption of BBB in AD mouse models [1]. In the present study, we conducted the first investigation of the dynamic effects of decreasing Aβ levels in blood on the soluble Aβ levels in ISF. The compelling finding of the present study was that the ISF-soluble Aβ levels were decreased with the reduction of the blood Aβ levels during peritoneal dialysis. Our findings suggest that the transport of Aβ across the BBB is functional in AD mice, and this lays the foundation for therapeutic development focusing on peripheral Aβ clearance approaches to reduce brain Aβ burden.
Is the removal of blood Aβ by peritoneal dialysis the only explanation for the reduction of Aβ from the brain? To address the question, we calculated the total Aβ removal via peritoneal dialysis. Total Aβ burden in the brain of 10-month-old AD mice is approximately 360 ng as calculated by brain Aβ concentration (1 ng/mg) × brain weight (360 mg). The total brain Aβ at the end of peritoneal dialysis is composed of the existing Aβ at the beginning of peritoneal dialysis and newly produced Aβ during the 30 days of peritoneal dialysis. The total Aβ removal in dialysis solution is 7.2 ng as calculated by the Aβ concentration in dialysis solution (200 pg/ml) × the volume of recovered dialysis solution (1.2 ml) × dialysis duration (30 day), while the total reduction of brain Aβ after peritoneal dialysis = brain Aβ concentration (1 ng/mg) × brain weight (360 mg) × the percentage decrease in brain Aβ burden (20.44%) = 72 ng. Thus, Aβ removal in dialysis solution was only 10% of the reduction in brain Aβ burden, suggesting that other Aβ clearing pathways may be enhanced through peritoneal dialysis. In our present study, chronic peritoneal dialysis increased the expression of LRP-1 and decreased the expression of RAGE in the brain of AD mice; these changes could favour the net efflux of Aβ across BBB from the brain into the blood. In addition to the removal of Aβ, the convection between dialysate and blood produced by hypertonic dialysis solution can also promote the efflux of other metabolites into the peritoneal dialysate through the peritoneum and omentum. Thus, peritoneal dialysis could significantly improve the internal environment of the brain, as reflected by the decreased pro-inflammatory cytokines and increased anti-inflammatory cytokines in the brain, suggesting that there might be a shift of microglia polarization state from M1 to M2. Enhancement of the Aβ phagocytic ability of microglial cells reflects the improvement of microglia functions in the brain after peritoneal dialysis. Furthermore, as the level of Aβ in the omentum in the peritoneal dialysis-treated mice was much higher than the controls, entrapment of Aβ in omentum also contributes to the reduction of brain Aβ burden. Taken together, peritoneal dialysis clears brain Aβ through multiple pathways.
Previous studies suggest that Aβ levels in the blood are not correlated with that in the brain and disease severity of AD, partially due to the difference in the Aβ solubility and the many confounding factors which influence the brain-derived Aβ levels in the blood such as binding of Aβ with albumin, blood cells and catabolism of Aβ in the periphery by enzymes, tissues and organs [31]. But in the present study the changes of soluble Aβ levels in ISF were closely correlated with that in blood during peritoneal dialysis, suggesting that the soluble brain Aβ pool and blood Aβ pool are communicative. Our recent study suggests that the physiological Aβ clearance in the periphery plays a significant role in removing brain Aβ, and the clearance of Aβ in the periphery is a potential route for brain Aβ clearance [44]. In addition, previous studies suggest that Aβ can also deposit in the peripheral tissues, such as intestine, skin and heart, of AD patients [21, 40]. In our present study, we provided the evidence that Aβ also accumulates in the omentum in AD mice. The higher levels of Aβ42 over Aβ40 in the omentum might be because that Aβ42 is more prone to deposit in tissues than Aβ40. Moreover, previously we found that blood Aβ levels were significantly increased in patients with peripheral disorders, such as hepatic and renal failures [25, 42], chronic obstructive pulmonary disease [3], and systemic infection [4, 40]. These findings suggest that the disturbance of Aβ clearance in the periphery may contribute to AD pathogenesis, and AD may be a disorder related not only to the brain but also to the peripheral system.
