Abstract
Introduction
To evaluate the effect of antenatal magnesium sulfate (MgSO4) on mortality and morbidity outcomes related to the gastrointestinal system (GI) in preterm infants.
Methods
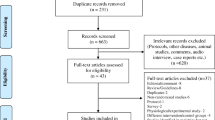
Data sources: A systematic literature search was conducted in November 2022. PubMed, CINAHL Plus with Full Text (EBSCOhost), Embase (Elsevier), and CENTRAL (Ovid) were searched. There were 6695 references. After deduplication, 4332 remained. Ninety-nine full-text articles were assessed and forty four articles were included in the final analysis.
Study eligibility criteria
Randomized or quasi-randomized clinical trials and observational studies that evaluated at least one of the pre-specified outcomes were included. Preterm infants whose mothers were given antenatal MgSO4 were included and whose mothers did not receive antenatal MgSO4 were the comparators. The main outcomes and measures were: Necrotizing enterocolitis (NEC) (stage ≥ 2), surgical NEC, spontaneous intestinal perforation (SIP), feeding intolerance, time to reach full feeds, and GI-associated mortality.
Study appraisal and synthesis methods
A random-effects model meta-analysis was performed to yield pooled OR and its 95% CI for each outcome due to expected heterogeneity in the studies. The analysis for each predefined outcome was performed separately for adjusted and unadjusted comparisons. All included studies were assessed for methodological quality. The risk of bias was assessed using elements of the Cochrane Collaboration’s tool 2.0 and the Newcastle–Ottawa Scale for randomized controlled trials (RCTs) and non-randomized studies (NRS), respectively. The study findings were reported as per PRISMA guidelines.
Results
A total of thirty-eight NRS and six RCTs involving 51,466 preterm infants were included in the final analysis. There were no increased odds of stage ≥2 NEC, (NRS : n = 45,524, OR: 0.95; 95% CI: 0.84–1.08, I2- 5% & RCT’s: n = 5205 OR: 1.00; 95% CI: 0.89–1.12, I2- 0%), SIP (n = 34,186, OR: 1.22, 95% CI: 0.94–1.58, I2–30%), feeding intolerance (n = 414, OR: 1.06, 95% CI: 0.64–1.76, I2–12%) in infants exposed to antenatal MgSO4. On the contrary, the incidence of surgical NEC was significantly lower in MgSO4 exposure infants (n = 29,506 OR:0.74; 95% CI: 0.62–0.90, ARR: 0.47%). Studies assessing the effect on GI-related mortality were limited to make any conceivable conclusion. The certainty of evidence (CoE) for all outcomes was adjudged as ‘very low’ as per GRADE.
Conclusion
Antenatal magnesium sulfate did not increase the incidence of gastrointestinal-related morbidities or mortality in preterm infants. With the current evidence concerns, regarding the adverse effects of MgSO4 administration leading to NEC/SIP or GI-related mortality in preterm infants should not be a hurdle in its routine use in antenatal mothers.
Similar content being viewed by others
Introduction
Magnesium sulfate (MgSO4) is widely used in obstetric patients for indications such as treatment for preeclampsia, tocolysis, and neuroprotection when preterm delivery is imminent [1, 2]. Recent evidence suggests that antenatal magnesium sulfate is effective in the reduction of moderate to severe cerebral palsy by up to 40% [3, 4]. The American College of Obstetricians and Gynecologists (ACOG) in 2010 and the World Health Organization (WHO) in 2015 endorsed the use of MgSO4 administration to antenatal women to reduce the risk of cerebral palsy (CP) when delivery is anticipated before 32 weeks [5, 6]. A recent meta-analysis found a number needed to treat of 42 to prevent CP [7]. When used as a tocolytic in preterm deliveries, MgSO4 was shown to prolong the delivery despite its safety concerns as compared to other tocolytic agents such as calcium channel blockers and betamimetics [8]. MgSO4 in preeclampsia is known to reduce the risk of seizures by 50% which makes it an effective treatment in women to prevent eclampsia [9].
When administered to antenatal mothers, MgSO4 can rapidly cross the placenta resulting in an increase in fetal and newborn magnesium levels in the serum [10]. Magnesium is known to produce fecal impaction and intestinal atony in infants by depressing the cholinergic activity in mothers exposed to high levels of magnesium [11, 12]. Hypotonia, hypercalciuria, parathyroid gland suppression, abnormal bone mineralization, and neutrophil dysfunction are some of the side effects reported in infants when mothers are exposed to MgSO4 [13,14,15,16]. Intestinal hypomotility with increased meconium overload can lead to increased intraluminal pressure and thinning of intestinal walls which could be exaggerated and increase the risk of NEC or SIP when concomitantly exposed to steroids [17, 18]. MgSO4 acts as a competitive antagonist against calcium and decreases the intracellular calcium and impedes intestinal smooth muscle contractility by interfering with actin-myosin interaction [19]. Studies have shown maternal exposure to MgSO4 resulted in a decrease in intestinal blood flow and an increase in feeding intolerance in infants [11, 20, 21]. The alterations in intestinal blood flow can affect feeding intolerance and subsequent development of necrotizing enterocolitis (NEC) or spontaneous intestinal perforation (SIP) [22,23,24,25,26]. MgSO4 has been associated with increased incidence of NEC in smaller infants and higher doses in recent studies [27, 28]. A few studies also provided evidence to the contrary [29,30,31]. A recent meta-analysis by Sheperd et al. [32] did not show an association between antenatal MgSO4 and neonatal adverse outcomes.
