Abstract
Amyloid-beta (Aβ) plays a central role in the pathogenesis of Alzheimer’s disease (AD), and it is a major therapeutic target for AD. It is proposed that removal of Aβ in blood can facilitate Aβ clearance from the brain, representing a promising therapeutic approach for AD. However, the efficacy and mechanisms for Aβ clearance by peripheral organs and tissues remain largely unknown. In the present study, 47 chronic kidney disease (CKD) patients (16 newly diagnosed patients who had never been dialyzed and 31 patients who were receiving dialysis) and 43 normal controls (NC) were enrolled. We found that serum Aβ levels were significantly higher in CKD patients than NC. CKD patients who were receiving dialysis had lower serum Aβ levels than patients without receiving dialysis, being comparable to NC. Furthermore, serum Aβ levels were correlated with renal functions reflected by estimated glomerular filtration rate (eGFR) and residual GFR (rGFR). Our study suggests that kidney is involved in peripheral clearance of Aβ, and dialysis might be a potential therapeutic approach of Aβ removal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD), defined by a gradual loss of renal function, and Alzheimer’s disease (AD) are both common diseases in the elderly [1, 2]. Amyloid-beta (Aβ) has been suggested to play a pivotal or causative role in the development of AD. Clearance of Aβ from the brain represents a major therapeutic strategy for AD. It has been proposed that removal of serum Aβ can facilitate Aβ efflux from the brain, thereby reducing brain Aβ accumulation [3]. Previously, we have proposed that removal of Aβ in periphery represents a promising therapeutic approach for removing brain Aβ [4–6]. However, the efficacy and mechanisms for Aβ clearance in peripheral organs and tissues remain largely unknown. Recently, CKD was found to be independently associated with dementia [7], suggesting that kidney might be a potential organ for peripheral clearance of Aβ [4]. In the present study, we investigated whether it is involved in Aβ clearance in a cohort of CKD patients.
Subjects and Methods
Subjects
The present study was approved by Institutional Review Board of Daping Hospital (Chongqing, China). Briefly, consecutive patients aged 40 years and over treated at the Department of Nephrology at Daping Hospital were recruited for the study. Between November 2013 and January 2014, all patients were enrolled, including 16 newly diagnosed CKD patients who had never been treated with peritoneal dialysis and 31 CKD patients who were receiving peritoneal dialysis. Age and gender-matched normal controls (NC) were recruited from the health examination center of the same hospital. Subjects were excluded from the study if they experienced symptomatic cerebral vascular events, liver dysfunction, or and inflammatory diseases. Additionally, subjects with abnormal cognition, assessed using the Chinese version of the mini-mental state examination (MMSE) and the activities of daily living (ADL) scale, were excluded. Both of these instruments were validated in our previous studies in Chinese elderly people [8].
Clinical Assessment and Blood Sampling
Demographic data including age, sex, educational level, and occupation were collected on admission. The medical history was collected as per our previous study from medical records and a formal questionnaire including current medications [8]. The data included prior head trauma and surgery, prior gas poisoning, schizophrenia, hypothyroidism, coronary heart diseases, atrial fibrillation, cerebrovascular diseases, chronic obstructive pulmonary disease, chronic hepatitis, chronic renal insufficiency, hypertension, diabetes mellitus, hypercholesterolemia, Parkinson’s disease, and regular use of non-steroidal anti-inflammatory or prescription drugs. Consents for blood sampling were obtained from all participants or their legal representatives. Fasting blood was sampled between 07:00 and 09:00 in order to take in account a possible circadian rhythm effect. A portion of fasting blood samples was aliquoted for measuring complete blood cell count, fasting glucose, thyroxin, creatinine, urea, uric acid, transaminase, and total cholesterol. Serum cystatin C (cysC) levels of CKD patients were also measured. For another portion of blood, serum was separated within 30 min after sampling and stored at −80 °C until further analysis of Aβ. Estimated glomerular filtration rate (eGFR), representing general GFR was calculated using the CKD-EPI equation based on serum creatinine levels [9]. The residual GFR (rGFR), representing the residual renal functions and “endogenous” GFR, was calculated based on cysC levels [10].
Quantification of Serum Aβ Levels
Serum Aβ40 and Aβ42 levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Covance, USA) according to the manufacturer’s instructions. Samples and standards were measured in duplicate, and the means of the duplicates were used for statistical analyses.
Statistics
In univariate analysis, continuous variables were tested for normal distribution with the Kolmogorov-Smirnov test. Differences in demographic characteristics and serum Aβ levels between groups were assessed using analysis of variance, Kruskal-Wallis tests, and Chi square tests, where appropriate. Specifically, partial correlation analysis was used to examine the correlations between Aβ levels and eGFR or rGFR. The data was expressed as the mean ± SD and significance was achieved when p < 0.05. All statistical analyses were performed with the statistical analysis software SPSS 18.0.
