Abstract
Metabolic syndrome is a cluster of conditions that increase the risk of cardiovascular diseases, and comprises obesity, hypertension, impaired glucose metabolism and dyslipidaemia. It is well recognised that the mineralocorticoid receptor (MR) plays an important role in blood pressure regulation via its effect on salt and water retention in renal tubules, with hypertension being a key feature in primary aldosteronism patients with excess adrenal production of aldosterone, the primary ligand for MRs in the epithelial tissues. MRs are also expressed in a number of non-epithelial tissues including adipose tissue; in these tissues, glucocorticoids or cortisol can also activate MRs due to low levels of 11-beta-hydroxysteroid-dehydrogenase type 2 (11-βHSD2), the enzyme which inactivates cortisol. There is increasing evidence suggesting that over-activation of MRs plays a role in the pathophysiology of the other components of metabolic syndrome, promoting adiposity, inflammation and glucose intolerance, and that MR antagonists may confer beneficial effects on energy and substrate homeostasis and cardiometabolic diseases. This review discusses the advances in the literature shedding light on the MR as an emerging player in metabolic syndrome.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) and cardiovascular diseases (CVDs) represent major causes of morbidity and mortality globally. MetS is defined by the constellation in a person of three or more of the following risk factors for CVDs—obesity, hypertension, impaired glucose metabolism (elevated fasting glucose or insulin resistance or type 2 diabetes mellitus) and dyslipidaemia (elevated triglycerides or low high density lipoprotein, HDL) [1]. The burden of MetS is escalating worldwide, concurrent with the progressive soar in the prevalence of obesity; 25% of the world population, i.e. over a billion people in the world, are estimated to have MetS [2].
The pathophysiology of MetS and associated CVDs is complex with various contributing factors. Hormones and their receptors underpin the pathophysiological changes surrounding energy and substrate metabolism and cardiovascular function and hence disease progression. It is well recognised that individuals with excess glucocorticoids (GCs), hypothyroid state or hypogonadism with low levels of sex steroid hormones often suffer features of MetS and an increased risk of CVDs.
Mineralocorticoids (MCs) such as aldosterone are adrenal hormones classically known for their effects on salt and water retention and blood pressure, acting via the mineralocorticoid receptors (MRs) in renal tubules. MRs belong to the steroid nuclear receptor superfamily of ligand-dependent transcription factors, are widely expressed in various tissues including heart, blood vessels, adipose tissue, brain and immune cells, and non-classical effects of MRs are emerging with implications for CVDs which are independent of its effect on blood pressure [3, 4]. In these non-epithelial tissues, MRs may also be activated by GCs [5, 6]. There is accumulating evidence supporting the pathophysiological role of MR activation in the other components of MetS such as obesity and dysregulation in glucose metabolism, and the potential benefits of MR antagonists (MRAs) for management of cardiometabolic diseases. This review focuses on the recent literature examining the role of MR in MetS.
We reviewed the relevant articles published in English between January 2005 and November 2019, which were retrieved from the PubMed using the search terms “mineralocorticoid”, “aldosterone”, “metabolism” and “MetS”.
Association between increased MR activation and metabolic syndrome
Primary aldosteronism (PA) is a condition where there is autonomous and excessive production of aldosterone by the adrenal gland(s) [7], and represents a classic model illustrating the consequences of excess MR activation. MetS is more frequent in patients with PA than those with essential hypertension [8, 9] or than age, gender, BMI and BP-matched control subjects [10]. A recent systematic review and meta-analysis has reported that PA increases the risk of MetS by 1.5 times and of diabetes by 1.3 times, compared to those with essential hypertension [11]. MetS and dysglycaemia improved after treatment of PA with an MR antagonist or removal of aldosterone-producing adenoma by surgery [9, 10, 12,13,14], indicating that increased MR activity in PA likely underlies the development of MetS.
The association between the activity of the mineralocorticoid (MC) system and MetS has also been observed among the general population. In a prospective longitudinal study involving >1000 people, baseline plasma aldosterone concentration predicted new onset obesity, hypertension and type 2 diabetes over a 4-year follow-up period [15]. In another large multiethnic longitudinal study, a 1-SD increase in log-aldosterone was associated with a 44% higher risk of incident diabetes over 10.5 years [16]. In cross-sectional analyses, plasma aldosterone concentration and adipose tissue MR expression are increased in obese individuals [17,18,19,20], and plasma/urinary aldosterone correlates with MetS, visceral adiposity, BMI and insulin resistance [16, 21,22,23,24].