Objective
We conducted a systematic review and meta-analysis to address three questions: Is there an association between antenatal MgSO4 administration and adverse gastrointestinal outcomes (GI) like NEC, SIP, feeding intolerance, time to reach full feeds, and GI-related mortality in preterm infants? In extreme preterm (<28 weeks) and very preterm infants (<32 weeks) does the administration of antenatal MgSO4 have any adverse gastrointestinal outcomes (GI)? And thirdly, is the association based on the different types of indications of MgSO4 like for neuroprotection, preeclampsia, or tocolysis?
Methods
The systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines [33]. Our protocol was registered on the PROSPERO (CRD42022367246), the international prospective register for systematic reviews.
Eligibility criteria
Randomized or quasi-randomized clinical trials and non-randomized studies that reported GI-related morbidities when mothers of preterm infants were given MgSO4 for either neuroprotection, tocolysis, or preeclampsia were included. Studies that did not report prespecified neonatal outcomes were excluded. Preterm infants whose mothers did not receive antenatal magnesium sulfate were considered as comparators. The following outcomes were included in this meta-analysis:
-
1.
Necrotizing enterocolitis (stage ≥ 2 NEC) (defined as per modified Bell’s staging) [34]
-
2.
Surgical necrotizing enterocolitis (those needing surgical intervention)
-
3.
Spontaneous intestinal perforation (SIP) (defined as per international standards) [35]
-
4.
Feeding intolerance—as defined by authors.
-
5.
Time to reach full feeds—Minimum of 150 ml/kg/d
-
6.
Gastrointestinal (GI) related mortality—mortality related to NEC/SIP as reported by authors.
Information sources and search strategy
A systematic literature search was conducted in November 2022 using the following databases: PubMed, CINAHL Plus with Full Text (EBSCOhost), Embase (Elsevier), and the Cochrane Central Register of Controlled Trials, or CENTRAL (Ovid). The references of the identified studies were also screened. No date or language restrictions or search filters were employed. Searches were run by a medical librarian (N.A.) in consultation with authors A.P. and S.D. Details of the search strategy are provided in the online Supplementary File. Results were deduplicated using the SR-Accelerator Deduplicator tool.
Study selection
The deduplicated results were transferred to Rayyan software (www.rayyan.ai) for screening. Title and abstract screening and full-text review of articles were done independently by A.P. and S.D. Any discrepancies were resolved by discussion and consensus (P.C). If necessary, trial authors were contacted by email correspondence to request missing data.
Assessment of risk of bias
All included studies were assessed for methodological quality. The risk of bias was assessed using elements of the Cochrane Collaboration tool 2.0 for randomized studies [36]. For non-randomized studies, the risk of bias for included studies was assessed using a modified Newcastle–Ottawa scale [37]. The following domains were evaluated: selection, comparability, and outcome. A priori, a score of >7/9 was deemed low risk, a score of 4–6/9 was deemed a moderate risk, and a score of ≤3/9 was deemed a high risk of bias. Two authors (A.P. and S.D.) performed the risk of bias independently; conflicts were resolved after discussion and consensus. Similarly, A.P. and S.D. assessed the certainty of evidence (confidence in the estimate of effect) for each outcome based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework [38]. Any discrepancies were resolved by discussion and consensus.
Data synthesis
All the studies were combined and analyzed using Review Manager V.5.3 (Cochrane Collaboration, Nordic Cochrane Center, Copenhagen, Denmark). The mean difference with 95% CI was calculated for continuous outcomes. For dichotomous outcomes, the OR with 95% CI was calculated from the data provided in the studies. Adjusted odds ratios for potential confounders were extracted from the studies reporting these data. Studies reporting continuous variables as median and range or interquartile range were converted to mean and SD using a published calculator [39]. The random effects model was used to calculate summary statistics owing to anticipated heterogeneity. For some variables like gestational age, a fixed outcome model was used. Statistical heterogeneity was assessed by use of the Cochran Q statistic and by use of the I2 statistic, which is derived from the Q statistic and describes the proportion of total variation that is due to heterogeneity beyond chance. We used the Egger regression test and funnel plots to assess publication bias if more than 10 studies were available for that particular outcome.
Sensitivity analysis was performed for studies based on the different type of indications for MgSO4 (neuroprotection, preeclampsia, tocolysis) and based on gestational age (<28 weeks, <32 weeks)
Results
Study selection and Study characteristics
A total of 6695 articles were identified through database searching. After duplicates were removed, 4332 articles underwent title and abstract screening. Ninety-nine full-text articles were assessed. A final total of 44 articles including 6 RCTs [40,41,42,43,44,45] and 38 non-randomized studies [20, 21, 27, 29,30,31, 46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76] were deemed eligible to be included in the final meta-analysis (Summary Table 1). The PRISMA flow diagram [33] is shown in Fig. 1a. A list of excluded studies and search strategies are provided in the Online Supplementary File.
