Abstract
Background
Fat embolism syndrome (FES) is commonly reported in the setting of long bone and pelvic fractures, but the etiology and pathogenesis are unclear. The aim of this study was to identify clinical characteristics and laboratory findings that may place orthopedic trauma patients at a higher risk of developing FES.
Methods
Electronic medical records were reviewed of all patients aged 18–89 years from 2015 to 2020 with a mention of FES in the patient chart who met Gurd and Wilson’s criteria for diagnosis after experiencing orthopedic trauma. A 3:1 matched pair analysis was performed between FES patients and those with similar age, gender, and FES-associated fracture (femur, tibia, humerus, or pelvis fracture).
Results
18 patients with FES who met inclusion criteria were identified. Hypomagnesemia (OR = 7.43), hyperphosphatemia (OR = 6.24), hypoalbuminemia (OR = 3.78), blunt traumatic mechanism of injury (OR = 7.16) and a greater number of bones fractured (Avg/SD = 2.89/1.53) were seen more often in FES patients (all p-values < 0.05).
Conclusion
Findings of this study suggest that patients with hypomagnesemia, hyperphosphatemia, hypoalbuminemia, a blunt trauma mechanism of injury, and an increased number of bones fractured are at increased risk for the development of FES. This may be related to their roles in physiologic oncotic pressure and inflammatory response, and thus further investigation of these variables is necessary for the evaluation of FES prevention.
Level of evidence
Level 3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fat embolism syndrome (FES) is a clinical syndrome that demonstrates a typical pattern of clinical findings related to the presence of fat globules in the microcirculation. While FES has established major and minor clinical symptoms associated with it, its pathogenesis is poorly defined and not well understood. FES is most often associated with long bone and pelvic fractures, and the classic symptoms of FES include respiratory insufficiency, cerebral symptoms, and a petechial rash [1]. Although diagnostic criteria have been well described, many of these criteria mimic other conditions or occur concomitantly in polytrauma patients. This leads to difficulty with utilization of diagnostic criteria, and FES is typically overlooked as an initial diagnosis.
Fat microglobulinemia is a common finding in trauma patients, but very few patients go on to develop the constellation of symptoms associated with fat embolism syndrome (FES) [2, 3]. Two main theories have evolved to explain the occurrence of FES. The mechanical theory has foundations in vascular perfusion with vascular occlusion causing symptom development [4, 5]. As such, the development of FES may be more likely in patients who have conditions associated with diseased vasculature such as diabetes mellitus or hypertension. Alternatively, patients with more fat microglobule release into the bloodstream would be at increased risk for vascular occlusion with the resulting symptoms of FES. The biochemical theory has foundations in pro-inflammatory states and the inflammatory response induced by free fatty acids [4, 5]. Patients with pro-inflammatory states such as hypomagnesemia, Factor V Leiden, antiphospholipid syndrome, hyperphosphatemia, Protein C/S deficiency, or antithrombin 3 deficiency are theoretically at increased risk for FES development. Furthermore, inflammatory markers such ESR and CRP are likely increased and serve as markers of active inflammatory response.
The lack of known pathophysiology, wide range of symptoms, and lack of confirmatory testing make the clinical diagnosis difficult. The Gurd and Wilson criteria offer a solution to the difficulty with diagnosis through their criteria, which requires two major symptoms or one of the major symptoms and four minor symptoms with the presence of fat microglobulinemia. Major symptoms include petechial rash, unexplained neurologic deficits or altered mental status, and pulmonary deficits causing significant unexplained hypoxemia. The minor criteria include tachycardia, fever, emboli on fundoscopy, jaundice, renal signs, lipiduria, thrombocytopenia, anemia, elevated ESR, and fat globules in sputum [1, 6, 7]. Their characterization has shown an incidence ranging from 0.9 to 11% in trauma patients who suffer long bone or pelvic fractures [8]. A major shortcoming of the Gurd and Wilson criteria is that many of the criteria are nonspecific and can be attributed to other diagnoses. For example, polytrauma patients may have respiratory insufficiency due to concomitant lung trauma with pelvic fracture. Altered mental status may not be measurable due to sedation for intubation. As a result, these main findings may not be appropriately recorded as criteria for FES. Few additional laboratory or objective clinical characteristics have been identified as supportive factors that can be used when the clinical picture overlaps with other patient conditions. To date, there are no confirmatory tests, reliably effective laboratory findings, or other characteristics that definitively differentiate FES from other clinical manifestations related to polytrauma.