Human adipocytes can indeed secrete MC releasing factors [25] and various adipokines such as leptin, which can stimulate the adrenal production of aldosterone [26,27,28]. In addition, adipose tissue can produce angiotensinogen and possesses a local renin-angiotensin-system (RAS) [29]. Plasma and adipose tissue MC activity have been reported to be reduced significantly after successful weight loss by calorie restriction [20, 30].
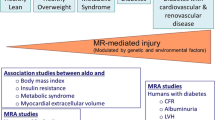
These findings together support the concept of increased MC activity as a mediator of MetS and obesity, which in turn contribute to the maintenance of high MC activity with resultant progression of these conditions (Fig. 1).
Role of MR in adipose tissue metabolism and inflammation
Adipose tissue plays a vital role in energy and substrate homeostasis. Adipose tissue can be differentiated into two forms: brown adipose tissue (BAT) and white adipose tissue (WAT). BAT is a thermogenic tissue with a unique protein called uncoupling protein-1 (UCP1), which dissipates nutrient energy as heat, and protects against obesity and improves insulin sensitivity, unlike WAT which stores energy as lipid, the accumulation of which over time leads to obesity, insulin resistance and metabolic diseases [31]. A number of studies suggest that the MR plays a role in the biology of both BAT and WAT.
BAT
MR is expressed in BAT [32]. In rodents, MCs inhibit the function of brown adipocytes [33, 34]. The inhibitory effect of aldosterone on the expression of thermogenic protein UCP1 and glucose uptake in brown adipocytes was dose-dependent and mediated via MR as well as the glucocorticoid receptor (GR) [33, 34]. On the other hand, Armani et al. studied the effects of MRAs (spironolactone and drosperinone) on BAT in mice fed high-fat diet over 3 months, and found that MRAs upregulated glucose uptake and UCP1 expression of interscapular BAT, and abated diet-induced weight gain and glucose intolerance [35]. MRAs also induced similar changes in the inguinal fat depots, indicative of “browning of WAT”, that is, formation of brown-like adipocytes within WAT [35].
In humans, it was previously thought that brown fat becomes obsolete after infancy. It has been realised only over the past decade that active brown fat persists into adult life [31]. We discovered for the first time in humans that the MRA, spironolactone (100 mg/day for 2 weeks), significantly enhances the function of BAT while suppressing postprandial lipid synthesis [36]. Spironolactone increased BAT metabolic activity by 50% and volume by more than twofold within 2 weeks. These findings are in agreement with the observations from rodent studies, suggesting that MR antagonism may indeed impart similar benefits in humans as in rodents, diverting nutrient energy from storage towards wastage as heat. The degree of enhancement in BAT activity by spironolactone may translate into a 5% decrease in body fat mass and 50% improvement in insulin sensitivity over time [31]. The study was however of short duration and not powered to define the long term metabolic effects.
WAT
In white adipocytes, aldosterone promotes intracellular lipid accumulation and adipogenesis in a dose-dependent manner in vitro [37]. The upregulation of MR produces a similar effect increasing lipid storage in white adipocytes and adiposity [17]. On the other hand, deletion [38] or downregulation of MR [39] or treatment with MRAs [17, 40] counteracts this.
In addition to the effects on adipose tissue metabolism, the MR is also implicated in adipose tissue inflammation. Obesity leads to chronic inflammation of adipose tissue with local infiltration of macrophages and secretion of pro-inflammatory cytokines such as tumour necrotic factor-α (TNF-α), interleukins and monocyte chemoattractant protein-1 [41]. This obesity-associated adipocyte dysfunction is believed to play a crucial role in the development of insulin resistance [41]. Aldosterone stimulates pro-inflammatory adipokines [33, 38], and the beneficial metabolic effects of MR antagonism has been shown to be mediated via amelioration of these inflammatory changes in adipose tissue [42, 43].
Taken together, there is convincing evidence demonstrating the role of MR in adipose tissue metabolism and inflammation, favouring storage of nutrient energy as WAT and promoting inflammation while suppressing the function of energy-burning BAT.
