Abstract
Diosmetin is a flavonoid compound with various pharmacological activities, which plays a vital role in alleviating the development of asthma and atopic dermatitis. However, the role of diosmetin in allergic rhinitis is unclear. Our study aimed to investigate the effects of diosmetin on AR progression and the underlying molecular mechanisms. The allergic rhinitis murine model was established via ovalbumin challenge, followed by administration with diosmetin or dexamethasone. Before mice were sacrificed, nasal symptoms were evaluated. The histopathological examination of nasal mucosa was performed through hematoxylin–eosin and toluidine blue staining. The levels of histamine, ovalbumin-specific IgE, and ovalbumin-specific IgG1 in serum of mice and the levels of Th1/Th2-related cytokines and pro-inflammatory cytokines in nasal lavage fluid of mice were determined by enzyme-linked immunosorbent assay. The protein levels of silent information regulator 1 (SIRT1) and nuclear factor kappa B (NF-κB) pathway-related molecules were detected by western blotting. Diosmetin improved nasal symptoms, and downregulated the serum levels of histamine, IgE, and IgG1 in allergic rhinitis mice. Diosmetin attenuated eosinophil and mast cell infiltration in nasal mucosa tissues, decreased the migration of inflammatory cells into the nasal lavage fluid, and improved the Th1/Th2 cytokine imbalance in nasal lavage fluid. Diosmetin upregulated SIRT1 and inactivated the NF-κB pathway in allergic rhinitis mice. Furthermore, treatment with an SIRT1 inhibitor (EX-527) overturned the effects of diosmetin on the SIRT1/NF-κB signaling, Th1/Th2 cytokine imbalance, and nasal inflammation in allergic rhinitis mice. Diosmetin ameliorates nasal inflammation and Th1/Th2 imbalance by regulating the SIRT1/NF-κB signaling in allergic rhinitis mice.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allergic rhinitis (AR) is a type I hypersensitivity reaction mediated by immunoglobulin E (IgE) after individual exposure to allergens. Following antigen-IgE stimulation, mast cells, eosinophils, and T-lymphocytes secrete allergic mediators including histamine, cytokines, and chemokines to induce hypersensitivity reactions (Hemmati et al. 2019). The incidence of AR has increased dramatically over the past decade, and epidemiologic data show that AR influences approximately 40% of the global population (Hoyte and Nelson 2018). Even though AR is not life-threatening, its typical symptoms, including rhinorrhea, paroxysmal sneezing, nasal congestion, and itching, severely impact the patient’s life quality (Zhang et al. 2021). The current agents widely used for the treatment of AR include corticosteroids, antihistamines, mast cell stabilizers, nasal decongestants, and antileukotrienes. However, prolonged use of these drugs brings various adverse side effects, such as endocrine disorders, damage to the liver and kidneys, and inhibition of the central nervous system (Meltzer and Bukstein 2011). Therefore, it is urgently required to develop novel better drugs with high safety and less side-effects.
In recent decades, anti-inflammatory agents from natural products have attracted people’s attention. Numerous herbal medicines have shown their potential safety and efficacy in the prevention and treatment of AR. Flavonoids, as the most common and widely distributed plant secondary metabolite, belong to the polyphenolic compounds that are characterized by the structure of benzo-γ-pyrone. The relationship between the hydroxyl groups in the structure of flavonoid molecules and their antioxidant activity including the ability to scavenge free radicals or to reduce iron ions have been widely reported (Hyun et al. 2010). Until now, many flavonoids have been revealed to play effective roles in protecting against the development of AR. For example, baicalin, a flavonoid compound extracted from Scutellaria baicalensis Georgi, Lamiaceae, has shown its anti-allergic and anti-inflammatory activities in lipopolysaccharide-stimulated human mast cells and ovalbumin (OVA)-induced AR guinea pigs (Zhou et al. 2016). Tangeretin, a polymethoxylated flavonoid found in the fruit peels of Citrus aurantium L., Rutaceae, has been demonstrated to alleviate airway inflammation, improve allergic symptoms, and promote regulatory T cell responses in an OVA-induced AR animal model (Xu et al. 2019). Diosmetin (1) is an O-methylated flavone (3′,5,7-trihydroxy-4′-methoxyflavone) found in the leaves of the olive tree (Olea europaea L., Oleaceae) and the fruits