The aim of this study was to identify clinical characteristics and objective findings outside the traditional Gurd and Wilson criteria that are associated with increased risk of developing FES in patients with long bone or pelvic fracture. The authors of this study hypothesize that factors contributing to the biochemical and mechanical theories will increase the risk of FES development. These factors include electrolyte abnormalities associated with an inflammatory state, medical comorbidities associated with vascular pathology or that place patients at high risk for thrombus formation, and injury characteristics that may increase fat microglobule release into the bloodstream. The objective of this study was to use this knowledge to contribute to the working theories on the pathophysiology of FES. This information may prove valuable for clinicians to diagnose and treat FES prior to the development of more severe sequelae.
Materials and Methods
This study was a retrospective chart review of patients from an urban inner city academic hospital with IRB approval (26316-0002). Inpatient records were retrospectively reviewed from January 2015 to March 2020 for a diagnosis of FES after a long-bone or pelvic fracture. All collected data was evaluated based on available data at the time of presentation or by documentation on prior admissions.
Patients that were diagnosed with FES were identified based on the ICD-10 code T-79.1XXS. Additional patients that were not assigned the specific ICD-10 code were gathered by having a mention of FES in a patient note and were subsequently evaluated for 2 major or 1 major and 4 minor criteria for the diagnosis of FES based on the Gurd and Wilson criteria by two independent reviewers [1]. All patients included in the FES group met Gurd and Wilson’s criteria for diagnosis. Findings not evaluated for in these patients due to lack of data availability included retinal embolism, jaundice, and fat globules in the sputum.
Inpatient records were also retrospectively reviewed from the same time period for patients without FES. A 3:1 matched pair analysis was performed between those with and without FES based on age, gender, and FES-associated bone that was fractured. FES-associated bones fractured included the femur, tibia, and humerus long bones and the pelvis. Age, gender, smoking status, open or closed fracture, mechanism of injury, presence of chronic inflammatory states, hypercholesterolemia with a cholesterol level greater than 200, height, weight, presence of increased prothrombin time (PT) or activated partial thromboplastin time (aPTT), presence of thrombocytopenia, hypomagnesemia, hyperphosphatemia, and hypoalbuminemia were recorded for patients in the study. Comorbidities recorded included coronary artery disease, stroke or transient ischemic attack, hypertension, deep vein thrombosis or pulmonary embolus history, malignancy history, chronic liver disease, and osteoporosis. Clinical symptoms were also analyzed including neurologic dysfunction, shortness of breath (SOB) with respiratory dysfunction, hypoxemia, lipiduria, fever, shock, right heart failure signs, and hypotension. Genetic hypercoagulable states assessed for included antithrombin 3 deficiency, protein C/S deficiencies, Factor V Leiden, and antiphospholipid syndrome. Conditions included in the chronic inflammation group included asthma, systemic sclerosis, rheumatoid arthritis, chronic inflammatory demyelinating polyneuropathy, malignancy, sarcoidosis, and chronic osteomyelitis.
Patients were excluded from analysis if they were younger than 18 years of age or older than 89 years of age, pregnant, prisoners, or had been diagnosed with FES following an atraumatic cause.
Statistical analyses were conducted using chi square analysis for categorical variables and student’s t test for continuous variables. Statistical significance was defined as p < 0.05. Categorical testing with Chi-square analysis was based on proportions of patients in each group with each finding. Student’s t test was used to assess the difference between means for continuous variables. Two independent reviewers were responsible for data extraction from the electronic medical record. A statistician associated with our institution assisted with statistical analysis.
Results
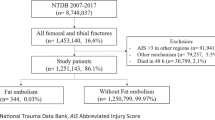
There were a total of 72 patients available for evaluation. 18 FES patients were identified and met diagnostic criteria. The remaining 54 patients consisted of those matched to the FES patients based on age, gender, and major FES-associated fractured bone. Table 1 shows the patient demographics and medical comorbidities of both the FES and non-FES group. There were no demographic differences between those who did and did not develop FES.
