Abstract
An easy, verified spectrofluorimetric approach was established for the investigation of moxifloxacin in pure forms, pharmaceutical preparations, and biological fluids. The approach involves forming a binary complex of moxifloxacin and eosin Y in an acetate buffer with a pH of 3.6. The highest quenching of eosin Y with moxifloxacin occurs at 545 nm. Several factors, such as pH, buffer type and concentration, and eosin Y concentration, were carefully studied. The calibration graph showed a linear relationship between fluorescence intensity and moxifloxacin concentrations between 0.2 and 10 µg mL−1 with a correlation coefficient of 0.998. It was determined that the detection and quantification limits were 0.0322 µg mL−1 and 0.0976 µg mL−1, respectively. The impact of common excipients was investigated, but no interferences were discovered. Standard forms of moxifloxacin, pharmaceuticals, and biological samples have all been studied using the established methodology. The method, which successfully complied with ICH requirements, was used for the analysis of moxifloxacin in its pure form, pharmaceutical dosage forms, and biological samples. The percentage recoveries obtained were ranged from 99.50 to 102.50% for pharmaceutical preparations and from 100.50 to 102.50% for human blood plasma and urine.
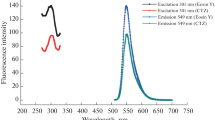
Graphical abstract
Proposed mechanisms for the reaction between moxifloxacin and eosin Y

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluoroquinolones are among the most effective antibacterial drugs available to humans. They have antibacterial efficacy against Gram-positive and Gram-negative bacteria via inhibiting DNA gyrase, as well as mycobacteria, mycoplasmas, and rickettsias [1].
Moxifloxacin is a fourth-generation 8-methoxy fluoroquinolone that was created to treat community-acquired pneumonia and infections of the upper respiratory tract. Gram-negative pathogens, Gram-positive cocci, aerobic intracellular bacteria, atypical species, and anaerobic bacteria are all targets [2, 3].
Several methods for determining moxifloxacin in pharmaceutical formulations and biological fluids have been documented. These methods include HPLC [4,5,6,7,8,9], differential spectrophotometry [10], UV–Vis spectrophotometry [11, 12], capillary electrophoresis [13], differential pulse polarography [14], differential pulse voltammetry [15], and spectrofluorimetric methods [16, 17].
The published methods for determining moxifloxacin are costly, require expensive reagents, and have a limited range of application to biological materials, low sensitivity, and interferences. Therefore, it is crucial to create a straightforward and affordable method for finding moxifloxacin in pharmaceutical formulations and biological fluids.
In comparison, only a few spectrofluorimetric methods for determining moxifloxacin in pharmaceutical formulations and biological materials have been documented. For the determination of moxifloxacin using eosin Y, no spectrofluorimetric approach has been published. The goal of this study is to develop a spectrofluorimetric method for quantifying moxifloxacin in pharmaceutical formulations and biological fluids that is simple, sensitive, and cost-effective. The proposed method relies on the formation of a binary complex between moxifloxacin and eosin Y in acidic conditions, and the intensity of their fluorescence is evaluated at 545 nm following excitation at 301 nm.
The developed spectrofluorimetric method is superior to the earlier reported methods in its better sensitivity, shorter measurement time and little cost. In addition, the proposed procedure is very simple, as the sample is dissolved in water and the formed ion pair was directly measured in the aqueous solution without the need for extraction with organic solvents. As a result, no volatile solvents but only water is used throughout the work which make the method meet the requirements of green chemistry.
Experimental
Instrument
The Fluorescence Spectrometer (Perkin Elmer LS 45) is outfitted with an emission grating monochromator, an excitation, a 150 Watt Xenon discharge lamp, and a 1 cm quartz cell. The slit widths for excitation and emission were both adjusted to 10 nm. Anthracene/Naphthalene, p-terphenyl, Ovalene, E11, Tetraphenyl butadiene, and Rhodamine were among the Certified Reference materials used to calibrate the instrument. The instruments utilized in this study included a JENWAY 3505 pH metre, an OHUAS Corporation USA digital analytical balance, a SONICOR SC-101TH sonicator, and a microcentrifuge.