of C. aurantium. Diosmetin contains benzene rings in its molecular structure, and the presence of hydroxyl group on the benzene ring endows diosmetin with strong antioxidant property, which can effectively scavenge free radicals and protect cells from oxidative damage (Sordon et al. 2019). Furthermore, diosmetin has been proved to possess many other pharmacological activities, including anti-cancer, anti-inflammatory, antihyperglycemic, antihyperlipidemic, anti-virulence, and free radical scavenging effects (Li et al. 2022). Notably, no adverse effect was detectable in the acute toxicity study of diosmetin, suggesting its safety for use in humans. In several recent studies, diosmetin was discovered to play an effective role in alleviating the development of allergic diseases. In OVA-challenged asthmatic mice, administration of diosmetin improves airway remodeling and hyperresponsiveness as well as relieves collagen deposition and inflammatory cell infiltration in lungs (Ge et al. 2015). Treatment with diosmetin decreases pro-inflammatory cytokine production and suppresses macrophage infiltration into the atopic dermatitis lesion in dinitrochlorobenzene-induced atopic dermatitis mouse models (Lee et al. 2020). To date, whether diosmetin plays an anti-allergic role in AR has not been investigated.

Silent information regulator 1 (SIRT1) is an NAD( +)-dependent deacetylase that removes acetylation modifications from many different proteins, including histones, transcription factors, and structural proteins. SIRT1 is involved in many cellular physiological and pathological processes, such as cell cycle regulation, DNA repair, glucose metabolism, fatty acid metabolism, apoptosis, and senescence (Fang and Nicholl 2014). Activation of SIRT1 inhibits inflammatory response, reduces cellular stress, and improves cellular antioxidant capacity. SIRT1 has been identified to negatively regulate nuclear factor kappa B (NF-κB) signaling pathway by reducing the transcriptional activity of NF-κB (Chen et al. 2020a). The NF-κB is a family of inducible transcription factors and consists of five different members in mammals: NF-κB1 (p105 and p50), NF-κB2 (p100 and p52), RelA (p65), RelB, and c-Rel. Due to its ability to modulate the transcription of genes involved in immune response and inflammation, NF-κB is regarded as a crucial regulator of the inflammatory response. SIRT1 can deacetylate lysine 310 of RelA/p65 subunit, which favors the association of p65/p50 complex with the NF-κB inhibitor IκBα and triggers the transport of the NF-κB complex from the nucleus back to the cytoplasm, affecting its transcriptional activity and inhibiting the expression of its pro-inflammatory target genes. Numerous studies have elucidated that inhibiting the NF-κB signaling pathway through activating SIRT1 contributes to alleviating the inflammatory response in multiple human diseases, including allergic diseases. For example, bergenin activates SIRT1 to deacetylate NF-κB and hinder its nuclear translocation, thereby suppressing the production of proinflammatory cytokines and improving airway inflammation in asthma (Huang et al. 2022). Loganin administration effectively reduces macrophage M1 polarization and the expression of pro-inflammatory cytokines in colon tissues of ulcerative colitis mouse models by upregulating SIRT1 expression and inhibiting NF-κB p65 acetylation (Liu et al. 2020). Interestingly, diosmetin was previously reported to alleviate colon inflammation and oxidative damage in the mouse model of colitis by inhibiting NF-κB signaling pathway through activating the circ-SIRT1/SIRT1 axis (Li et al. 2022). However, whether diosmetin participates in AR progression by regulating the SIRT1/NF-κB remains unclear.
Herein, we aim to figure out the influence of diosmetin on the OVA-induced AR murine model. We hypothesize that diosmetin improves Th1/Th2 cell–released cytokine imbalance and ameliorates nasal inflammation in AR mice via modulating the SIRT1/NF-κB signaling pathway. The present study might provide evidence that diosmetin is a promising and safe drug for AR treatment.
Materials and Methods
Animals
Totally 40 male BALB/c mice (6-week-old, 21–23 g weight range) purchased from Damool Science (Daejeon, Korea) were included in our study. Mice were housed in a specific pathogen-free facility under a 12-h light/dark cycle with free access to standard food and water. The Institutional Animal Care and Use Committee of Hubei Provincial Hospital of TCM (Wuhan, China) approved all experimental protocols.