Table 2 compares the injury characteristics of patients with and without FES. Patients with FES exhibited an increased prevalence of a blunt traumatic mechanism of injury (p value = 0.037, OR = 7.16) as well as a greater number of bones fractured (p value = 0.028).
Table 3 examines the clinical findings observed of patients with and without FES. Statistically significant values that differed among the FES and non-FES group included presence of respiratory symptoms (p value = 0.001, OR = 14.52), neurological dysfunction (p value = 0.011, OR = 4.09), hypotension (p value = 0.016, OR = 4.27), and fever (p value ≤ 0.001, OR = 71.09). No patients observed had the previously mentioned genetic hypercoagulable states. Additionally, osteoporosis was seen in 4 patients and was not statistically different between those with and without FES development.
Table 4 shows laboratory findings of patients with and without FES. Patients with FES were more likely to have hypomagnesemia (p value = 0.014, OR = 7.43), hyperphosphatemia (p value = 0.003, OR = 6.24), hypoalbuminemia (p value = 0.021, OR = 3.78), and thrombocytopenia (p value = 0.015, OR = 3.91).
Discussion
The findings of this study reflect the study’s patient selection method in reference to the Gurd and Wilson criteria. As expected, the Gurd and Wilson criteria that were present and recorded for the majority of patients were more often seen in those with FES than in the control patients. This study also showed that patients with FES had statistically significant differences in hypomagnesemia, hyperphosphatemia, hypoalbuminemia, mechanism of injury, and the number of bones fractured. Magnesium has been shown to play a role in the inflammatory response. Mazur et al. showed that low levels of magnesium induce a clinical inflammatory syndrome characterized by leukocyte and macrophage activation, release of pro-inflammatory cytokines, and excessive production of free radicals. Furthermore, increased levels of magnesium were associated with a decreased inflammatory response [9]. This directly relates to the role of the inflammatory response in FES development as predicted by the biochemical theory.
Hyperphosphatemia has been correlated with an increase in the expression of proinflammatory cytokines, such as TNF-a [10]. Increased expression of pro-inflammatory cytokines leads to endothelial cell activation and damage which leads to some of the common symptoms associated with FES. Moreover, chronic increases in phosphate have been shown to increase mineral deposition in smooth muscle possibly disrupting vessel perfusion and decreasing vessel patency [11]. As such, the role of hyperphosphatemia in FES development supports a hybrid model involving both the biochemical and mechanical theories.
Albumin, being the main protein found in the plasma, plays an important role in fluid balance. Hypoalbuminemia has long been associated with edema due to a decrease in intravascular oncotic pressure and an increase in interstitial pressure [12]. The authors of this study hypothesize that the mechanical force generated by the third-spacing of fluid secondary to hypoalbuminemia may increase the fat globule release from the bone marrow into the circulation. This would increase the opportunity for vaso-occlusion or fat-induced inflammation as hypothesized by the mechanical and biochemical theories. Nonetheless, an increase in fat globule release would augment the development of FES as fat microglobulinemia is the key component of FES development.
The development of FES is known to be related to fat emboli in the bloodstream as previously mentioned above. The findings of this study suggest that an increased number of fractured bones is associated with FES development. This is supported by Stein et al. [13] who found that multiple fractures increased a patient’s risk for the development of FES as compared to isolated fractures. Increased number of fractured bones may have been associated with FES development due to increasing the volume of medullary cavity fat tissue that can be released into the bloodstream. The authors of this study hypothesize that an increased number of fractured bones would increase the opportunity for a higher volume of fat microglobules to enter the bloodstream and cause FES. Though multiple fracture sites would increase the opportunity for fat microglobule leakage from a singular bone, it may not increase the total fat capable of leaking into the bloodstream since it originates from the same medullary canal. Further studies are warranted to determine the relationship of FES development with fat leakage from multiple fracture sites within one bone as opposed to multiple fractured bones. The clinical importance of this distinction is related to the injury setting for patients. Patients with multiple injuries or who experience polytrauma incidents would theoretically be at increased risk for FES development when compared to those with isolated injuries.
