Abstract
A simple, fast, sensitive, cost-effective, and well-proven spectrofluorimetric method has been developed for the quantification of cetirizine dihydrochloride (CTZ). The quenching effect of CTZ on the fluorescence intensity of Eosin Y was utilized to generate an ion-pair complex that can be detected at 549 nm using a 301 nm excitation wavelength in the presence of glycine buffer (pH 4.0) for the method development. The factors that influence reactions were thoroughly examined and optimized. With a determination coefficient of 0.9996, the fluorescence quenching value was linear to CTZ concentration in the range of 1–40 µg/mL. The estimated detection and quantification limits were determined to be 0.02 and 0.08 µg/mL, respectively. The method selectivity was validated by analyzing the effects of excipients, and no interference was found. The established approach was applied to determine the presence of CTZ in products available on the market as well as in biological samples. The methodology was validated using guidelines from the International Conference on Harmonization, and percent recoveries for pharmaceutical items varied from 90.6 to 103.5% and for biological fluids from 97.9 to 102.5%. The method has been successfully used for the content uniformity test with high percent recovery and low relative standard deviation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The major regulator of an allergic response is histamine. Histamine levels are especially high in mast cells found in the respiratory system, gastrointestinal tract, and skin. Histamine produces itching, promotes nasal mucosa secretion, contracts smooth muscle in the bronchi. Antihistamines of type H1 are commonly used in patients to treat symptoms caused by histamine release, which is common in allergic diseases. Cetirizine dihydrochloride (CTZ) is a second-generation H1 antihistaminic drug [1] which relieves the physical symptoms of allergic rhinitis, rhinoconjunctivitis, chronic urticaria, and other allergic illnesses. This drug is classified as a weak acid since it is easily soluble in water. It has three pKa values, i.e., 2.19, 2.93, and 8.00 [2]. It was first patented in 1981, and it was first used in medicine in 1987 under the name Zyrtec® [3]. The product is identified as (±)-[2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy] acetic acid potent long acting antihistaminic H1 receptor antagonist drug [4, 5]. Piperazines are antihistamines that have moderate sedative and antiemetic properties. CTZ, cyclizine, and hydroxyzine are piperazine derivatives. It has a lower sedative effect than the other members of this class [6].
One of the most significant instruments for pharmaceutical quality control is drug determination. As a result, the development of a simple, sensitive, quick, and efficient technique for identifying drug concentration is essential [7]. The British Pharmacopeia has offered a potentiometric titration approach for the determination of CTZ in pure form, whereas for CTZ oral solution and tablets, it suggested a n HPLC method [8]. Recent and comparative literature studies have been described for CTZ assay such as reversed-phase-HPLC [9], high-performance thin-layer chromatography [10], capillary electrophoresis . Among them, spectrofluorimetry is a very simple, cost-effective, and sensitive method. However, only a limited number of spectrofluorimetric methods have been cited in the literature for the detection of CTZ in pharmaceutical formulations and biological fluids [11].
Eosin Y was applied for the determination of several medicines using spectrophotometric and/or spectrofluorimetric techniques to generate colorful or fluorescent ion association complexes. Spectrofluorimetry is a very sensitive analytical approach that is a type of molecular emission spectroscopy which is used to assay a wide range of pharmaceutical substances [12]. Keeping in view the advantages of spectrofluorimetry in drug determination, a study was designed to develop an efficient, cost effective, and fast method for the determination of CTZ in pharmaceutical formulations and biological fluids using Eosin Y as a probe. Optimization of various reaction parameters was also among the objectives of the study.
EXPERIMENTAL
Apparatus. A fluorescence spectrometer was used to measure the fluorescence quenching (Perkin Elmer LS 45) which was equipped with a 150-W xenon discharge lamp, 1 × 1 cm quartz cells, and a grating monochromator.