Experimental Design
All mice were randomly allocated into 5 groups (n = 8/each group): control, OVA, OVA + diosmetin, OVA + dexamethasone (Dex), and OVA + diosmetin + EX-527 groups. To induce the mouse model of AR, mice were sensitized with 50 μg OVA (Sigma-Aldrich, St. Louis, MO, USA) and 1 mg aluminum hydroxide (Vetec, Rio de Janeiro, RJ, Brazil) on days 0, 7, and 14 by intraperitoneal injection. On days 15–28, the OVA-sensitized mice in diosmetin and DEX groups were daily administered by oral gavage with 200 μl diosmetin (0.5 mg/kg; purity: ≥ 98%, batch number: PS010395, purchased from Chengdu Push Bio-technology Co., Ltd., Chengdu, Sichuan, China) or DEX (positive control; 2.5 mg/kg; Sigma-Aldrich). The dosages of diosmetin (Ge et al. 2015) and DEX (Piao et al. 2020b) were determined as previously described. Mice in OVA and control groups received the same volume of saline once a day. On days 21–28, the OVA-sensitized mice in OVA, diosmetin, and DEX groups were challenged daily with 200 μg OVA by intranasal instillation. For EX-527 treatment, mice were intraperitoneally injected with SIRT1 antagonist EX-527 (10 mg/kg; Sigma-Aldrich) 10 min before OVA challenge on day 21 (Zou et al. 2021). On day 29, mice were lightly anesthetized by inhaling with ether and sacrificed by cervical dislocation. A schematic diagram of AR mouse model establishment and diosmetin or DEX administration is depicted in Fig. 1A.
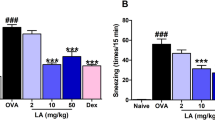
Influence of diosmetin (1) on nasal symptoms in allergic rhinitis mice. A Experimental protocol for murine model of allergic rhinitis. B, C Recording of sneezing and nasal rubbing events within 20 min of ovalbumin intranasal challenge on day 28. D–F Detection of ovalbumin-specific IgE, ovalbumin-specific IgG1, and histamine level in serum of mice using ELISA kits. n = 8/each group. **p < 0.01, ***p < 0.001
Nasal Symptom Evaluation
On day 29, the events of sneezing and nasal rubbing occurring over a 20-min period after the last OVA challenge were recorded by two blinded observers, from which the nasal symptoms were evaluated.
Histopathological Examination
Mice heads were fixed in 10% neutral formalin for 2 days, followed by decalcification in Calci-Clear Rapid histological decalcifying agent (National Diagnostics, Atlanta, GA) for 3 days. The fixed tissues were embedded in paraffin and then sectioned in 4-µm-thick segments. The slides were stained with hematoxylin–eosin (H&E) and toluidine blue solution and observed under light microscopy to detect the infiltration of eosinophils and mast cells, respectively. Digital photographs were taken by using the Moticam 5.0 MP camera, and the morphometric analysis of the histological findings was performed by using KS400 software.
Collection of Blood Sample and Nasal Lavage Fluid
After mice were anesthetized, blood specimens were harvested from retro-orbital plexus. Nasal lavage fluid (NALF) was collected by cannulating the upper part of the trachea into the nasal cavity direction and lavaging with 1 ml saline. The blood and NALF were centrifuged at 1000 × g for 10 min at 4 °C to obtain the serum and supernatant, respectively, which were stored at − 80 °C for further use.
Enzyme-Linked Immunosorbent Assay
The levels of OVA-specific immunoglobulin E (IgE), OVA-specific immunoglobulin G1 (IgG1), and histamine in serum of mice and levels of Th1-related cytokines including interferon-gamma (IFN-γ) and interleukin-12 (IL-12); Th2-related cytokines including interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-13 (IL-13); and pro-inflammatory cytokines including interleukin-6 (IL-6), interleukin-1beta (IL-1β), and tumor necrosis factor-alpha (TNF-α) in NALF of mice were measured by using ELISA kits following the manufacturer’s instructions. The ELISA kits were bought from R&D Systems Inc. (Minneapolis, MN, USA) and BD Biosciences (San Diego, CA, USA).