Materials and reagents
In this study, analytical-grade reagents and compounds were used. A pharmaceutical company provided the standard moxifloxacin medication, and marketable descriptions were obtained on the local market. Dissolving 0.0138 g of Eosin Y disodium salt (Merck, Darmstadt, Germany) in 100 mL of distilled water yielded 4.0 × 10–4 mol L−1 of eosin Y disodium salt solution. A known volume of 0.1 mol L−1 sodium acetate and 0.1 mol L−1 acetic acid were combined to create an acetate buffer (0.1 mol L−1), and the pH was then adjusted using a pH meter.
Pharmaceutical formulations
Marketable products containing 400 mg per tablet of moxifloxacin (Fotiflox, manufactured by Helix Pharma (Pvt) Ltd., Karachi, Pakistan; Xefecta, manufactured by Hilton Pharma (Pvt) Ltd., Korangi Industrial Era, Karachi, Pakistan; Moxifo, Moxifo made by Tabros Pharma (Pvt) Ltd., L-20/B, Sector 22, F.B. Industrial Area, Karachi, Pakistan, and Oxaquin made by Highnoon Laboratories Ltd., Multan Road, Lahore-Pakistan) were purchased from the local pharmaceutical shop.
Standard drug solutions
The standard solution for moxifloxacin (100 µg mL−1) was prepared by dissolving 0.005 g of moxifloxacin in 50 mL of distilled water. By diluting the necessary volume of the standard solution with distilled water, different working solutions of the required concentrations were created. When refrigerated, the typical stock solution remained stable for one week.
Procedure in general
The necessary volume of 100 µg mL−1 standard moxifloxacin solution was added to a series of volumetric flasks along with 1.0 mL of acetate buffer solution (pH 3.6) and 1.0 mL of eosin Y (4.0 × 10–4 mol/L). Using distilled water, the volume of the resultant solution was increased to 10 mL. The relative fluorescence intensity was assessed at 549 nm following excitation at 300 nm in comparison to a reagent blank.
Procedure for tablets
A carefully weighed amount of powdered pharmaceutical tablets, equivalent to 100 µg mL−1 moxifloxacin, was weighed, dissolved in distilled water, and the volume was completed to 50 mL with distilled water. Different volumes of sample solution equivalent to 0.2, 0.4, and 0.6 µg mL−1 were obtained and evaluated in 10 mL volumetric flasks using the same approach as indicated above under “Procedure in General.”
Applications to biological samples
Separately, 1.0 mL of blood plasma and 1.0 mL of urine were combined with 2.5 mL of the 100 µg mL−1 standard moxifloxacin solution. The plasma and urine samples were then deproteinized using 6.0 mL of acetonitrile. The mixed solution was centrifuged for 10 min at 3000 rpm. The clear liquid was transferred to a 50 mL volumetric flask, and the required volume of distilled water was added afterward. The same process was used to assess the volume of this solution that would produce 0.2, 0.4, and 0.6 µg mL−1 of moxifloxacin.
Results and discussion
Eosin Y is a green-fluorescent acidic dye with a yellowish-red color. In acidic environments, the fluorescence of Eosin Y is quenched by the formation of a stable combination with cationic medicines [18]. Eosin Y has been widely employed in the development of precise and accurate analytical procedures for determining a wide range of pharmaceutical substances using spectrofluorimetric and/or spectrophotometric measurements [19,20,21,22,23].