Results
Characteristics of Subjects
The demographic characteristics of subjects are shown in Table 1. Briefly, 47 CKD patients (31 dialysis patients and 16 non-dialysis patients) and 43 age and gender-matched NCs were enrolled into the present study. There were no differences in age, gender, frequency of hyperlipidemia, or diabetes mellitus (DM) between dialysis CKD, non-dialysis CKD, and NC. However, CKD patients were more frequent in hypertension compared with NC. As expected, CKD patients had lower eGFR, higher serum creatinine, urea, and cysC levels. Dialyzed CKD patients had lower markers of renal functions compared to non-dialyzed CKD patient as reflected by serum creatinine and urea levels. However, there was no difference in serum cysC levels and rGFR between dialysis and non-dialysis CKD patients.
Serum Aβ Levels Were Increased in CKD Patients
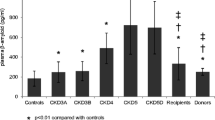
In the present study, we found that serum Aβ40 (NC vs CKD 66.59 ± 47.31 pg/ml vs 91.78 ± 50.21 pg/ml, p = 0.017) and Aβ42 levels (NC vs CKD 53.94 ± 37.97 pg/ml vs 84.46 ± 48.57 pg/ml, p = 0.001) were significantly higher in CKD patients. We further divided the CKD group into two subgroups according to the status of dialysis. We found that non-dialysis CKD patients had significantly higher serum Aβ40 (non-dialysis vs dialysis 124.38 ± 59.60 pg/ml vs 73.31 ± 32.61 pg/ml, p < 0.001; non-dialysis vs NC 124.38 ± 59.60 pg/ml vs 66.59 ± 47.31 pg/ml, p < 0.001) and Aβ42 levels (non-dialysis vs dialysis 120.53 ± 49.48 pg/ml vs 65.84 ± 36.63 pg/ml, p < 0.001; non-dialysis vs NC 120.53 ± 49.48 pg/ml vs 53.94 ± 37.97 pg/ml, p < 0.001) than dialysis CKD patients and NC. Interestingly, dialysis CKD patients had comparable serum Aβ40 (p = 0.613) and Aβ42 (p = 0.207) levels with NC (Fig. 1).
Correlations Between Serum Aβ Levels and Renal Functions
Partial correlation analysis was used to investigate the correlations of serum Aβ levels with eGFR and rGFR, with adjustment for age, gender, and comorbidities such as hyperlipidemia, hypertension, and DM. In the model including all subjects, both serum Aβ40 (γ = −0.388, p < 0.001) and Aβ42 (γ = −0.452, p < 0.001) levels were correlated with eGFR. In the model including CKD patients only, both serum Aβ40 (γ = −0.509, p = 0.001) and Aβ42 (γ = −0.567, p < 0.001) levels were also correlated with eGFR. Furthermore, both serum Aβ40 (γ = −0.509, p = 0.001) and Aβ42 (γ = −0.567, p < 0.001) levels were also correlated with rGFR.
Discussion
In the present study, we found that serum Aβ levels were significantly increased in CKD patients. Moreover, subgroup analysis indicated that the serum Aβ levels in dialysis CKD patients were lower than that of non-dialysis patients and were comparable to that of NC. Serum Aβ levels were correlated with renal functions in CKD patients, as monitored by eGFR and rGFR (Fig. 2).
Insufficient clearance of brain Aβ contributes to the initiation and progression of sporadic AD [11]. As brain Aβ equilibrates with Aβ in serum, peripheral clearance of Aβ provides a potential approach to facilitate efflux of Aβ from the brain [4]. It is worthy to note that adverse effects subsequent to the entry of therapeutic agents into the brain might also compromise the therapeutic effects of Aβ-targeting trials [6]. In this regard, removing Aβ in periphery might represent a safer approach for clearing Aβ from the brain [4]. However, the capacity of Aβ clearance by peripheral organs and their contribution to the development of AD remains largely unknown. Aβ is known to be a normal component of human urine [12], indicating that the urinary system may participate in Aβ clearance. A previous study demonstrated that serum creatinine levels were positively correlated with Aβ levels, indicating that renal functions are involved in Aβ homeostasis [13]. To test this hypothesis, we further analyzed the association between serum Aβ levels and renal functions. As dialysis significantly affects serum creatinine levels, eGFR reflects the efficacy of dialysis but not the endogenous renal functions of dialyzed CKD patients. In this regard, we further estimated rGFR of CKD patients according to serum cysC levels, which is not influenced by dialysis [14]. Interestingly, we found that serum Aβ levels were correlated with both eGFR and rGFR. These findings support the significant role of kidney in Aβ hemostasis and the idea that impairment of renal functions might lead to insufficient Aβ clearance and thus contribute to the development of AD [4]. Indeed, CKD increases risks for developing dementia including AD [15].