Materials and reagents. Reagents and chemicals used were of analytical grade with high purity. A solution (0.1 mM) of Eosin Y was prepared by dissolving 0.0034 g of Eosin Y in 50 mL of distilled water. Glycine buffer (0.25 M) was also prepared by combining suitable quantities of 0.25 M glycine with 0.25 M hydrochloric acid, and the measured pH was 4.0. Alliance Pharmaceutical Industry Hayatabad, Pakistan, donated CTZ standard. From a local medical store, CTZ formulations were purchased, i.e., Zyrtec 10 mg/capsule made by Glaxo Smith Kline (F/268 S.I.T.E., Karachi, Pakistan), Rigix 10 mg/capsule made by AGP B-23-C, S.I.T.E., Karachi, Pakistan), Alerzine 10 mg/capsule made by Medera Pharmaceuticals (249/A, Industrial Triangle, Kahuta Road, Islamabad Industries, Pakistan), and Sedil 10 mg/capsule made by Sami Pharmaceuticals (F-95, S.I.T.E., Karachi, Pakistan).
Standard drug solutions preparation. The standard solution of CTZ (100 µg/mL) was prepared by dissolving 0.005 g of CTZ in distilled water, then diluting to 50 mL. Working solutions of small concentrations were prepared from the stock solution.
Sample solution preparation. First of all, ten tablets from each sample (10 mg) were weighed, finely powdered, and the average weight of one tablet was found. The powder form was dissolved in 10 mL of distilled water in a 50 mL calibrated volumetric flask and shaken for 5 min. The contents were sonicated for about 5 min before being diluted to the desired concentration with the same solvent. The solution was filtered using a filter paper. Working solutions with lower concentrations were prepared by diluting the suitable amount with distilled water.
Methodology of analysis. Standard solution (1–40 µg/mL) of CTZ was added to 10 mL volumetric flasks along with 2.0 mL of glycine buffer (pH 4.0) and 2.5 mL of Eosin Y (6.0 × 10–5 M) solution. Flasks were filled to the mark with distilled water. The relative fluorescence intensity was measured at 549 nm following a 301 nm excitation wavelength, compared to a blank solution prepared in the same fashion but adding water instead of drug.
Pharmaceutical preparations analysis. Using distilled water, appropriate volumes of CTZ sample solutions were diluted and assessed in the same way as described above to get concentrations within the working range. Regression equation was used to determine the specified CTZ concentrations in each marketed product.
Biological samples spiking analysis. In this experiment, 2.0 mL of CTZ standard solution (100 µg/mL) was mixed with 1.0 mL of plasma or urine. The sample was then deproteinized using 6.0 mL of acetonitrile. The mixture was centrifuged for 20 min at 3000 rpm, and the clear liquid volume was adjusted to 50 mL with distilled water. As suggested previously, the correct volume of this solution was studied via the usual analytical technique.
Procedure for determining content uniformity. For ten tablets, the procedure was repeated as described above, but each tablet was tested separately.
RESULTS AND DISCUSSION
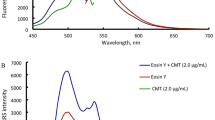
Eosin Y, one of the acidic dyes, gives a yellowish-red color through fluorescence quenching. In an acidic solution, due to the formation of ion-pair complex with cationic basic nitrogenous drugs, the fluorescence of Eosin Y can be decreased. By creating binary or ternary complexes, Eosin Y was used to identify numerous drugs utilizing spectrophotometric, spectrofluorimetric, or combined spectrophotometric and spectrofluorimetric methods [13]. Due to the formation of a binary complex of Eosin Y with CTZ, a quenching effect was observed on the native fluorescence of Eosin Y at λex/λem of 301/549 nm (Fig. 1). In the range of 1–40 µg/mL, quenching was directly proportional to CTZ concentration. The anionic part of Eosin Y in acidic medium interacted with the cationic side of CTZ and was primarily responsible for complex formation.