-
i.
Medical necrotizing enterocolitis (Stage ≥ 2 NEC) was reported in 40 included studies [20, 21, 29,30,31, 40,41,42,43,44, 46,47,48,49,50,51, 53,54,55,56,57,58,59,60,61,62,63,64, 68, 72] involving 51,466 infants. The pooled effect estimate favored the MgSO4 group but was not statistically significant between the two groups in non-randomized studies (n = 45,524, OR: 0.95; 95% CI: 0.84–1.08, I2- 5%) and randomized controlled trials (n = 5205 OR:1.24; 95% CI: 0.99–1.56, I2- 0%) (shown in Fig. 2a, b). Sensitivity analysis of infants <28 weeks reported in 5 studies (n = 5133, OR: 1.0; 95% CI: 0.83 to 1.20, I2– 0%) reported no difference between the two groups (shown in Fig. 3a)
-
ii.
Surgical NEC was reported in 5 studies [48, 63, 70, 71, 75] of the 44 studies involving 29,506 infants. The incidence of surgical NEC was significantly lower in the MgSO4 group compared with controls (n = 29,506 OR:0.74; 95% CI: 0.62–0.90, ARR: 0.47%) (shown in Fig. 2c). There was no heterogeneity noted between the studies. Sensitivity analysis revealed no significance for infants <32 weeks including 3 studies [70, 71, 75] (n-1163, OR: 0.60; 95% CI: 0.36 to 1.01, I2- 0%) (shown in Fig. 3c).
-
iii.
Spontaneous intestinal perforation was reported in 8 out of the 44 studies [27, 29, 31, 48, 63, 70, 71, 75] including 34,186 infants. Antenatal exposure of MgSO4 did not increase the incidence of SIP in preterm infants (n = 34,186, OR: 1.22, 95% CI: 0.94–1.58, I2–30%) (shown in Fig. 2d). Subgroup analysis of the incidence of SIP in infants <32 weeks was analyzed in 5 studies [27, 70, 71, 74, 75] and there was no difference between groups (n-1, 610 OR: 1.54, 95% CI: 0.67 to 3.55, I2 – 34%) (shown in Fig. 3d).
-
iv.
Time to reach full feeds was also analyzed and was reported in 8 [21, 52, 59, 65, 68, 71,72,73] of the 44 included studies involving 3,258 infants. Full enteral feeds were defined as a minimum of 150 cc/kg/day for analysis. There was no difference in time taken to achieve full feeds (20.3 d vs 20.7 d in the MgSO4 group vs the control group (MD:1.48: (95% CI: −0.76 to 3.72) (shown in Fig. 2e).
-
v.
Feeding intolerance was reported in 3 studies [21, 29, 73] out of the 44 involving 414 infants (n = 414, OR: 1.06 (95% CI: 0.64–1.76, I2- 12%) (shown in Fig. 2f).
-
vi.
GI-related mortality (NEC/SIP related) was reported in only one study (n = 4355) [29]. There was no statistically significant difference between the two groups (4.6% vs 3.8%, p - 0.18) in mortality. Stratified analysis based on gestational age (22–25 weeks & 26–27 weeks) did not show any significance either between the two groups.
Flowchart of search results (adapted from PRISMA 2021) [33] GI-gastrointestinal, NEC-Necrotizing enterocolitis, SIP-Spontaneous intestinal perforation; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
a Necrotizing enterocolitis (Stage ≥ 2) (≤28 weeks). b Necrotizing enterocolitis (Stage ≥ 2) (≤32 weeks). c Surgical Necrotizing enterocolitis (≤32 weeks). d Spontaneous intestinal perforation (SIP) (≤32 weeks). e Necrotizing enterocolitis (Stage ≥ 2) (Neuroprotection & ≤32 weeks). f Necrotizing enterocolitis (Stage ≥ 2) (Neuroprotection & Preeclampsia & ≤32 weeks). g Spontaneous intestinal perforation (Neuroprotection). h Spontaneous intestinal perforation (≤32 weeks). i Time to reach full feeds (150 cc/kg/d) (≤32 weeks). j Time to reach full feeds (150 cc/kg/d) (Neuroprotection).
Sensitivity and adjusted analysis
Sensitivity analysis was performed separately for all studies including infants less than 32 weeks of gestation. Seventeen studies involving 10,021 infants were analyzed [40, 43,44,45, 54, 55, 58,59,60,61,62, 64, 66,67,68,69,70,71,72,73,74,75]. The incidence of medical NEC was similar among the MgSO4 group as compared to controls (OR: 0.84; 95% CI: 0.66 to 1.09) (shown in Fig. 3b). Studies that used MgSO4 for neuroprotection alone (13 studies, n = 10,471) were analyzed, and neuroprotection or preeclampsia (5 studies, n = 4870) were analyzed separately. Both analyses did not show any statistical difference between the two groups (shown in Fig. 3e, f). For the outcome of SIP, sensitivity analysis was performed for very preterm infants and studies that used MgSO4 for neuroprotection. A total of 3 studies for neuroprotection [27, 31, 74] (shown in Fig. 3g) and seven studies [27, 29, 31, 70, 71, 74, 75] for <32 weeks were included (shown in Fig. 3h) and there was no difference between the two groups. For other indications like tocolysis and preeclampsia, not enough studies were available for metanalysis. For the outcome of time to reach full feeds subgroup analysis on preterm infants <32 weeks [52, 59, 71,72,73, 77] (shown in Fig. 3i) and studies that used MgSO4 for neuroprotection [52, 59, 77] (shown in Fig. 3j) showed no difference between two groups.