At 545 nm emission and 300 nm excitation wavelengths, the eosin Y solution’s fluorescence peak was seen. Eosin Y's fluorescence was significantly reduced by the addition of moxifloxacin (Fig. 1). Collisional or dynamic quenching and static quenching are the two key types of quenching process. Static quenching occurs when a fluorophore and quencher form a non-fluorescent complex before excitation of fluorophore [24]. The proposed reaction occurs on the mechanism of static quenching as moxifloxacin form an ion-pair complex with eosin Y before excitation of eosin Y (Fluorophore). The fluorescence of Eosin Y was found to be quenched through the formation of a stable non-fluorescence ion-pair complex with moxifloxacin.
Optimization of the reaction conditions
Several characteristics were thoroughly examined to determine the best circumstances for the complex development. The pH of the solution, the concentration of eosin Y, the solvents employed for dilution, the reaction stability, and the effect of temperature were all examined.
The influence of pH and buffer concentration
The impact of pH was observed in the pH range of 2.8 to 4.0. At pH 3.6, the greatest ∆F was measured (Fig. 2). Because a pH greater or lower than 3.6 can cause the quenching effect to gradually diminish, pH 3.6 was chosen. The effects of other buffers, including Mcllvaine, acetate, citrate, and phosphate, were also examined. The acetate buffer yielded the highest ∆F value. The effect of different acetate buffer concentrations was also investigated, and it was discovered that a 0.1 mol L−1 acetate buffer solution with a pH of 3.6 generated the most quenching. Investigations on the impact of acetate buffer volume (pH 3.6) produced the best results when using 1.0 mL of 0.1 mol L−1 acetate buffer.
The effect of eosin Y concentration
Eosin Y concentrations ranging from 1.0 × 10–4 to 6.0 × 10–4 mol L−1 were examined for their effects. Eosin Y concentrations of 4.0 × 10–4 mol L−1 show the highest fluorescence intensity (∆F) (Fig. 3). The influence of eosin Y volume was also tested, and the maximum ∆F value was found with 1.0 mL of 4.0 × 10–4 mol L−1 eosin Y solution.
Diluting solvent’s effect
The ion-pair complex of MOX and eosin Y was diluted in a variety of solvents including acetone, chloroform, dichloromethane, dioxane, ethanol, methanol, and distilled water (Fig. 4). With distilled water, the highest ∆F values were attained. As a result, throughout the analysis, distilled water is used as a solvent.
The product’s stability
The influence of time on the stability of the ion-pair complex generated between MOX and eosin Y was investigated. The product's fluorescence intensity was monitored at 10-min intervals for up to 2 h. According to the findings (Fig. 5), the substance remains stable for up to two hours after dilution.
Eosin Y and moxifloxacin reaction stoichiometry
The Job's method of continuous variations [25] was used to calculate the molar ratio between the moxifloxacin and Eosin Y using 4.0 × 10–4 mol L−1 master equimolar solutions. The ratio of Eosin to moxifloxacin was found to be 1:1 (Fig. 6). The results of Job's approach studies conformed to the postulated reaction mechanism [26, 27]. The complex was produced by electrostatic interaction between Eosin Y's acidic carboxylate group and the moxifloxacin tertiary amine. Figure 7 depicts the proposed reaction process.
Temperature influences
The combination was heated for 15 min in an electrical thermostatic water bath at 50, 60, 70, 80, 90, and 100 °C to determine the impact of temperature. The mixture was then cooled and diluted to 10 mL with distilled water. The quenching effect is reduced as the temperature rises. As a result, more testing was done at the ambient temperature.
Analytical figures of excellence
The dye's fluorescence quenching rises in a linear relationship with moxifloxacin concentration. Under optimal experimental conditions, the calibration graph of fluorescence intensity versus moxifloxacin concentration was linear in the range of 0.2–10 µg mL−1, with an exceptional correlation coefficient of 0.992 (Fig. 8). The limit of detection (LOD) was estimated using the formula LOD = 3.3 σ/S, where S is the slope of the calibration curve and σ is the standard deviation of the intercept. The LOD was found to be 0.0322 µg mL−1. The limit of quantification (LOQ) was calculated using the formula LOQ = 10/S and determined to be 0.0976 µg mL−1. The results of calculating a number of analytical parameters using the linear regression equation are displayed in Table 1. In Table 2, the proposed method's sensitivity was compared to that of other approaches in the literature. The results reveal that the proposed method has a higher sensitivity than the previously described methods.