Reaction conditions optimization. Several factors were carefully studied by changing one parameter while keeping others constant such as concentration, pH, buffer type, reaction duration, temperature, Eosin Y concentration, and dilution solvent.
pH and buffer effect. Several buffer solutions in the pH range of 3.0 to 4.6 were prepared, and the influence of pH on fluorescence quenching was evaluated. The maximum fluorescence quenching (ΔF) was found by using glycine buffer at pH 4.0 (Fig. 2). Solutions of different concentrations of various buffers such as acetate, glycine, citrate, and phosphate were prepared (Fig. 3). The best results were obtained with 0.25 M glycine buffer at pH 4.0. The effect of glycine buffer (pH 4.0) volume on fluorescence intensity was also investigated; 2.0 mL of buffer solution yielded the highest fluorescence intensity.
Effect of concentration of Eosin Y. Different concentrations (2.0 × 10–5 to 9.0 × 10–5 M) of Eosin Y were used to test the impact of Eosin Y concentration; the highest fluorescence intensity was generated with 6.0 × 10–5 M Eosin Y (Fig. 4), as fluorescence quenching is directly proportional to CTZ concentration in the range of 1–40 µg/mL, so Eosin Y with the concentration of 6.0 × 10–5 M formed a stable complex with the drug (CTZ); therefore, this was maintained as optimized concentration throughout the research. The volume effect on 6.0 × 10–5 M Eosin Y fluorescence intensity was also investigated; the highest fluorescence intensity was produced by 2.5 mL of 6.0 × 10–5 M Eosin Y solution.
Reaction product stability. The reaction between CTZ and Eosin Y was carried out at room temperature. At regular intervals of 10 min, the product fluorescence intensity was monitored up to 2 h to evaluate the influence of time on the complex stability (Fig. 5). After 2 h of dilution, even when the fluorescence intensity was calculated, there was no influence of time on the results.
Solvent diluting effect. The influence of several types of solvents on the fluorescence qualities of the produced product was studied using distilled water, methanol, acetonitrile, 1,4-dioxane, and acetone. Maximum quenching was achieved using distilled water which is a cost-effective and eco-friendly diluting solvent (Fig. 6). As a result, distilled water was used to dilute the samples throughout the experiment.
Temperature effects. The reaction mixture was heated in an electrical thermostatic water bath for 15 min at 40, 50, 60, 70, 80, 90, and 100°C to study the influence of temperature (Fig. 7) before diluting to 10 mL with distilled water. The complex became unstable at high temperatures, diminishing the quenching action. As a result, the research was conducted at room temperature.
Method applicability. According to the International Conference on Harmonization (ICH) methodology, the validity of the suggested procedure was reviewed. The analytical parameters that were evaluated were limit of detection (LOD), limit of quantitation (LOQ), linearity, precision, accuracy, selectivity, and robustness [14].
Linearity and range. CTZ solutions of various concentrations were analyzed using the optimal parameters and the general analytical technique. Calibration curves were developed by plotting fluorescence quenching values against CTZ concentrations. Linear regression was performed on the data, and several analytical properties were found (Table 1). The calibration curve of fluorescence quenching value against CTZ concentration revealed linearity in the range of 1.0–40 µg/mL with the linear regression equation of y = 9.335x + 14.865 and a strong determination coefficient of 0.9996. The suggested method was compared with existing published methods for sensitivity (Table 2). Results showed that the suggested method sensitivity was higher than that of other methods that have already been reported [8, 10, 19].
Detection and quantification limits. The lowest concentration of an analyte that can be consistently distinguished from background levels by an analytical technique is the limit of detection, while the lowest concentration of the standard curve that can be measured with acceptable accuracy, precision, and variability is known as the limit of quantification [16]. Using the formulas LOD = 3.3σ/S and LOQ = 10σ/S, LOD and LOQ were calculated, where S is the slope of the calibration curve, and σ is the standard deviation of the intercept. The LOD and LOQ were determined to be 0.025 and 0.08 µg/mL, respectively (Table 1). The method remarkable sensitivity was demonstrated by nano-level LOD and LOQ values.