Do MRs mediate the adverse metabolic effects of glucocorticoids?
MRs have a similar affinity for GCs as they do for MCs (aldosterone) [44]. The MR is protected from binding to GCs only in tissues possessing 11β-hydroxysteroid-dehydrogenase type 2 (11β-HSD2) enzyme, which inactivates GCs [5, 6]. Adipose tissues do not display significant levels of 11βHSD2 while expressing high levels of 11β-HSD1 which converts inactive GCs to their active form [39, 45], raising the possibility that GCs can act on MRs in adipose tissue.
Obesity and MetS are common complications of exposure to excess GCs as seen in individuals with Cushing’s syndrome and those taking exogenous GCs such as prednisolone. It has been demonstrated that the MR mediates detrimental metabolic effects of GCs [18, 38, 39]. GC-induced adipocyte dysfunction can be reversed by MRA (eplerenone), but not by the GR antagonist (GRA) RU486, in vitro [18]. MR, but not GR, knockdown inhibits GC-induced adipogenesis and expression of pro-inflammatory adipokines [38, 39]. A combined MRA/GRA provides improvements (predominantly in body fat) in diet-induced metabolic dysregulation compared to GRA alone in rodents [46]. In humans, we have found that MRA (spironolactone) exerts opposing effects to those of GC (prednisolone) on adipose tissue metabolism in vivo; prednisolone suppressed the function of BAT and promoted postprandial lipid synthesis [47] while spironolactone enhanced BAT function and suppressed lipid synthesis [36]. These observations suggest that the MR likely plays a significant role in GC-induced adverse metabolic effects and that MRAs may have protective cardiometabolic effects in patients exposed to excess GCs although clinical evidence for this is yet to be established.
Role of MR in pancreatic β-cell function
Failure of pancreatic islet β-cell function and insulin secretion is recognised as an important element in the progression of type 2 diabetes in individuals with obesity and insulin resistance [48]. Increased prevalence of diabetes or dysglycaemia in PA has been observed by several investigators [8,9,10,11, 49]. Plasma aldosterone predicts development of diabetes in the general community [15, 16]. Insulin resistance or reduced insulin sensitivity has been a widely reported feature in PA [12, 13, 50, 51], and patients with PA have also been shown to exhibit impaired insulin secretory response [52, 53]. The observations have been reported even in the absence of hypokalaemia [12].
There is evidence that aldosterone can directly suppress pancreatic islet cell function [54,55,56], but the effect seems to be non-MR-mediated. Luther et al. reported that excess aldosterone induces dysfunction and apoptosis of pancreatic β-cells of murine islets and decreases glucose-stimulated insulin secretion, but the effects were not blocked by MRAs [55]. Chen et al. reported that the effect of aldosterone on islet cell was mediated via the GR based on their observations that in clonal β-cells, the expression level of MR was low compared to that of GR, and that a GRA prevented the impairment of β-cells by aldosterone [56]. The above findings indicate that MR regulation of glucose metabolism occurs predominantly via alterations in insulin sensitivity/resistance, rather than pancreatic islet cell function.
Do the metabolic effects of MR at tissue level translate into the whole-body level?
There is an array of in vivo evidence in rodents demonstrating that MR exerts metabolic effects at the whole-body level, influencing, for example, body fat mass, liver fat and glucose metabolism. Mice with upregulated MR expression in adipocytes develop obesity, insulin resistance and MetS [17, 57]. On the other hand, MRAs attenuate adiposity, hepatic steatosis and inflammation in high fat diet-fed mice [35, 58, 59]. MRAs also reverse insulin resistance and glucose intolerance in obese mice [35, 42, 43, 58, 59]. These beneficial effects were observed with various MRAs including spironolactone, eplerenone and drosperinone.
It is worth noting that the dosages employed in the preclinical studies have been relatively high; for instance, spironolactone was used at 16–20 mg/kg per day in rodents [35, 58], equating to a human equivalent daily dose of ~1.2–1.5 mg/kg (i.e. 84–105 mg/day of spironolactone for a 70 kg person) and eplerenone at a daily dosage of 100 mg/kg in rodents, equating to 7.5 mg/kg in humans [43]. In the case of spironolactone and drospirenone, which can have progestogenic and anti-androgenic effects at high dosages [60], this raises the question as to whether the beneficial effects were actually via progesterone (PR) and/or androgen receptors (AR), rather than the MR. This was demonstrated to be unlikely by Caprio et al. in vitro by showing the anti-adipogenic effect of droperinone on 3T3-L1 pre-adipocytes and adipocytes which virtually lack PR, and by demonstrating that testosterone, i.e. AR agonist, not AR antagonism, actually inhibits adipogenesis [40].