Based on the peripheral “sink” hypothesis, clearance of serum Aβ may represent a potential approach for AD prevention or treatment, which might avoid the adverse effects associated with the entry of Aβ-targeted agents into the brain [4]. A recent study found that a single nucleotide polymorphism (SNP) of CD33, which reduces the capacity of Aβ endocytosis in circulating monocytes, increases the risk of AD [16]. This finding implies the importance of peripheral Aβ clearance in AD. Consistently, removal of blood Aβ by enhancing Aβ metabolism in liver or long-term expression of Aβ degrading enzyme neprilysin can potentially reduce brain Aβ burden in an AD mouse model [17, 18], although recent studies suggest that periodical injection of recombinant neprilysin reduced Aβ in blood but not in brain [19, 20]. In the present study, we found that dialysis can significantly reduce serum Aβ in CKD patients, suggesting that dialysis might be a new therapeutic approach to remove Aβ from blood. Removal of brain Aβ via this peripheral approach can avoid adverse effects related to brain entry of Aβ removing agents [6]. Thus, the effectiveness of peripheral Aβ clearance becomes an important issue to be addressed in the future [5].
As Aβ plays a pivotal or causative role in the pathogenesis of AD, Aβ-targeting therapies are among the main-stream therapeutic strategies of AD. However, currently Aβ-centered therapies have not led to positive outcomes so far in clinical trials. The failures of these trials might be attributed to that the intervention is given too late to reverse the disease. Meanwhile, targeting of Aβ alone might not be enough to hold or slow the disease progression, as multiple mechanisms are involved in AD pathogenesis [5].
The present study suggests that the kidney is involved in peripheral clearance of Aβ, suggesting that decrease in the capacity of peripheral Aβ clearance in peripheral organs may also contribute to the development of AD. Maintenance and restoration of renal function might be protective against AD development.
References
Coresh J et al (2007) Prevalence of chronic kidney disease in the United States. JAMA 298(17):2038–2047
James BD et al (2014) Contribution of Alzheimer disease to mortality in the United States. Neurology 82(12):1045–1050
Lemere CA et al (2003) Evidence for peripheral clearance of cerebral Abeta protein following chronic, active Abeta immunization in PSAPP mice. Neurobiol Dis 14(1):10–18
Liu YH, et al (2014) Clearance of Amyloid-Beta in Alzheimer's Disease: Shifting the Action Site from Center to Periphery. Mol Neurobiol
Wang YJ (2014) Alzheimer disease: Lessons from immunotherapy for Alzheimer disease. Nat Rev Neurol 10(4):188–189
Liu YH et al (2012) Immunotherapy for Alzheimer disease: the challenge of adverse effects. Nat Rev Neurol 8(8):465–469
Miwa K et al (2014) Chronic kidney disease is associated with dementia independent of cerebral small-vessel disease. Neurology 82(12):1051–1057
Li J et al (2011) Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology 76(17):1485–1491
Levey AS et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Hoek FJ et al (2007) Estimation of residual glomerular filtration rate in dialysis patients from the plasma cystatin C level. Nephrol Dial Transplant 22(6):1633–1638
Wang YJ, Zhou HD, Zhou XF (2006) Clearance of amyloid-beta in Alzheimer's disease: progress, problems and perspectives. Drug Discov Today 11(19–20):931–938
Ghiso J et al (1997) Alzheimer's soluble amyloid beta is a normal component of human urine. FEBS Lett 408(1):105–108
Arvanitakis Z et al (2002) Serum creatinine levels correlate with plasma amyloid Beta protein. Alzheimer Dis Assoc Disord 16(3):187–190
Delaney MP et al (2008) Relationship of serum cystatin C to peritoneal and renal clearance measures in peritoneal dialysis: a cross-sectional study. Am J Kidney Dis 51(2):278–284
Helmer C et al (2011) Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology 77(23):2043–2051
Bradshaw EM et al (2013) CD33 Alzheimer's disease locus: altered monocyte function and amyloid biology. Nat Neurosci 16(7):848–850
Sehgal N et al (2012) Withania somnifera reverses Alzheimer's disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc Natl Acad Sci U S A 109(9):3510–3515
Liu Y et al (2010) Circulating neprilysin clears brain amyloid. Mol Cell Neurosci 45(2):101–107
Henderson SJ et al (2014) Sustained peripheral depletion of amyloid-beta with a novel form of neprilysin does not affect central levels of amyloid-beta. Brain 137(Pt 2):553–564
Walker JR et al (2013) Enhanced proteolytic clearance of plasma Abeta by peripherally administered neprilysin does not result in reduced levels of brain Abeta in mice. J Neurosci 33(6):2457–2464
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30973144), PLA healthcare research grant (13BJZ31), Chinese postdoctor scientific grant (2013 T60955) and Chongqing postdoctor research grant (XM201342).
Conflicts of Interests
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yu-Hui Liu and Yang Xiang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, YH., Xiang, Y., Wang, YR. et al. Association Between Serum Amyloid-Beta and Renal Functions: Implications for Roles of Kidney in Amyloid-Beta Clearance. Mol Neurobiol 52, 115–119 (2015). https://doi.org/10.1007/s12035-014-8854-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8854-y