Selectivity. The interfering impact of tablet excipients was investigated using the standard addition technique to validate method selectivity. Ascorbic acid, glucose, starch, sucrose, magnesium stearate were all included in the research. Different concentrations of each excipient were added in the ratios of 1 : 1, 1 : 2, 1 : 4, 1 : 6, 1 : 8, 1 : 10, 1 : 20, 1 : 50, and 1 : 100 to 2.0 mL of standard CTZ solution. Then, 2.5 mL of 6.0 × 10–5 M Eosin Y and 2.0 mL of 0.25 M glycine buffer (pH 4.0) were mixed together. These excipients did not cause any interference at lower concentration (Fig. 8).
Reaction mechanism. To measure the molar ratio between CTZ and Eosin Y, Job’s method of continuous variation was used [17]. Both the drug and Eosin Y were produced in master equimolar solutions (6.0 × 10–5 M). In 10 mL calibrated flasks, 1.0-mL aliquots of CTZ and Eosin Y master solutions with varying complementary ratios (0 : 1.0, 0.1 : 0.9, 0.9 : 0.1, 1.0 : 0) were added. When the optimum volume of glycine buffer (pH 4.0) was added and the volume was completed to 10 mL, the fluorescence intensity was evaluated. Fluorescence intensities (ΔF) decreased and were plotted against the CTZ mole fraction (Fig. 9). Eosin Y and CTZ were found to be in a 1 : 1 ratio in this methodology. A proposed mechanism for the reaction pathway between CTZ and Eosin Y based on the obtained molar ratio is shown in Fig. 10.
Precision and accuracy. Through two levels of precision, namely, intra- and inter-day, the suggested approach was tested. To determine intra-day precision (repeatability), three different concentrations of standard CTZ solutions were evaluated in triplicate during the same day at three consecutive times. Analysis was carried out on three days in a row for inter-day (intermediate) accuracy. Table 3 shows the calculated relative standard deviation (RSD) values. The suggested method excellent accuracy was confirmed by its lower relative standard deviation value. The method accuracy was tested by measuring commercial CTZ formulations in triplicate at three different concentrations within the calibration limits. Table 4 shows the calculated percentage recoveries. The percent recoveries close to 100% suggested that the proposed methodology was accurate. The proposed approach accuracy was also tested using the standard addition method. Zyrtec, Rigix, Alerzine, and Sedil were among four CTZ pharmaceutical items studied, each having 10 mg of CTZ per tablet. The recoveries calculated varied from 90.6 to 103.5% (Table 5). The percent recoveries close to 100% suggested that the proposed methodology for CTZ determination in pharmaceutical products was extremely accurate.
Robustness. Robustness was assessed by evaluating the effect of slight changes in the selected experimental parameters to establish the validity of the planned technique for pharmaceutical analysis during normal use. The robustness of four parameters, i.e., pH, buffer volume, dye volume, and reaction duration, was studied. Table 6 shows that small modifications in these variables do not have a significant impact on the given method results.
Application to the biological samples. The approach was successfully used to determine the active component of CTZ in four distinct pharmaceutical formulations. The approach was very sensitive, had good precision and accuracy, and due to the lack of interference from excipients, this could also be used to calculate CTZ in spiked human urine and plasma samples. The recovery was between 97.9 and 102.5% (Table 7).
Application for content uniformity test. To evaluate the quality of a batch in a medicinal product, specification content uniformity is used. This test ensures that a medicinal product strength is maintained within acceptable limits [18]. To assess the consistency of Zyrtec® tablet (10 mg) dosage units according to the United States Pharmacopeia criteria [19], a content uniformity test was used. Table 8 summarizes the findings of the study, including recovery and RSD for each tablet. Among the 10 tablets, the acceptance value was less than or equal to the maximum allowed acceptance value (L1), the content of the tested 10 tablet dose was considered uniform, as indicated in the table.