It is worthy to note that CKD patients are different from AD mice in the present study. The first difference is that patients were suffering from chronic renal failure while renal functions of AD mice were normal. Our previous study suggests that the Aβ clearance capacity of the kidney is impaired in CKD patients [25]. In the present study, we found that peritoneal dialysis is potent in removing Aβ from the blood in CKD patients. While in AD mice, the blood Aβ levels were dramatically higher than that of wild-type mice, suggesting that the renal function in AD mice is not sufficient to remove Aβ in blood. In this regard, enhancement of Aβ clearance from blood is a necessary therapeutic approach for AD, even though their renal functions are normal. The second difference between CKD patients and AD mice is different procedures of peritoneal dialysis. CKD patients usually received continuous ambulatory peritoneal dialysis (CAPD) which consists of three daytime exchanges (i.e. 4–6-h dwell-time) and one nightly (i.e. 8–12-h dwell-time) in clinical settings. While in our study AD mice received only 2 h of dialysis per day. Even with this shorter time of dialysis, significant reduction of brain Aβ burden was also achieved in AD mouse, suggesting that CAPD would be more potent in removing brain Aβ if it is applied for AD patients. It is of importance to note that brain Aβ deposition is lower in CDK patients who received hemodialysis than those who did not, suggesting that reducing blood Aβ by dialysis would be a promising and effective therapeutic approach for AD [34].
In conclusion, the efficacy of peritoneal dialysis for clearing brain Aβ in our present study provides proof-of-concept evidence that the restoration of the AD brain microenvironment and the clearance of brain Aβ could be realized via peripheral approaches. The findings of the present study could also provide implication for the prevention and treatment of other neurodegenerative diseases, such as Parkinson’s disease, Huntington’s disease and amyotrophic lateral sclerosis, through peripheral approaches.
References
Bien-Ly N, Boswell CA, Jeet S, Beach TG, Hoyte K, Luk W et al (2015) Lack of widespread BBB disruption in Alzheimer’s disease models: focus on therapeutic antibodies. Neuron 88:289–297. doi:10.1016/j.neuron.2015.09.036
Brody DL, Magnoni S, Schwetye KE, Spinner ML, Esparza TJ, Stocchetti N et al (2008) Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science 321:1221–1224. doi:10.1126/science.1161591
Bu XL, Cao GQ, Shen LL, Xiang Y, Jiao SS, Liu YH et al (2015) Serum amyloid-beta levels are increased in patients with chronic obstructive pulmonary disease. Neurotox Res 28:346–351. doi:10.1007/s12640-015-9552-x
Bu XL, Yao XQ, Jiao SS, Zeng F, Liu YH, Xiang Y et al (2015) A study on the association between infectious burden and Alzheimer’s disease. Eur J Neurol 22:1519–1525. doi:10.1111/ene.12477
Busche MA, Grienberger C, Keskin AD, Song B, Neumann U, Staufenbiel M et al (2015) Decreased amyloid-beta and increased neuronal hyperactivity by immunotherapy in Alzheimer’s models. Nat Neurosci 18:1725–1727. doi:10.1038/nn.4163
Carrano A, Hoozemans JJ, van der Vies SM, Rozemuller AJ, van Horssen J, de Vries HE (2011) Amyloid beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid Redox Signal 15:1167–1178. doi:10.1089/ars.2011.3895
Chai GS, Duan DX, Ma RH, Shen JY, Li HL, Ma ZW et al (2014) Humanin attenuates Alzheimer-like cognitive deficits and pathological changes induced by amyloid beta-peptide in rats. Neurosci Bull 30:923–935. doi:10.1007/s12264-014-1479-3
Chakraborty A, de Wit NM, van der Flier WM, de Vries HE (2017) The blood brain barrier in Alzheimer’s disease. Vascul Pharmacol 89:12–18. doi:10.1016/j.vph.2016.11.008
Cirrito JR, May PC, O’Dell MA, Taylor JW, Parsadanian M, Cramer JW et al (2003) In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci 23:8844–8853
Erickson MA, Banks WA (2013) Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J Cereb Blood Flow Metab 33:1500–1513. doi:10.1038/jcbfm.2013.135