Based on the findings of this study, the mechanism of injury was an important risk factor for the development of FES. Blunt trauma was associated with an increased risk of developing FES as compared to penetrating trauma. One possible suggestion for this could be the prevalence of microfractures with blunt trauma. The widened impact area of blunt trauma may cause an increased number of microfractures in affected bones, which could lead to more fat microglobule leakage into the bloodstream with FES development. Another theory proposed by Bolliger et al. [14] in their study on the development of pulmonary fat emboli (PFE) is that damaged subcutaneous adipose tissue can contribute to the development of PFE, which they note can lead to FES. Their findings suggest that blunt trauma may be associated with an increased risk of FES development due to additional trauma to the subcutaneous tissue. The same level of adipose tissue damage is unlikely to be seen with penetrating trauma due to a smaller impact zone when penetrating trauma occurs. The underlying mechanism for both of these suggestions is the increased volume of fat globules that can enter the circulation and cause FES either by vascular occlusion directly or through augmentation of the inflammatory cascade.
One surprising finding of this study was the absence of any medical comorbidities associated with FES development. Based on the mechanical theory as previously described, medical conditions that decrease blood vessel patency such as hypercholesterolemia with atherosclerosis, hypertension, peripheral vascular disease, or diabetes mellitus would predispose patients to FES development. One difficulty of the patient population studied is adequate access and utilization of healthcare services. The patients cared for at our institution often have conditions that go undiagnosed as a result of these barriers to care. Subsequently, the authors of this study are hesitant to definitively say that these medical comorbidities are not associated with FES development or that the absence of their association is evidence contradicting the mechanical theory for FES development.
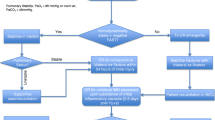
Studies have also varied on the true incidence of FES ranging from 1 to 29% after long bone fracture [7]. This study identified only 10 patients based on the ICD10 code at our institution since the utilization of electronic medical records. The prevalence at our institution is much lower than the 1% low-end range quoted by Shaikh, which suggests that many patients are misdiagnosed with another cause for the FES manifestations. Current diagnostic criteria may be difficult to identify due to patient sedation or intubation. The diagnostic criteria have largely remained unchanged since Gurd and Wilson’s criteria were established. Though imaging and laboratory findings are unreliable for diagnosis independently, the novel findings suggested by this study may prove to be important diagnostic factors in patients with FES. Updating the current clinical criteria to include these new findings will likely improve sensitivity of Gurd and Wilson’s criteria by offering objective laboratory findings unaffected by patient sedation or stabilization treatment. The mechanism of injury and number of bones fractured also offer objective criteria that can be assessed without concern for patient sedation interfering with interpretation. Furthermore, correction of the laboratory values may prove effective forms of prophylaxis or treatment by reducing their roles on inflammation and edema as previously mentioned. The increased sensitivity from adding these findings to Gurd and Wilson’s criteria would allow for earlier identification, monitoring, and treatment for patients with FES, thereby reducing mortality. The future directions of this study are to evaluate the use of these novel findings in the clinical setting for diagnosis and to determine the clinical benefit of electrolyte and albumin correction through a prospective experimental study. Nonetheless, the findings of this study suggest that a lower threshold should be utilized for assessing FES as a diagnosis when these factors are present. Doing so may identify patients at an earlier stage of disease progression and reduce the morbidity and mortality associated with the pulmonary and neurologic manifestations.
The major limitation of this study relates to the scarcity of the condition. Patients were first gathered using the ICD10 code for FES entered by paramedical staff, which resulted in a very small cohort. This was addressed by using the electronic medical record engine to search for patients with a mention of FES in a clinical note. Patients from the ICD10 code recruitment method and the FES-search in the engine were then included if Gurd and Wilson’s criteria for diagnosis were met. However, the scarcity of clinical recognition prevented any large additions to the study size, which resulted in a small sample size.
Conclusion
FES remains a poorly recognized, rare clinical entity without adequate tests for diagnosis and assessment. Early diagnosis for treatment may be necessary for the prevention of deadly sequelae. The findings of this study suggest that hypomagnesemia, hyperphosphatemia, and hypoalbuminemia are associated with an increased risk for FES development. The role of hypomagnesemia and hyperphosphatemia suggests an inflammatory component to the underlying pathophysiology while the fluid dysregulation of hypoalbuminemia suggests a mechanical component to the pathophysiology, supporting a hypothesis that the pathogenesis of FES likely involves both theories. Additionally, patients who suffer blunt trauma or multiple bone fractures are at increased risk for FES development. These factors should be considered when evaluating for FES as they may be indicators of FES development earlier in disease or assist with differential diagnosis. The addition of these findings to Gurd and Wilson’s criteria would likely improve the sensitivity of diagnostic criteria and lead to quicker supportive care in patients who develop severe sequelae. Further studies are needed to assess the roles of these factors in FES development and the therapeutic benefits of laboratory abnormality correction.