We performed an adjusted odds ratio analysis for medical NEC (5 studies) and surgical NEC (3 studies) and SIP (4 studies) (shown in Fig. 4a–c). None of the analysis showed any statistical significance between the two groups. Analysis for superior mesenteric artery (SMV) blood flow velocity (cm/s) indices like mean velocity (MV), peak systolic velocity (PSV), and end-diastolic velocity (EDV) was reported in only 2 studies [21, 73]. There was no difference between the two groups for all the indices (online supplementary file).
Risk of bias (RoB) and Certainty of Evidence (CoE)
All the non-randomized studies were assessed for quality of evidence using the Newcastle-Ottawa scale [37]. All 38 studies were scored as good (>6) (Online supplementary file). The risk of bias assessment for RCTs was performed as per the Cochrane Risk of Bias 2.0 tool [78] in five domains (shown in Fig. 5). Of the six RCTs included, two studies were adjudged as high risk of bias [41, 42], one with some concerns [43], and two studies with low risk of bias (shown Fig. 5) [40, 45].
The certainty of evidence (CoE) as per GRADE [38] for all outcomes is provided in Table 2. For outcomes of medical and surgical NEC, SIP, and GI-related mortality, the CoE was determined to be “very low”.
Neither visual inspection of funnel plots nor the Egger test suggested publication or selection bias for the outcome of medical NEC [20, 21, 30, 31, 40,41,42,43,44,45,46,47,48,49,50,51, 53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72, 74,75,76] (41 studies) (shown in Fig. 6). The number of studies was insufficient to evaluate publication bias for the other outcomes.
Discussion
Our review demonstrates that there is no difference in the incidence of medical necrotizing enterocolitis SIP, feeding intolerance, or time to reach full feeds in infants exposed to antenatal magnesium sulfate as compared to no exposure. We did not find any adverse association between the use of antenatal MgSO4 and GI morbidities in extreme preterm or very preterm infants. On the contrary, infants born to mothers who received antenatal MgSO4 had a lower incidence of surgical NEC by 26%. There was no difference in outcomes of NEC based on the type of indication like neuroprotection, preeclampsia, or tocolysis. Antenatal MgSO4 exposure did not increase gastrointestinal-related mortality in preterm infants.
Magnesium Sulfate has been used in imminent preterm deliveries less than 32 weeks for neuroprotection as a routine practice for the past decade after the large clinical trials [42, 44] and endorsement by ACOG and WHO [5, 6]. Its role in preeclampsia and as a tocolytic had been established long before [8, 9]. Concerns about maternal adverse effects were highlighted for the last 50 years but red flags were raised for its association with NEC and SIP in preterm infants since 2014 [27]. MgSO4 at higher cumulative doses and smaller infants (<25 weeks) increased the odds of NEC and SIP by 2–9 times [27, 28]. Contrasting evidence with absolutely no association was found in large observational studies [29, 63]. This difference could be explained by the adjustment of confounding factors for SIP/NEC in the later studies, for example, the use of steroids, indomethacin, small for gestational, and use of mechanical ventilation. We found no association between MgSO4 and NEC when adjusted odds ratio (aOR) was analysed, with a caveat that the adjusted confounding factors were not uniform across all studies and only 7 studies [29, 53, 54, 63, 66, 70, 75] reported the adjusted data (Shown in Fig. 4).
Various hypotheses were proposed for the association between antenatal MgSO4 and adverse GI-outcomes. These include calcium antagonism and decreasing actin-myosin interaction, intestinal hypomotility, increased resistance, and reduction of blood flow in the mesenteric artery. Our results are consistent with the results of the previous meta-analysis [4, 32]. Zeng et al reported on cerebral palsy and overall mortality with ten studies included and Shepherd et al reported-on NEC, SIP and NEC/SIP-related death as a part of an overall review of neonatal outcomes of antenatal MgSO4. However the previous reviews [4, 32] were not specific to the GI-outcomes, the number of included studies were significantly less compared to the current review which justifies the need for extensive and updated review.
We found that the antenatal MgSO4 group had decreased the incidence of surgical NEC by 26%. This finding is contrary to the conventional reports from previous studies [27, 28]. The reduced incidence of surgical NEC in preterm infants exposed to antenatal MgSO4 had been reported in previous observational studies [31, 63]. This positive effect could be due to the attenuation of peroxide-induced vasoconstriction by inhibiting thromboxane synthesis [79]. Thromboxane is an inciting agent for NEC [34]. MgSO4 also increases the blood flow by vasodilatation [79]. We also attribute the beneficial effects due to the overall benefit of MgSO4 in preterm infants and the fact that infants in the MgSO4 group could be less sick and more hemodynamically stable. This observation needs further exploration by larger studies.