In humans, the evidence of a regulatory role of MR on whole-body energy and substrate metabolism is relatively primordial and long-term studies have been mainly observational. Cross-sectional observational studies have reported that plasma aldosterone level correlates with visceral fat mass and BMI in patients with PA [61] and in obese women [22, 62]. In an open labelled uncontrolled study, MRAs (eplerenone 25–100 mg daily or spironolactone 12.5–100 mg daily) reduces visceral fat mass, but not insulin resistance or serum lipids, over 12 months of treatment vs baseline in patients with PA [63]. In other cohort studies, treatment of PA with MRAs or surgery improved insulin sensitivity [12] and glucose tolerance [10] at 6–12 months. On the other hand, short term clinical trials assessing the effects of spironolactone (50 mg daily for 6 weeks) [64] or eplerenone (50 mg daily for 2 weeks) [65] on insulin sensitivity and fasting/postprandial glucose levels respectively in non-hypertensive non-PA subjects did not find any changes, albeit this was rather unsurprising given the short duration of intervention.
With regards to hepatic steatosis, a significant correlation between plasma aldosterone and liver fat has been reported in individuals with PA [66] and those with HIV [67]. Interventional studies investigating the effect of MRAs on liver fat have yielded inconsistent findings. Polyzos et al. reported a significant improvement in liver fat score over 1 year in 31 subjects with biopsy-proven non-alcoholic fatty liver disease randomised to spironolactone (25 mg daily) plus vitamin E compared to vit E alone [68], while in a recent randomised controlled trial by Johansen et al. involving 140 patients with longstanding type 2 DM (80% of whom were already on angiotensin receptor blocker or angiotensin converting enzyme inhibitor treatment), there was no change in liver fat after 26 weeks of treatment with eplerenone (50–200 mg daily) [69].
Overall, the question as to whether the metabolic effects of MR at tissue levels translate into changes at the whole-body level remains to be resolved in humans.
Future directions
There is a need for further long term interventional clinical studies specifically aimed at defining the effects of MRAs on whole-body metabolism. To date, the efficacy of MRAs in the management of MetS has been demonstrated mainly in animal models as discussed. In order to replicate the preclinical findings, future clinical studies will need to carefully take into account the dose-dependency of effects of MRAs on metabolism [40] and the selection of representative target groups (obese, MetS model) and to be of longer duration. There is evidence from a recent study in patients with PA to suggest that higher dosages of MRAs (sufficient to unsuppress plasma renin levels) are also likely necessary to abrogate the excess cardiometabolic risk associated with that condition [49].
The demonstrated metabolic benefits of MR antagonism, both in vitro and in vivo, have been limited to steroidal MRAs. Steroidal MRAs are generally safe for use by the general population, proven by the safety record of the long term usage in high dosages in women with hirsutism [70]. However, cross reactivity of older MRAs such as spironolactone with sex steroid receptors can lead to side effects such as gynaecomastia in men, menstrual irregularities in women and reduced libido. While this issue has been at least partly addressed by the introduction of more specific MRAs such as eplerenone, use of that agent is not yet subsidised in many countries, making treatment expensive. Furthermore, administration of steroidal MRAs can result in hyperkalaemia in some vulnerable groups such as those with renal impairment, and given the availability of new, non-steroidal MRAs with lower side effect profiles [71], it would be of interest to examine whether non-steroidal MRAs can confer the beneficial effects on adipose tissue function and metabolism demonstrated with steroidal MRAs. Uncovering the key signalling pathways, which mediate the metabolic effects of MR activation and which positively respond to MRAs also may allow identification of downstream targets for future research and development.
Another area of great interest is the clarification of the role of MR in mediating the adverse metabolic effects of GCs. Available evidence suggests a potential interaction between MRs and GCs in the regulation of adipose tissue function and lipid storage as discussed. However, firm evidence of clinical significance of the contribution by MR to GC-induced metabolic dysfunction in vivo and whole-body metabolism is yet to be established. The potential for MRAs to confer protective cardiometabolic effects in people exposed to excess GCs by preventing the occupation of MRs by GCs remains to be explored.