CONCLUSIONS
A simple, selective, and economical spectrofluorimetric approach was developed for the quantification of CTZ in its pure form, pharmaceutical products, and biological fluids (human blood plasma and urine). Compared to other methods, the proposed method has several advantages. The reagents used are inexpensive, and a spectrofluorimeter which is readily available in most quality control laboratories may be utilized. The method is straightforward, selective, and environmentally friendly, and it can be used to measure CTZ in its pure form, commercial formulations, as well as human blood plasma and urine samples, yielding complementary findings. The proposed method is very sensitive with low limit of detection and quantification, when compared to other existing methods. Finally, the suggested approach was verified according to ICH rules.
REFERENCES
Corsico, A.G., Leonardi, S., Licari, A., Marseglia, G., Miraglia del Giudice, M., Peroni, D.G., and Ciprandi, G., Multidiscip. Respir. Med., 2019, vol. 14, p. 40.
Bajerski, L., Sangoi, M.D.S., Barth, T., Diefenbach, I.F., Dalmora, S.L., and Cardoso, S.G., Quim. Nova, 2010, vol. 33, no. 1, p. 114.
Leistner, A., Haerling, S., Kreher, J.D., Becker, I., Jung, D., and Holzgrabe, U., J. Pharm. Biomed. Anal., 2020, vol. 189, p. 113425.
Ibrahim, F., Sharaf El-Din, M.K., Eid, M., and Wahba, M.E.K., Int. J. ChemTech Res., 2011, vol. 2, no. 8, p. 2056.
Jaber, A.M.Y., Al Sherife, H.A., Al Omari, M.M., and Badwan, A.A., J. Pharm. Biomed. Anal., 2004, vol. 36, no. 2, p. 341.
El-Kommos, M.E., El-Gizawy, S.M., Atia, N.N., and Hosny, N.M., Anal. Chem. Res., 2015, vol. 3, p. 1.
Patil, R.H., Hegde, R.N., and Nandibewoor, S.T., Colloids Surf., B., 2011, vol. 83, no. 1, p. 133.
Aly, F.A., Nahed, E.E., Elmansi, H., and Nabil, A., Chem. Cent. J., 2017, vol. 11, p. 99.
Karakuş, S., Küçükgüzel, İ., and Küçükgüzel, Ş.G., J. Pharm. Biomed. Anal., 2008, vol. 46, no. 2, p. 295.
Ulu, S.T., J. Food Drug Anal., 2010, vol. 18, no. 6, p. 440.
Bhatia, N.M., Ganbavale, S.K., and More, H.N., Asian J. Pharm., 2008, vol. 2, no. 3.
Rahman, H., Int. J. Pharm. Pharm. Sci., 2017, vol. 9, p. 1.
Khan, M.N., Irum, and Mursaleen, M., Luminescence, 2021, vol. 36, no. 2, p. 515.
Derayea, S.M., Gahlan, A.A., Omar, M.A., Saleh, G.A., and Haredy, A.M., Luminescence, 2020, vol. 35, no. 7, p. 1028.
Kabra, P., Nargund, L.V.G., and Murthy, M.S., Trop. J. Pharm. Res., 2014, vol. 13, no. 7, p. 1141.
Dhongle, P.S., Sahare, S.J., Dhongle, S.S., Mundhey, A.S., and Wate, S.P., Res. J. Pharm. Technol., 2011, vol. 4, no. 9, p. 1471.
El-Didamony, A.M. and Ramadan, G.M., SN Appl. Sci., 2020, vol. 2, no. 4, p. 723.
Williams, R.L., Adams, W.P., Poochikian, G., and Hauck, W.W., Pharm. Res., 2002, vol. 19, no. 4, p. 359.
The United States Pharmacopeia 30, the National Formulary 25, Rockville: US Pharmacopeial Convention, 2007.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Shah Mansoor, Jan, F.A. & Ullah, N. Determination and Quantification of Cetirizine Dihydrochloride in Pharmaceutical Formulations and Biological Fluids Through Fluorescence Quenching of Eosin Y: Application to Content Uniformity Test. J Anal Chem 78, 965–974 (2023). https://doi.org/10.1134/S1061934823080129
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934823080129