The effect of MgSO4 on intestinal blood flow indices is inconclusive. Theoretically, MgSO4 will induce vasodilatation by calcium antagonism, attenuation of peroxide-induced vasoconstriction, inhibition of platelet activation induced by adenosine diphosphate, and release of vasoactive substances [79, 80]. Studies reporting on blood flow velocities in mesenteric arteries were limited [20, 21, 73] and none reported any significant change in mean velocity, end-diastolic velocity, peak systolic velocity, or the resistivity index between the two groups (online supplementary file).
Conclusions and implications
Our study findings do not support any clear association between antenatal magnesium sulfate administration and adverse gastrointestinal morbidities or gastrointestinal-related mortality in preterm infants. Concerns regarding the adverse effects of antenatal MgSO4 administration on GI morbidity or mortality should not be a hurdle in its routine use when indicated. This review should encourage clinicians to not deviate from feeding practices in the neonatal units when premature infants are exposed to antenatal MgSO4. The biological plausibility of MgSO4 causing NEC or SIP by decreased blood flow in mesenteric arteries needs further studies.
Strengths and limitations
The strength of this meta-analysis was that we used a comprehensive search strategy for our literature search, published our protocol a priori, and assessed the certainty of evidence using GRADE. To our knowledge, this is the largest meta-analysis on adverse neonatal GI outcomes and antenatal MgSO4. Nonetheless, there were several limitations. The majority of the studies were non-randomized, studies were heterogeneous, variable doses of MgSO4 were used in different studies and dose-related associations were not evaluated.
Data availability
All data included in the analysis are available in public domain. The details including search strategy, list of excluded studies with reason for exclusion, risk of bias assessment and quality assessment of included studies are all available in the online supplementary file.
References
Mittendorf R. Magnesium sulfate tocolysis: time to quit. Obstet Gynecol. 2007;109:1204–5.
Nelson KB, Grether JK. Can magnesium sulfate reduce the risk of cerebral palsy in very low birthweight infants? Pediatrics. 1995;95:263–9.
Costantine MM, Weiner SJ, Eunice Kennedy Shriver National Institute of Child H, Human Development Maternal-Fetal Medicine Units N. Effects of antenatal exposure to magnesium sulfate on neuroprotection and mortality in preterm infants: a meta-analysis. Obstet Gynecol. 2009;114:354–64.
Zeng X, Xue Y, Tian Q, Sun R, An R. Effects and safety of magnesium sulfate on neuroprotection: a meta-analysis based on PRISMA guidelines. Medicine (Baltimore). 2016;95:e2451.
Committee Opinion No. 455. Magnesium sulfate before anticipated preterm birth for neuroprotection. Obstet Gynecol. 2010;115:669–71.
WHO recommendations on interventions to improve preterm birth outcomes. Geneva, Switzerland: World Health Organization; 2015.
Crowther CA, Middleton PF, Voysey M, Askie L, Duley L, Pryde PG, et al. Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: an individual participant data meta-analysis. PLoS Med. 2017;14:e1002398.
Wilson A, Hodgetts-Morton VA, Marson EJ, Markland AD, Larkai E, Papadopoulou A, et al. Tocolytics for delaying preterm birth: a network meta-analysis (0924). Cochrane Database Syst Rev. 2022;8:CD014978.
Duley L, Gulmezoglu AM, Henderson-Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;2010:CD000025.
Cruikshank DP, Pitkin RM, Reynolds WA, Williams GA, Hargis GK. Effects of magnesium sulfate treatment on perinatal calcium metabolism. I. Maternal and fetal responses. Am J Obstet Gynecol. 1979;134:243–9.
Lu JF, Nightingale CH. Magnesium sulfate in eclampsia and pre-eclampsia: pharmacokinetic principles. Clin Pharmacokinet. 2000;38:305–14.
Santafe MM, Garcia N, Lanuza MA, Tomas M, Besalduch N, Tomas J. Presynaptic muscarinic receptors, calcium channels, and protein kinase C modulate the functional disconnection of weak inputs at polyinnervated neonatal neuromuscular synapses. J Neurosci Res. 2009;87:1195–206.
Riaz M, Porat R, Brodsky NL, Hurt H. The effects of maternal magnesium sulfate treatment on newborns: a prospective controlled study. J Perinatol. 1998;18:449–54.
Rantonen T, Kaapa P, Jalonen J, Ekblad U, Peltola O, Valimaki I, et al. Antenatal magnesium sulphate exposure is associated with prolonged parathyroid hormone suppression in preterm neonates. Acta Paediatr. 2001;90:278–81.
Malaeb SN, Rassi AI, Haddad MC, Seoud MA, Yunis KA. Bone mineralization in newborns whose mothers received magnesium sulphate for tocolysis of premature labour. Pediatr Radio. 2004;34:384–6.
Mehta R, Petrova A. Intrapartum magnesium sulfate exposure attenuates neutrophil function in preterm neonates. Biol Neonate. 2006;89:99–103.