A transpiring topic is the gender differences in MR activity and organ damage in the setting of obesity. There is evidence showing that obese females are more susceptible than male counterparts to aldosterone-induced hypertension and cardiovascular damage, and various mechanisms have been proposed including higher adipose tissue-derived leptin resulting in increased RAS activity and MR activation [72]. MRAs are also reportedly more efficacious in females compared to males for cardiac protection [73]. The question as to whether these phenomena also apply to other components of MetS remains open.
Finally, there seems to be a bidirectional interplay between the MC system activity and adiposity as discussed earlier and as illustrated in Fig. 1. The finding from a recent retrospective cross-sectional study of PA from Japan of a higher prevalence of obesity in idiopathic/bilateral hyperaldosteronism (despite lower plasma aldosterone concentrations) vs aldosterone-producing adenoma raises an intriguing question concerning the potential role of adiposity in the pathogenesis of bilateral forms of PA [74]. Additional studies are warranted to further explore this relationship.
Conclusions
An array of preclinical evidence supports a regulatory role of MR in metabolism (illustrated in Fig. 2) and the beneficial effects of MRAs. A central contributory role of MR in the pathophysiology of hypertension, adiposity and glucose dysregulation likely underpins the close connections observed between the various components of MetS and cardiovascular health. In humans, MR activation is associated with metabolic disturbances. The metabolic benefits of MRAs are yet to be clearly defined in humans, particularly for long term treatment. The clinical relevance of MR in metabolic pathophysiology and therapeutic potential of MRAs in the management of adiposity and MetS deserve further investigation.
References
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5.
Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20:12.
Bender SB, McGraw AP, Jaffe IZ, Sowers JR. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes. 2013;62:313–9.
Hawkins UA, Gomez-Sanchez EP, Gomez-Sanchez CM, Gomez-Sanchez CE. The ubiquitous mineralocorticoid receptor: clinical implications. Curr Hypertens Rep. 2012;14:573–80.
Edwards CR, Stewart PM, Burt D, Brett L, McIntyre MA, Sutanto WS, et al. Localisation of 11 beta-hydroxysteroid dehydrogenase-tissue specific protector of the mineralocorticoid receptor. Lancet. 1988;2:986–9.
Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242:583–5.
Thuzar M, Young K, Ahmed AH, Ward G, Wolley M, Guo Z, et al. Diagnosis of Primary Aldosteronism by Seated Saline Suppression Test-Variability Between Immunoassay and HPLC-MS/MS. J Clin Endocrinol Metab. 2020;105:e477–83.
Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. 2006;91:454–9.
Turchi F, Ronconi V, di Tizio V, Ceccoli L, Boscaro M, Giacchetti G. Primary aldosteronism and essential hypertension: assessment of cardiovascular risk at diagnosis and after treatment. Nutr Metab Cardiovas. 2014;24:476–82.
Hanslik G, Wallaschofski H, Dietz A, Riester A, Reincke M, Allolio B, et al. Increased prevalence of diabetes mellitus and the metabolic syndrome in patients with primary aldosteronism of the German Conn’s Registry. Eur J Endocrinol. 2015;173:665–75.
Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50.
Catena C, Lapenna R, Baroselli S, Nadalini E, Colussi G, Novello M, et al. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab. 2006;91:3457–63.
Giacchetti G, Ronconi V, Turchi F, Agostinelli L, Mantero F, Rilli S, et al. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J Hypertens. 2007;25:177–86.
Wu VC, Chueh SJ, Chen L, Chang CH, Hu YH, Lin YH, et al. Risk of new-onset diabetes mellitus in primary aldosteronism: a population study over 5 years. J Hypertens. 2017;35:1698–708.
Buglioni A, Cannone V, Sangaralingham SJ, Heublein DM, Scott CG, Bailey KR, et al. Aldosterone Predicts Cardiovascular, Renal, and Metabolic Disease in the General Community: a 4-Year Follow-Up. J Am Heart Assoc. 2015;4:e002505.