The method’s reliability
The method's precision was verified by measuring moxifloxacin in standard and commercial formulations in triplicate at three distinct concentrations (0.2, 0.4, and 0.6 µg mL−1). Table 3 shows the results for the standard form, while Table 4 shows the results for pharmaceutical formulations. The percent recovery obtained ranged from 101.29% to 102.71% for the standard and from 98.83% to 102.50% for pharmaceutical formulations with smaller relative standard deviations, demonstrating the reproducibility of the suggested procedure.
The developed method's repeatability and reproducibility were assessed by looking at intra-day and inter-day precision. To evaluate intra-day precision, three separate standard moxifloxacin concentrations were examined in triplicate during the course of the same day at three different times. The identical solutions were examined on three successive days for inter-day precision. Table 5 summarizes the findings. The lower RSD values imply that the established method is repeatable and reproducible.
The proposed method's accuracy was tested using a standard addition method with four distinct brands of pills containing 40 mg of moxifloxacin: Oxaquin, Fotiflox, Moxifo, and Xefecta. The known quantity of tablet solutions was mixed with three different concentrations of standard moxifloxacin solutions and analysed using the procedure specified. The percentage recoveries ranged from 98.50 to 101.16 percent and were calculated by comparing the findings before and after the addition of standard moxifloxacin solution (Table 6).
Selectivity
Interferents may be present in a real sample, which might reduce or amplify the fluorescence signal of the analyte under investigation. The effects of glucose, sucrose, lactose, ascorbic acid, Mg-stearate, starch, and fructose, all of which are commonly found in pharmaceutical products, were investigated. The given process was used to generate and analyse a sample containing a set dose of moxifloxacin (0.2 µg mL−1) and varied concentration ratios of excipients of 1:1, 1:2, 1:4, 1:6, 1:8, 1:10, 1:20, 1:40, and 1:100. The criterion for error was set at 5%. There has been no evidence that any of these excipients create interference (Fig. 9).
Application of the developed method
The proposed method was used to determine the active components of moxifloxacin in four distinct pharmaceutical formulations with great effectiveness. The results were comparable to the quantities on the labels (Table 7). The new approach was also successfully used to determine moxifloxacin in spiked human plasma and urine due to the absence of excipient interference. The percent recoveries for plasma samples varied from 100.50% to 101.50%, while those for urine samples ranged from 101.25% to 102.50% (Table 8).
Conclusions
A spectrofluorimetric approach that is relatively simple, selective, and economical was devised for the analysis of moxifloxacin in pure form, pharmaceutical formulations, and biological fluids. In comparison to previously published methods, it was discovered that the novel methodology had a wider linear range and lower detection and quantification limits. These characteristics made the devised approach more useful than previously published methods for quantifying moxifloxacin active components in pharmaceutical formulations, human blood plasma, and urine samples. Finally, the study was unaffected by excipients commonly seen in medicinal preparations.