Gordon PV, Price WA, Stiles AD, Rutledge JC. Early postnatal dexamethasone diminishes transforming growth factor alpha localization within the ileal muscularis propria of newborn mice and extremely low-birth-weight infants. Pediatr Dev Pathol. 2001;4:532–7.
Gordon PV, Paxton JB, Herman AC, Carlisle EM, Fox NS. Igf-I accelerates ileal epithelial cell migration in culture and newborn mice and may be a mediator of steroid-induced maturation. Pediatr Res. 2004;55:34–41.
Iams J. CR, In: Creasy RK RR, Iams J, Lockwood, C MTe. Preterm labor and delivery. Maternal-Fetal Medicine: Principles and Practice. 2004. pp 623–61.
Havranek T, Ashmeade TL, Afanador M, Carver JD. Effects of maternal magnesium sulfate administration on intestinal blood flow velocity in preterm neonates. Neonatology. 2011;100:44–9.
Gursoy T, Imamoglu EY, Ovali F, Karatekin G. Effects of antenatal magnesium exposure on intestinal blood flow and outcome in preterm neonates. Am J Perinatol. 2015;32:1064–9.
Fang S, Kempley ST, Gamsu HR. Prediction of early tolerance to enteral feeding in preterm infants by measurement of superior mesenteric artery blood flow velocity. Arch Dis Child Fetal Neonatal Ed. 2001;85:F42–5.
Robel-Tillig E, Knupfer M, Pulzer F, Vogtmann C. Blood flow parameters of the superior mesenteric artery as an early predictor of intestinal dysmotility in preterm infants. Pediatr Radio. 2004;34:958–62.
Maruyama K, Koizumi T, Tomomasa T, Morikawa A. Intestinal blood-flow velocity in uncomplicated preterm infants during the early neonatal period. Pediatr Radio. 1999;29:472–7.
Coombs RC, Morgan ME, Durbin GM, Booth IW, McNeish AS. Abnormal gut blood flow velocities in neonates at risk of necrotising enterocolitis. J Pediatr Gastroenterol Nutr. 1992;15:13–9.
Kempley ST, Gamsu HR. Superior mesenteric artery blood flow velocity in necrotising enterocolitis. Arch Dis Child. 1992;67:793–6.
Rattray BN, Kraus DM, Drinker LR, Goldberg RN, Tanaka DT, Cotten CM. Antenatal magnesium sulfate and spontaneous intestinal perforation in infants less than 25 weeks gestation. J Perinatol. 2014;34:819–22.
Kamyar M, Clark EA, Yoder BA, Varner MW, Manuck TA. Antenatal magnesium sulfate, necrotizing enterocolitis, and death among neonates <28 weeks gestation. AJP Rep. 2016;6:e148–54.
Shalabi M, Mohamed A, Lemyre B, Aziz K, Faucher D, Shah PS, et al. Antenatal exposure to magnesium sulfate and spontaneous intestinal perforation and necrotizing enterocolitis in extremely preterm neonates. Am J Perinatol. 2017;34:1227–33.
Wiswell TE, Caddell JL, Graziani LJ, Kornhauser MS, Spitzer AR. Maternally-administered magne- sium sulfate (MgSO4) decreases the incidence of severe necrotizing enterocolitis (NEC) in preterm infants: a prospective study. Pediatr Res. 1996;39:1501.
Mikhael M, Bronson C, Zhang L, Curran M, Rodriguez H, Bhakta KY. Lack of evidence for time or dose relationship between antenatal magnesium sulfate and intestinal injury in extremely preterm neonates. Neonatology. 2019;115:371–8.
Shepherd E, Salam RA, Manhas D, Synnes A, Middleton P, Makrides M, et al. Antenatal magnesium sulphate and adverse neonatal outcomes: a systematic review and meta-analysis. PLoS Med. 2019;16:e1002988.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201.
Meyer CL, Payne NR, Roback SA. Spontaneous, isolated intestinal perforations in neonates with birth weight less than 1000 g not associated with necrotizing enterocolitis. J Pediatr Surg. 1991;26:714–7.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins J, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142.
Wells G, Shea B, O’Connell D, Peterson J, Welch v, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2012.
Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice. Z Evid Fortbild Qual Gesundh. 2009;103:391–400.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Crowther CA, Hiller JE, Doyle LW, Haslam RR. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. Jama. 2003;290:2669–76.
Gupta NGR, Gupta A, Garg R, Mishra S. Magnesium sulfate for fetal neuroprotection in women at risk of preterm birth: analysis of its effect on cerebral palsy. J South Asian Feder Obst Gynae. 2021;13:90–3.
Marret S, Marpeau L, Zupan-Simunek V, Eurin D, Leveque C, Hellot M-F, et al. Magnesium sulphate given before very-preterm birth to protect infant brain: the randomised controlled PREMAG trial*. Bjog. 2007;114:310–8.
Paradisis M, Osborn DA, Evans N, Kluckow M. Randomized controlled trial of magnesium sulfate in women at risk of preterm delivery-neonatal cardiovascular effects. J Perinatol. 2012;32:665–70.
Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359:895–905.
Wolf HT, Brok J, Henriksen TB, Greisen G, Salvig JD, Pryds O, et al. Antenatal magnesium sulphate for the prevention of cerebral palsy in infants born preterm: a double-blind, randomised, placebo-controlled, multi-centre trial. BJOG. 2020;127:1217–25.
de Veciana M, Porto M, Major CA, Barke JI. Tocolysis in advanced preterm labor: impact on neonatal outcome. Am J Perinatol. 1995;12:294–8.
Schanler RJ, Smith LG Jr, Burns PA. Effects of long-term maternal intravenous magnesium sulfate therapy on neonatal calcium metabolism and bone mineral content. Gynecol Obstet Invest. 1997;43:236–41.
Kimberlin DF, Hauth JC, Goldenberg RL, Bottoms SF, Iams JD, Mercer B, et al. The effect of maternal magnesium sulfate treatment on neonatal morbidity in < or = 1000-gram infants. Am J Perinatol. 1998;15:635–41.
Elimian A, Verma R, Ogburn P, Wiencek V, Spitzer A, Quirk JG. Magnesium sulfate and neonatal outcomes of preterm neonates. J Matern Fetal Neonatal Med. 2002;12:118–22.
Jazayeri A, Jazayeri MK, Sutkin G. Tocolysis does not improve neonatal outcome in patients with preterm rupture of membranes. Am J Perinatol. 2003;20:189–93.
Yokoyama K, Takahashi N, Yada Y, Koike Y, Kawamata R, Uehara R, et al. Prolonged maternal magnesium administration and bone metabolism in neonates. Early Hum Dev. 2010;86:187–91.
Basu SK, Chickajajur V, Lopez V, Bhutada A, Pagala M, Rastogi S. Immediate clinical outcomes in preterm neonates receiving antenatal magnesium for neuroprotection. J Perinat Med. 2011;40:185–9.
Lee NY, Cho SJ, Park EA. Influence of antenatal magnesium sulfate exposure on perinatal outcomes in VLBW infants with maternal preeclampsia. Neonatal Med. 2013;20:28–34.
Weisz DE, Shivananda S, Asztalos E, Yee W, Synnes A, Lee SK, et al. Intrapartum magnesium sulfate and need for intensive delivery room resuscitation. Arch Dis Child Fetal Neonatal Ed. 2015;100:F59–65.
James AT, Corcoran JD, Hayes B, Franklin O, El-Khuffash A. The effect of antenatal magnesium sulfate on left ventricular afterload and myocardial function measured using deformation and rotational mechanics imaging. J Perinatol. 2015;35:913–8.
Suh BS, Ko KH, Bang JS, Joung Y, Lee YJ, Lee JW, et al. Neonatal outcomes of premature infants who were delivered from mother with hypertensive disorders of pregnancy and effects of antihypertensive drugs and MgSO4. kjp. 2015;26:190–9.
Bouet PE, Brun S, Madar H, Baisson AL, Courtay V, Gascoin-Lachambre G, et al. Implementation of an antenatal magnesium sulfate protocol for fetal neuroprotection in preterm infants. Sci Rep. 2015;5:14732.
Bozkurt O, Eras Z, Canpolat FE, Oguz SS, Uras N, Dilmen U. Antenatal magnesium sulfate and neurodevelopmental outcome of preterm infants born to preeclamptic mothers. J Matern Fetal Neonatal Med. 2016;29:1101–4.
De Jesus LC, Sood BG, Shankaran S, Kendrick D, Das A, Bell EF, et al. Antenatal magnesium sulfate exposure and acute cardiorespiratory events in preterm infants. Am J Obstet Gynecol. 2015;212:94.e91–7.
Morag I, Okrent AL, Strauss T, Staretz-Chacham O, Kuint J, Simchen MJ, et al. Early neonatal morbidities and associated modifiable and non-modifiable risk factors in a cohort of infants born at 34-35 weeks of gestation. J Matern Fetal Neonatal Med. 2015;28:876–82.
Garcia Alonso L, Pumarada Prieto M, Gonzalez Colmenero E, Concheiro Guisan A, Suarez Albo A, Duran Fernandez-Feijoo C, et al. Prenatal therapy with magnesium sulfate and its correlation with neonatal serum magnesium concentration. Am J Perinatol. 2018;35:170–6.
Lloreda-Garcia JM, Lorente-Nicolás A, Bermejo-Costa F, Martínez-Uriarte J, López-Pérez R. Necesidad de reanimación en prematuros menores de 32 semanas expuestos a sulfato de magnesio para neuroprotección fetal. Rev Chil de Pediatría. 2016;87:261–7.
Downey LC, Cotten CM, Hornik CP, Laughon MM, Toila VN, Clark RH, et al. Association of in utero magnesium exposure and spontaneous intestinal perforations in extremely low birth weight infants. J Perinatol. 2017;37:641–4.
Jung EJ, Byun JM, Kim YN, Lee KB, Sung MS, Kim KT, et al. Antenatal magnesium sulfate for both tocolysis and fetal neuroprotection in premature rupture of the membranes before 32 weeks’ gestation. J Matern Fetal Neonatal Med. 2018;31:1431–41.