Joseph JJ, Echouffo Tcheugui JB, Effoe VS, Hsueh WA, Allison MA, Golden SH. Renin-Angiotensin-Aldosterone System, Glucose Metabolism and Incident Type 2 Diabetes Mellitus: MESA. J Am Heart Assoc. 2018;7:e009890.
Urbanet R, Nguyen Dinh Cat A, Feraco A, Venteclef N, El Mogrhabi S, Sierra-Ramos C, et al. Adipocyte Mineralocorticoid Receptor Activation Leads to Metabolic Syndrome and Induction of Prostaglandin D2 Synthase. Hypertension. 2015;66:149–57.
Hirata A, Maeda N, Nakatsuji H, Hiuge-Shimizu A, Okada T, Funahashi T, et al. Contribution of glucocorticoid-mineralocorticoid receptor pathway on the obesity-related adipocyte dysfunction. Biochem Biophys Res Commun. 2012;419:182–7.
Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, et al. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007;92:4472–5.
Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, et al. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356–62.
Bochud M, Nussberger J, Bovet P, Maillard MR, Elston RC, Paccaud F, et al. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension. 2006;48:239–45.
Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res. 1999;7:355–62.
Rossi GP, Belfiore A, Bernini G, Fabris B, Caridi G, Ferri C, et al. Body mass index predicts plasma aldosterone concentrations in overweight-obese primary hypertensive patients. J Clin Endocrinol Metab. 2008;93:2566–71.
Dudenbostel T, Ghazi L, Liu M, Li P, Oparil S, Calhoun DA. Body mass index predicts 24-hour urinary aldosterone levels in patients with resistant hypertension. Hypertension. 2016;68:995–1003.
Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, et al. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci USA. 2003;100:14211–6.
Luther JM. Aldosterone in vascular and metabolic dysfunction. Curr Opin Nephrol Hypertens. 2016;25:16–21.
Huby AC, Otvos L Jr., Belin de Chantemele EJ. Leptin induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms in obese female mice. Hypertension. 2016;67:1020–8.
Faulkner JL, Bruder-Nascimento T, Belin, de Chantemèle EJ. The regulation of aldosterone secretion by leptin: implications in obesity-related cardiovascular disease. Curr Opin Nephrol Hypertens. 2018;27:63–9.
Giacchetti G, Faloia E, Mariniello B, Sardu C, Gatti C, Camilloni MA, et al. Overexpression of the renin-angiotensin system in human visceral adipose tissue in normal and overweight subjects. Am J Hypertens. 2002;15:381–8.
Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med. 1981;304:930–3.
Thuzar M, Ho KK. Mechanisms in endocrinology: brown adipose tissue in humans: regulation and metabolic significance. Eur J Endocrinol. 2016;175:R11–25.
Zennaro MC, Le Menuet D, Viengchareun S, Walker F, Ricquier D, Lombes M. Hibernoma development in transgenic mice identifies brown adipose tissue as a novel target of aldosterone action. J Clin Investig. 1998;101:1254–60.
Kraus D, Jager J, Meier B, Fasshauer M, Klein J. Aldosterone inhibits uncoupling protein-1, induces insulin resistance, and stimulates proinflammatory adipokines in adipocytes. Horm Metab Res. 2005;37:455–9.
Viengchareun S, Penfornis P, Zennaro MC, Lombes M. Mineralocorticoid and glucocorticoid receptors inhibit UCP expression and function in brown adipocytes. Am J Physiol Endocrinol Metab. 2001;280:E640–9.
Armani A, Cinti F, Marzolla V, Morgan J, Cranston GA, Antelmi A, et al. Mineralocorticoid receptor antagonism induces browning of white adipose tissue through impairment of autophagy and prevents adipocyte dysfunction in high-fat-diet-fed mice. FASEB J. 2014;28:3745–57.
Thuzar M, Law WP, Dimeski G, Stowasser M, Ho KKY. Mineralocorticoid antagonism enhances brown adipose tissue function in humans: A randomized placebo-controlled cross-over study. Diabetes Obes Metab. 2019;21:509–16.
Rondinone CM, Rodbard D, Baker ME. Aldosterone stimulated differentiation of mouse 3T3-L1 cells into adipocytes. Endocrinology. 1993;132:2421–6.
Hoppmann J, Perwitz N, Meier B, Fasshauer M, Hadaschik D, Lehnert H, et al. The balance between gluco- and mineralo-corticoid action critically determines inflammatory adipocyte responses. J Endocrinol. 2010;204:153–64.