References
J.R. Johnson, Fluoroquinolones in urinary tract infection, in Fluoroquinolones antibiotics (milestones in drug therapy). ed. by A.R. Ronald, D.E. Low (Birkhauser Verlag, Berlin, 2003), p.107
H. Stass, A. Dalhoff, D. Kubitza, U. Schühly, Antimicrob. Agents Chemother. 42, 2060 (1998)
A. Dalhoff, H. Stass, Drugs 58, 239 (1999)
J. Sousa, G. Alves, A. Fortuna, A. Falcao, Anal. Bioanal. Chem. 403, 93 (2012)
D. Predrag, C. Andrija, D. Aleksandra, J.S. Milena, J. Pharm. Biomed. Anal. 50, 117 (2007)
H.A. Nguyena, J. Grelleta, B.B. Ba, C. Quentin, M.C. Saux, J. Chromatogr. B 810, 77 (2004)
F.U. Khan, F. Nasir, Z. Iqbal, I. Khan, N. Shahbaz, M. Hassan, F. Ullah, J. Chromatogr. B 1017–1018, 120 (2016)
J.D. Smet, K. Boussery, K. Colpaert, P.D. Suttera, P.D. Paepe, J. Decruyenaere, J.V. Bocxlaer, J. Chromatogr. B 877, 961 (2009)
A.P. Dewani, B.B. Barik, S.K. Kanungo, B.R. Wattyani, Am. Eurasian J. Sci. Res. 6, 192 (2011)
R. Kant, R. Bodla, R. Bhuthani, G. Kapoor, Int. J. Pharm. Pharm. Sci. 7, 316 (2015)
S.K. Motwani, S. Chopra, F.J. Ahmad, R.K. Khar, Spectrochim. Acta A Mol. Biomol. Spectrosc. 68, 250 (2007)
P. Patel, B. Suhagia, M. Patel, Indian Drugs 2, 155 (2005)
J.G. Möller, H. Stass, R. Heinig, G. Blaschke, J. Chromatogr. B Biomed. Sci. Appl. 716, 325 (1998)
R. Inam, H. Mercan, E. Yilmaz, B. Uslu, Anal. Lett. 40, 529 (2007)
N. Erk, Anal. Bioanal. Chem. 378, 1351 (2004)
J.A. Ocana, F.J. Barragan, M. Callejon, Analyst 125, 2322 (2000)
M.N. Khan, W. Ali, Z. Shah, M. Idrees, H. Gulab, Adnan, Anal. Sci. 36, 361 (2020)
R.W. Sabnis, Handbook of biological dyes and stains: synthesis and industrial applications (Wiley, New York, 2010), p.173
M.I. Walash, M.S. Rizk, M.I. Eid, M.E. Fathy, J. AOAC Int. 90, 1579 (2007)
M.E.K. Wahba, N. El-Enany, F. Belal, Anal. Methods 7, 10445 (2015)
F. Belal, A. El-Brashy, N. El-Enany, N. El-Bahay, J. AOAC Int. 91, 1309 (2008)
M.N. Khan, M. Mursaleen, Luminescence 36, 515 (2021)
S.M.S. Derayea, Anal. Methods 6, 2270 (2014)
H. Rahman, Int. J. Pharm. Pharm. Sci. 9, 1 (2017)
D. Harvey, Modern analytical chemistry (McGraw-Hill, New York, 2000)
M. Walash, F. Belal, N. El-Enany, H. Elmansi, Int. J. Biomed. Sci. 6, 327 (2010)
M.I. Walash, F.F. Belal, M.I. Eid, S.A.E. Mohamed, Chem. Cent. J. 5, 60 (2011)
R.M.W. Kazan, H. Seddik, M. Aboudane, Int. J. Acad. Sci. Res. 5, 67 (2017)
P. Djurdjevic, A. Ciric, A. Djurdjevic, M.J. Stankov, J. Pharm. Biomed. Anal. 50, 117 (2009)
Acknowledgements
The authors express their gratitude to Allama Iqbal Open University, Islamabad, Pakistan, for allowing them to perform this study. The authors also extend their appreciation to Bacha Khan University, Charsadda, Pakistan for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, M.N., Zaman, N., Mursaleen, M. et al. Eco-friendly approach for determination of moxifloxacin in pharmaceutical preparations and biological fluids through fluorescence quenching of eosin Y. ANAL. SCI. 38, 1541–1547 (2022). https://doi.org/10.1007/s44211-022-00192-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-022-00192-6