Narasimhulu D, Brown A, Egbert NM, Rojas M, Haberman S, Bhutada A, et al. Maternal magnesium therapy, neonatal serum magnesium concentration and immediate neonatal outcomes. J Perinatol. 2017;37:1297–303.
Stockley EL, Ting JY, Kingdom JC, McDonald SD, Barrett JF, Synnes AR, et al. Intrapartum magnesium sulfate is associated with neuroprotection in growth-restricted fetuses. Am J Obstet Gynecol. 2018;219:606.e601–606.e608.
Qasim A, Jain SK, Aly AM. Antenatal magnesium sulfate exposure and hemodynamically significant patent ductus arteriosus in premature infants. AJP Rep. 2019;9:e353–6.
Özlü F, Hacıoğlu C, Büyükkurt S, Yapıcıoğlu H, Satar M. Changes on preterm morbidities with antenatal magnesium. Cukurova Med J. 2019;44:502–8.
Gochi Valdovinos A, Arriaga-Redondo M, Dejuan Bitriá E, Pérez Rodríguez I, Márquez Isidro E, Blanco Bravo D. Terapia prenatal con sulfato de magnesio y obstrucción intestinal por meconio en recién nacidos pretérmino. An de Pediatría. 2022;96:138–44.
Hong JY, Hong JY, Choi YS, Kim YM, Sung JH, Choi SJ, et al. Antenatal magnesium sulfate treatment and risk of necrotizing enterocolitis in preterm infants born at less than 32 weeks of gestation. Sci Rep. 2020;10:12826.
Kim SH, Kim YJ, Shin SH, Cho H, Shin SH, Kim EK, et al. Antenatal magnesium sulfate and intestinal morbidities in preterm infants with extremely low gestational age. Pediatr Neonatol. 2021;62:202–7.
Üstün N, Hocaoğlu M, Turgut A, Ovalı F. Effects of antenatal magnesium sulfate use for neuroprotection on cardiorespiratory complications during the early neonatal period in preterm infants. J Surg Med [Internet]. 2021;5:843–7.
Chandran S, Tergestina M, Ross B, Joshi A, Rebekah G, Kumar M. Effects of antenatal magnesium sulfate on the gut function of preterm (<32 weeks) very low birth weight neonates: experience from a Tertiary Institute in South India. J Trop Pediatr. 2021;67:1–9.
Ayed M, Ahmed J, More K, Ayed A, Husain H, AlQurashi A, et al. Antenatal magnesium sulfate for preterm neuroprotection: a single-center experience from Kuwait Tertiary NICU. Biomed Hub. 2022;7:80–7.
Sung SI, Ahn SY, Choi SJ, Oh SY, Roh CR, Yang M, et al. Increased risk of meconium-related ileus in extremely premature infants exposed to antenatal magnesium sulfate. Neonatology. 2022;119:68–76.
Bansal V, Desai A. Efficacy of antenatal magnesium sulfate for neuroprotection in extreme prematurity: a comparative observational study. J Obstet Gynaecol India. 2022;72:36–47.
Özlü F, Hacıoğlu C, Büyükkurt S, Yapıcıoğlu H, Satar M. Changes on preterm morbidities with antenatal magnesium. Cukurova Med J. 2019;44:502–8.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Krinsky DL. Natural products: show me the data. Am J Health Syst Pharm. 1998;55:125.
Ghidini A, Espada RA, Spong CY. Does exposure to magnesium sulfate in utero decrease the risk of necrotizing enterocolitis in premature infants? Acta Obstet Gynecol Scand. 2001;80:126–9.
Author information
Authors and Affiliations
Contributions
AP: conceptualized, formulated the research methodology, performed formal analysis, curated data, and wrote the original draft, reviewed and edited the manuscript. Nell Aronoff participated in formulating the research methodology and provided resources for the literature search. She also contributed to writing, reviewing, and editing the manuscript. PC: administered the project and also contributed to manuscript writing, reviewing and editing. SD: conceptualized, formulated the research methodology, performed formal analysis, curated data, wrote the original draft, reviewed, edited the manuscript, supervised/administered the whole project and guarantor for the project. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
AP, NA, and SD: have no relevant conflicts to disclose. PC: was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; grant number: R01HD104909 and the National Institutes of Health (NIH)/National Heart Lung and Blood Institute (NHLBI; grant no.: K12 HL138052). The results of this manuscript are not supported or endorsed by any of the funding institutions.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prasath, A., Aronoff, N., Chandrasekharan, P. et al. Antenatal Magnesium Sulfate and adverse gastrointestinal outcomes in Preterm infants—a systematic review and meta-analysis. J Perinatol 43, 1087–1100 (2023). https://doi.org/10.1038/s41372-023-01710-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01710-8
- Springer Nature America, Inc.
This article is cited by
-
Antenatal exposure to magnesium sulfate and neonatal outcomes in very low birth weight infants: a multicenter study
Journal of Perinatology (2024)
-
Neuroprotective therapies in the NICU in preterm infants: present and future (Neonatal Neurocritical Care Series)
Pediatric Research (2024)