Caprio M, Feve B, Claes A, Viengchareun S, Lombes M, Zennaro MC. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J. 2007;21:2185–94.
Caprio M, Antelmi A, Chetrite G, Muscat A, Mammi C, Marzolla V, et al. Antiadipogenic effects of the mineralocorticoid receptor antagonist drospirenone: potential implications for the treatment of metabolic syndrome. Endocrinology. 2011;152:113–25.
Lehrke M, Lazar MA. Inflamed about obesity. Nat Med. 2004;10:126–7.
Hirata A, Maeda N, Hiuge A, Hibuse T, Fujita K, Okada T, et al. Blockade of mineralocorticoid receptor reverses adipocyte dysfunction and insulin resistance in obese mice. Cardiovasc Res. 2009;84:164–72.
Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, et al. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117:2253–61.
Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, et al. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–75.
Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Heintze U, Janke J, et al. Regulation of 11beta-HSD genes in human adipose tissue: influence of central obesity and weight loss. Obes Res. 2004;12:9–17.
Mammi C, Marzolla V, Armani A, Feraco A, Antelmi A, Maslak E, et al. A novel combined glucocorticoid-mineralocorticoid receptor selective modulator markedly prevents weight gain and fat mass expansion in mice fed a high-fat diet. Int J Obes (Lond). 2016;40:964–72.
Thuzar M, Law WP, Ratnasingam J, Jang C, Dimeski G, Ho KKY. Glucocorticoids suppress brown adipose tissue function in humans: A double-blind placebo-controlled study. Diabetes Obes Metab. 2018;20:840–8.
Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6.
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–9.
Sindelka G, Widimský J, Haas T, Prázný M, Hilgertová J, Skrha J. Insulin action in primary hyperaldosteronism before and after surgical or pharmacological treatment. Exp Clin Endocrinol Diabetes. 2000;108:21–5.
Widimský J,Jr., Sindelka G, Haas T, Prázný M, Hilgertová J, Skrha J. Impaired Insulin Action Prim Hyperaldosteronism. Physiol Res. 2000;49:241–4.
Mosso LM, Carvajal CA, Maiz A, Ortiz EH, Castillo CR, Artigas RA, et al. A possible association between primary aldosteronism and a lower beta-cell function. J Hypertens. 2007;25:2125–30.
Fischer E, Adolf C, Pallauf A, Then C, Bidlingmaier M, Beuschlein F, et al. Aldosterone excess impairs first phase insulin secretion in primary aldosteronism. J Clin Endocrinol Metab. 2013;98:2513–20.
Jin HM, Zhou DC, Gu HF, Qiao QY, Fu SK, Liu XL, et al. Antioxidant N-acetylcysteine protects pancreatic beta-cells against aldosterone-induced oxidative stress and apoptosis in female db/db mice and insulin-producing MIN6 cells. Endocrinology. 2013;154:4068–77.
Luther JM, Luo P, Kreger MT, Brissova M, Dai C, Whitfield TT, et al. Aldosterone decreases glucose-stimulated insulin secretion in vivo in mice and in murine islets. Diabetologia. 2011;54:2152–63.
Chen F, Liu J, Wang Y, Wu T, Shan W, Zhu Y, et al. Aldosterone induces clonal β-cell failure through glucocorticoid receptor. Sci Rep. 2015;5:13215.
Nguyen Dinh Cat A, Antunes TT, Callera GE, Sanchez A, Tsiropoulou S, Dulak-Lis MG, et al. Adipocyte-Specific Mineralocorticoid Receptor Overexpression in Mice Is Associated With Metabolic Syndrome and Vascular Dysfunction: role of Redox-Sensitive PKG-1 and Rho Kinase. Diabetes. 2016;65:2392–403.
Wada T, Kenmochi H, Miyashita Y, Sasaki M, Ojima M, Sasahara M, et al. Spironolactone improves glucose and lipid metabolism by ameliorating hepatic steatosis and inflammation and suppressing enhanced gluconeogenesis induced by high-fat and high-fructose diet. Endocrinology. 2010;151:2040–9.