Western Blotting
The nasal mucosa of mice was collected for total protein extraction with radioimmunoprecipitation assay buffer (Kaiji Biotech, China) containing protease inhibitors. The total protein concentrations were determined by bicinchoninic acid protein assay kit (Beyotime, Shanghai, China). The protein samples were loaded on 8–12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis for separation and subsequently transferred onto polyvinylidene fluoride membranes (Millipore). The membranes were then blocked with 5% skimmed milk for 2 h, followed by overnight culture at 4 °C with the primary antibody and 1 h incubation at 37 °C with the secondary antibody (ab97080, Abcam, Cambridge, UK). After three 10-min washes with tris-buffered saline with Tween-20 buffer, the bands were displayed using the enhanced chemiluminescence reaction kit (Millipore). The following primary antibodies were used: anti-SIRT1 (ab189494), anti-NF-kB p65 (ab32536, Abcam), anti-acetyl (ace)-NF-kB p65 (ab19870, Abcam), anti-phospho (p)-NF-kB p65 (ab76302, Abcam), anti-IκBα (#4812, Cell Signaling Technology (CST), Danvers, MA, USA), anti-p-IκBα (#2859, CST), and anti-GAPDH (ab181603, Abcam). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control.
Statistical Analysis
The statistical analysis was performed using SPSS 20.0 software (SPSS, Chicago, IL, USA), and the results for three independent experiments are represented as mean ± standard deviation. Comparisons between the groups were analyzed with two-tailed Student’s t-test or one-way analysis of variance. Statistical significance was set at p < 0.05.
Results and Discussion
Nasal Symptoms
To explore the anti-allergic role of diosmetin, the major symptoms of AR including sneezing, nasal congestion, and/or itching in OVA-challenged mice were evaluated. The sneezing and nasal rubbing events over a 20-min period after OVA intranasal challenge on day 28 were recorded. Compared with the control mice, AR mice presented obviously higher frequencies of sneezing and nasal rubbing. However, the administration of diosmetin or DEX effectively improved nasal symptoms in AR mice (Fig. 1B, C). Furthermore, the upregulated serum levels of OVA-specific IgE, OVA-specific IgG1, and histamine in AR mice were reduced by diosmetin or DEX treatment (Fig. 1D–F).
Eosinophil and Mast Cell Infiltration
The histopathological evaluation of nasal mucosa through H&E staining showed that the number of eosinophils was considerably increased in the OVA group compared with the control group. Diosmetin or DEX treatment led to a remarkable reduction in eosinophil numbers in nasal mucosa of AR mice (Fig. 2A, B). Toluidine blue staining was performed to detect the presence of mast cells in nasal mucosa tissues. Consistently, obvious recruitment of mast cells can be observed in the OVA group. Nevertheless, the infiltration of mast cells in the nasal mucosa of AR mice was ameliorated after administration of diosmetin or DEX (Fig. 2C, D).
Influence of diosmetin (1) on eosinophil and mast cell infiltration in nasal mucosa tissues. A, B HE staining of nasal mucosa tissues for the detection of eosinophil infiltration. C, D Toluidine Blue staining nasal mucosa tissues for the examination of mast cell infiltration (indicated by red arrows). n = 8/each group. **p < 0.01, ***p < 0.001
Inflammatory Cell Infiltration
Next, the numbers of total and differential inflammatory cells in NALF of mice were counted. A significant elevation in the numbers of total cells, eosinophils, and lymphocytes as well as the percentage of eosinophils was discovered in NALF of AR mice. However, diosmetin or DEX administration mitigated the migration of the above inflammatory cells into the NALF (Fig. 3A–D).
Imbalance of Cytokine Release
Compared with the control group, the OVA group displayed decreased levels of Th1-related cytokines (IFN-γ and IL-12) as well as increased levels of Th2-related cytokines (IL-4, IL-5, and IL-13) and pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α). After AR mice received diosmetin or DEX treatment, the levels of all the above cytokines in NALF were restored (Fig. 4A–H).