Glenner GG, Wong CW (1984) Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 122:1131–1135
Glenner GG, Wong CW (1984) Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120:885–890
Gloor SM, Wachtel M, Bolliger MF, Ishihara H, Landmann R, Frei K (2001) Molecular and cellular permeability control at the blood-brain barrier. Brain Res Brain Res Rev 36:258–264
Guan H, Liu Y, Daily A, Police S, Kim MH, Oddo S et al (2009) Peripherally expressed neprilysin reduces brain amyloid burden: a novel approach for treating Alzheimer’s disease. J Neurosci Res 87:1462–1473. doi:10.1002/jnr.21944
Henderson SJ, Andersson C, Narwal R, Janson J, Goldschmidt TJ, Appelkvist P et al (2013) Sustained peripheral depletion of amyloid-beta with a novel form of neprilysin does not affect central levels of amyloid-beta. Brain 137:553–64. doi:10.1093/brain/awt308
Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ et al (2016) Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540:230–235. doi:10.1038/nature20587
Iijima-Ando K, Hearn SA, Granger L, Shenton C, Gatt A, Chiang HC et al (2008) Overexpression of neprilysin reduces alzheimer amyloid-beta42 (Abeta42)-induced neuron loss and intraneuronal Abeta42 deposits but causes a reduction in cAMP-responsive element-binding protein-mediated transcription, age-dependent axon pathology, and premature death in Drosophila. J Biol Chem 283:19066–19076. doi:10.1074/jbc.M710509200
Jacobson I, Sandberg M, Hamberger A (1985) Mass transfer in brain dialysis devices–a new method for the estimation of extracellular amino acids concentration. J Neurosci Methods 15:263–268
Jiao SS, Bu XL, Liu YH, Zhu C, Wang QH, Shen LL et al (2016) Sex Dimorphism profile of Alzheimer’s disease-type pathologies in an APP/PS1 mouse model. Neurotox Res 29:256–266. doi:10.1007/s12640-015-9589-x
Jiao SS, Yao XQ, Liu YH, Wang QH, Zeng F, Lu JJ et al (2015) Edaravone alleviates Alzheimer’s disease-type pathologies and cognitive deficits. Proc Natl Acad Sci USA 112:5225–5230. doi:10.1073/pnas.1422998112
Joachim CL, Mori H, Selkoe DJ (1989) Amyloid beta-protein deposition in tissues other than brain in Alzheimer’s disease. Nature 341:226–230. doi:10.1038/341226a0
Kitaguchi N, Hasegawa M, Ito S, Kawaguchi K, Hiki Y, Nakai S et al (2015) A prospective study on blood Abeta levels and the cognitive function of patients with hemodialysis: a potential therapeutic strategy for Alzheimer’s disease. J Neural Transm (Vienna, Austria: 1996) 122:1593–1607. doi:10.1007/s00702-015-1431-3
Liu YH, Giunta B, Zhou HD, Tan J, Wang YJ (2012) Immunotherapy for Alzheimer disease: the challenge of adverse effects. Nat Rev Neurol 8:465–469. doi:10.1038/nrneurol.2012.118
Liu YH, Wang YR, Xiang Y, Zhou HD, Giunta B, Manucat-Tan NB et al (2015) Clearance of amyloid-beta in Alzheimer’s disease: shifting the action site from center to periphery. Mol Neurobiol 51:1–7. doi:10.1007/s12035-014-8694-9
Liu YH, Xiang Y, Wang YR, Jiao SS, Wang QH, Bu XL et al (2015) Association between serum amyloid-beta and renal functions: implications for roles of kidney in amyloid-beta clearance. Mol Neurobiol 52:115–119. doi:10.1007/s12035-014-8854-y
Macauley SL, Stanley M, Caesar EE, Yamada SA, Raichle ME, Perez R et al (2015) Hyperglycemia modulates extracellular amyloid-beta concentrations and neuronal activity in vivo. J Clin Invest 125:2463–2467. doi:10.1172/JCI79742
Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M (2010) Alzheimer’s disease: clinical trials and drug development. Lancet Neurol 9:702–716. doi:10.1016/S1474-4422(10)70119-8
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA 82:4245–4249
Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC et al (2010) Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330:1774. doi:10.1126/science.1197623
Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO (2003) Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med 9:448–452. doi:10.1038/nm840
Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M et al (2016) CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 15:673–684. doi:10.1016/S1474-4422(16)00070-3
Pipili C, Polydorou A, Pantelias K, Korfiatis P, Nikolakopoulos F, Grapsa E (2013) Improvement of hepatic encephalopathy by application of peritoneal dialysis in a patient with non-end-stage renal disease. Perit Dial Int 33:213–216. doi:10.3747/pdi.2011.00271
Roberts KF, Elbert DL, Kasten TP, Patterson BW, Sigurdson WC, Connors RE et al (2014) Amyloid-beta efflux from the central nervous system into the plasma. Ann Neurol 76:837–844. doi:10.1002/ana.24270
Sakai K, Senda T, Hata R, Kuroda M, Hasegawa M, Kato M et al (2016) Patients that have undergone hemodialysis exhibit lower amyloid deposition in the brain: evidence supporting a therapeutic strategy for Alzheimer’s disease by removal of blood amyloid. J Alzheimers Dis 51:997–1002. doi:10.3233/JAD-151139
Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M et al (2009) A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology 73:2061–2070. doi:10.1212/WNL.0b013e3181c67808
Sehgal N, Gupta A, Valli RK, Joshi SD, Mills JT, Hamel E et al (2012) Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc Natl Acad Sci USA 109:3510–3515. doi:10.1073/pnas.1112209109
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8:595–608. doi:10.15252/emmm.201606210
Siska Mortier NHL, De Vriese An S (2005) Animal models in peritoneal dialysis research: a need for consensus. Perit Dial Int 25:16–24
Tholen S, Schmaderer C, Chmielewski S, Forstl H, Heemann U, Baumann M et al (2016) Reduction of amyloid-beta plasma levels by hemodialysis: an anti-amyloid treatment strategy? J Alzheimers disease 50:791–796. doi:10.3233/jad-150662
Troncone L, Luciani M, Coggins M, Wilker EH, Ho CY, Codispoti KE et al (2016) Abeta amyloid pathology affects the hearts of patients with Alzheimer’s disease: mind the heart. J Am Coll Cardiol 68:2395–2407. doi:10.1016/j.jacc.2016.08.073
Walker JR, Pacoma R, Watson J, Ou W, Alves J, Mason DE et al (2013) Enhanced proteolytic clearance of plasma Abeta by peripherally administered neprilysin does not result in reduced levels of brain Abeta in mice. J Neurosci 33:2457–2464. doi:10.1523/JNEUROSCI.3407-12.2013
Wang YR, Wang QH, Zhang T, Liu YH, Yao XQ, Zeng F et al (2016) Associations between hepatic functions and plasma amyloid-beta levels-implications for the capacity of liver in peripheral amyloid-beta clearance. Mol Neurobiol 54:2338–2344. doi:10.1007/s12035-016-9826-1
Wu X, Bai Y, Tan T, Li H, Xia S, Chang X et al (2014) Lithium ameliorates autistic-like behaviors induced by neonatal isolation in rats. Front Behav Neurosci 8:234. doi:10.3389/fnbeh.2014.00234
Xiang Y, Bu XL, Liu YH, Zhu C, Shen LL, Jiao SS et al (2015) Physiological amyloid-beta clearance in the periphery and its therapeutic potential for Alzheimer’s disease. Acta Neuropathol 130:487–499. doi:10.1007/s00401-015-1477-1
Xiong H, Callaghan D, Wodzinska J, Xu J, Premyslova M, Liu QY et al (2011) Biochemical and behavioral characterization of the double transgenic mouse model (APPswe/PS1dE9) of Alzheimer’s disease. Neurosci Bull 27:221–232. doi:10.1007/s12264-011-1015-7
Yao XQ, Jiao SS, Saadipour K, Zeng F, Wang QH, Zhu C et al (2015) p75NTR ectodomain is a physiological neuroprotective molecule against amyloid-beta toxicity in the brain of Alzheimer’s disease. Mol Psychiatry 20:1301–1310. doi:10.1038/mp.2015.49
Acknowledgements
This study was supported by National Natural Science Foundation of China (Grant Number 81471296 and 81625007). W.S. is the holder of the Tier 1 Canada Research Chair in Alzheimer’s Disease.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jin, WS., Shen, LL., Bu, XL. et al. Peritoneal dialysis reduces amyloid-beta plasma levels in humans and attenuates Alzheimer-associated phenotypes in an APP/PS1 mouse model. Acta Neuropathol 134, 207–220 (2017). https://doi.org/10.1007/s00401-017-1721-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-017-1721-y