Wada T, Miyashita Y, Sasaki M, Aruga Y, Nakamura Y, Ishii Y, et al. Eplerenone ameliorates the phenotypes of metabolic syndrome with NASH in liver-specific SREBP-1c Tg mice fed high-fat and high-fructose diet. Am J Physiol Endocrinol Metab. 2013;305:E1415–25.
Garthwaite SM, McMahon EG. The evolution of aldosterone antagonists. Mol Cell Endocrinol. 2004;217:27–31.
Shibayama Y, Wada N, Baba S, Miyano Y, Obara S, Iwasaki R, et al. Relationship between visceral fat and plasma aldosterone concentration in patients with primary aldosteronism. J Endocr Soc. 2018;2:1236–45.
Li L, Hou X, Geng X, Xu Y. Body mass index predicts aldosterone production in hypertensive postmenopausal women. Clin Exp Hypertens. 2020;42:281–6.
Karashima S, Yoneda T, Kometani M, Ohe M, Mori S, Sawamura T, et al. Comparison of eplerenone and spironolactone for the treatment of primary aldosteronism. Hypertens Res. 2016;39:133–7.
Garg R, Kneen L, Williams GH, Adler GK. Effect of mineralocorticoid receptor antagonist on insulin resistance and endothelial function in obese subjects. Diabetes Obes Metab. 2014;16:268–72.
Krug AW, Stelzner L, Rao AD, Lichtman AH, Williams GH, Adler GK. Effect of low dose mineralocorticoid receptor antagonist eplerenone on glucose and lipid metabolism in healthy adult males. Metab Clin Exp. 2013;62:386–91.
Fallo F, Dalla Pozza A, Tecchio M, Tona F, Sonino N, Ermani M, et al. Nonalcoholic fatty liver disease in primary aldosteronism: a pilot study. Am J Hypertens. 2010;23:2–5.
Srinivasa S, Fitch KV, Quadri N, Maehler P, O’Malley TK, Martinez-Salazar EL, et al. Significant Association of Aldosterone and Liver Fat Among HIV-Infected Individuals With Metabolic Dysregulation. J Endocr Soc. 2018;2:1147–57.
Polyzos SA, Kountouras J, Mantzoros CS, Polymerou V, Katsinelos P. Effects of combined low-dose spironolactone plus vitamin E vs vitamin E monotherapy on insulin resistance, non-invasive indices of steatosis and fibrosis, and adipokine levels in non-alcoholic fatty liver disease: a randomized controlled trial. Diabetes Obes Metab. 2017;19:1805–9.
Johansen ML, Schou M, Rossignol P, Holm MR, Rasmussen J, Brandt N, et al. Effect of the mineralocorticoid receptor antagonist eplerenone on liver fat and metabolism in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial (MIRAD trial). Diabetes Obes Metab. 2019;21:2305–14.
Cumming DC, Yang JC, Rebar RW, Yen SS. Treatment of hirsutism with spironolactone. JAMA. 1982;247:1295–8.
Kolkhof P, Barfacker L. 30 Years of the mineralocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol. 2017;234:T125–t40.
Faulkner JL, Belin de Chantemèle EJ. Sex differences in mechanisms of hypertension associated with obesity. Hypertension. 2018;71:15–21.
Kanashiro-Takeuchi RM, Heidecker B, Lamirault G, Dharamsi JW, Hare JM. Sex-specific impact of aldosterone receptor antagonism on ventricular remodeling and gene expression after myocardial infarction. Clin Transl Sci. 2009;2:134–42.
Ohno Y, Sone M, Inagaki N, Yamasaki T, Ogawa O, Takeda Y, et al. Obesity as a key factor underlying idiopathic hyperaldosteronism. J Clin Endocrinol Metab. 2018;103:4456–64.
Acknowledgements
MT is supported by a Postdoctoral Fellowship from the Endocrine Society of Australia, a Project Grant from the Diabetes Australia and an Early Career Research Grant from the Metro South Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thuzar, M., Stowasser, M. The mineralocorticoid receptor—an emerging player in metabolic syndrome?. J Hum Hypertens 35, 117–123 (2021). https://doi.org/10.1038/s41371-020-00467-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-020-00467-3
- Springer Nature Limited
This article is cited by
-
Low-dose spironolactone ameliorates adipose tissue inflammation and apoptosis in letrozole-induced PCOS rat model
BMC Endocrine Disorders (2022)