References
Gurd, A. R., & Wilson, R. I. (1974). The fat embolism syndrome. Journal of Bone and Joint Surgery. British Volume, 56B(3), 408–416.
Levy, D. (1990). The fat embolism syndrome. A review. Clinical Orthopaedics and Related Research, 261, 281–286.
Talbot, M., & Schemitsch, E. H. (2006). Fat embolism syndrome: History, definition, epidemiology. Injury, 37(Suppl 4), S3-7. https://doi.org/10.1016/j.injury.2006.08.035 ((Erratum In: Injury 2007Oct;38(10):1224)).
Baker, P. L., Pazell, J. A., & Peltier, L. F. (1971). Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. Journal of Trauma, 11(12), 1026–1030. https://doi.org/10.1097/00005373-197112000-00006.
Gossling, H. R., & Pellegrini, V. D., Jr. (1982). Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clinical Orthopaedics and Related Research, 165, 68–82.
Scarpino, M., Lanzo, G., Lolli, F., & Grippo, A. (2019). From the diagnosis to the therapeutic management: Cerebral fat embolism, a clinical challenge. International Journal of General Medicine, 4(12), 39–48. https://doi.org/10.2147/IJGM.S177407.
Shaikh, N. (2009). Emergency management of fat embolism syndrome. Journal of Emergencies, Trauma, and Shock, 2(1), 29–33. https://doi.org/10.4103/0974-2700.44680.
Kwiatt, M. E., & Seamon, M. J. (2013). Fat embolism syndrome. International Journal of Critical Illness and Injury Science, 3(1), 64–68. https://doi.org/10.4103/2229-5151.109426.
Mazur, A., Maier, J. A., Rock, E., Gueux, E., Nowacki, W., & Rayssiguier, Y. (2007). Magnesium and the inflammatory response: potential physiopathological implications. Archives of Biochemistry and Biophysics, 458(1), 48–56. https://doi.org/10.1016/j.abb.2006.03.031 ((Epub 2006 Apr 19)).
Yamada, S., Tokumoto, M., Tatsumoto, N., Taniguchi, M., Noguchi, H., Nakano, T., et al. (2014). Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia. American Journal of Physiology. Renal Physiology, 306(12), F1418–F1428. https://doi.org/10.1152/ajprenal.00633.2013 ((Epub 2014 May 7)).
Jono, S., McKee, M. D., Murry, C. E., et al. (2000). Phosphate regulation of vascular smooth muscle cell calcification. Circulation Research, 87(7), e10.
Soeters, P. B., Wolfe, R. R., & Shenkin, A. (2019). Hypoalbuminemia: Pathogenesis and clinical significance. Journal of Parenteral and Enteral Nutrition, 43(2), 181–193. https://doi.org/10.1002/jpen.1451 ((Epub 2018 Oct 4)).
Stein, P. D., Yaekoub, A. Y., Matta, F., & Kleerekoper, M. (2008). Fat embolism syndrome. American Journal of the Medical Sciences, 336(6), 472–477. https://doi.org/10.1097/MAJ.0b013e318172f5d2.
Bolliger, S. A., Muehlematter, K., Thali, M. J., & Ampanozi, G. (2011). Correlation of fat embolism severity and subcutaneous fatty tissue crushing and bone fractures. International Journal of Legal Medicine, 125(3), 453–458. https://doi.org/10.1007/s00414-011-0563-8 ((Epub 2011 Mar 19)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard statement
Our instutition’s IRB approved a HIPPA waiver for this study on January 8, 2020 since it met all criteria for waiver approval.
Informed consent
For this type of study informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lowery, A., Naran, V., Ames, R. et al. Risk Stratification Algorithm for Orthopedic Trauma Patients at Risk for Fat Embolism Syndrome. JOIO 55, 879–885 (2021). https://doi.org/10.1007/s43465-021-00365-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43465-021-00365-x