SIRT1/NF-κB Signaling
To determine the potential molecular mechanism by which diosmetin inhibited nasal inflammation in AR mice, western blotting was conducted to analyze the expression of SIRT1 and NF-κB signaling–related molecules. The expression of ace-NF-κB p65, p-NF-κB p65, and p-IκBα was upregulated while the expression of SIRT1 and IκBα was downregulated in AR mice, suggesting the downregulation of SIRT1 and the activation of NF-κB signaling. However, compared with OVA-induced AR mice, diosmetin or DEX-treated AR mice presented reduced protein levels of ace-NF-κB p65, p-NF-κB p65, and p-IκBα and elevated protein levels of SIRT1 and IκBα, indicating that diosmetin or DEX activated SIRT1 and suppressed the NF-κB signaling in AR mice (Fig. 5A–F).
Effect of SIRT1 Inhibitor EX-527
To confirm whether diosmetin participates in regulating AR development by regulating the SIRT1/NF-κB signaling, SIRT1 inhibitor EX-527 was used. As shown in Fig. 6A–F, EX-527 overturned diosmetin-induced upregulation in SIRT1 and IκBα protein levels and downregulation in ace-NF-κB p65, p-NF-κB p65, and p-IκBα protein levels in nasal mucosa tissues of AR mice. This indicated that EX-527 antagonized the repression of diosmetin on SIRT1 expression and its activation on NF-κB signaling pathway in AR mice. Finally, whether EX-527 treatment influences diosmetin-mediated Th1/Th2 cytokine imbalance and nasal inflammation in AR mice was estimated. ELISA revealed that diosmetin-induced increase in the levels of Th1-related cytokines (IFN-γ and IL-12) and decrease in the levels of Th2-related cytokines (IL-4, IL-5, and IL-13) and pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α) in AR mice were counteracted by EX-527 treatment (Fig. 7A–H), suggesting that diosmetin ameliorates Th1/Th2 cytokine imbalance and nasal inflammation in AR mice through activating SIRT1 and inhibiting NF-κB signaling pathway.
SIRT1 inhibitor EX-527 reverses the inactivation of diosmetin (1) on the SIRT1/NF-κB signaling. A–F The protein levels of SIRT1, acetyl (ace)-NF-kB p65, phospho (p)-NF-kB p65, p-IκBα and IκBα in nasal mucosa tissues of mice were measured by western blotting. n = 8/each group. **p < 0.01, ***p < 0.001
SIRT1 inhibitor EX-527 offsets the improvement of diosmetin (1) on Th1/Th2 cytokine imbalance and nasal inflammation. The levels of A, B Th1-associated cytokines, C–E Th2-associated cytokines, and F–H pro-inflammatory cytokines in NALF of mice were estimated using ELISA kits. n = 8/each group. **p < 0.01
This study explored the influence of diosmetin, a natural flavonol-type flavonoid, on allergic inflammation in AR mice. OVA-challenged AR mice exhibited nasal allergy symptoms similar to humans, which makes them appropriate animal models for studying AR. OVA-challenged AR mice presented pronounced nasal allergy symptoms, eosinophil, and mast cell infiltration into the nasal cavity, inflammatory cell infiltration in NALF, and Th1/Th2 cytokine imbalance. However, we discovered that diosmetin treatment alleviated all these symptoms. In addition, the downregulation of SIRT1 and the activation of NF-κB signaling pathway in AR mice were also overturned by diosmetin. All these results suggested that diosmetin ameliorated nasal inflammation and Th1/Th2 cytokine imbalance in AR mice through modulating the SIRT1/NF-κB signaling pathway.
Allergic reactions primarily rely on the action of IgE antibody and other immunoglobulins such as IgG. OVA, as an exogenous antigen, can stimulate B cells to produce IgE. IgE upregulates the expression of FcεRI, thereby playing a pivotal role in mast cell activation. The activated mast cells release allergic mediators including histamine, chemokines, and pro-inflammatory cytokines, which are closely linked with the infiltration of inflammatory cells. These mediators trigger a series of allergy symptoms, such as nasal itching, sneezing, runny, and other symptoms. The overproduction of histamine is related to multiple pathological features of allergic inflammation, including tissue edema, mucus overproduction, and contraction of bronchial smooth muscle. Therefore, antihistamines have been widely utilized for the treatment of allergic diseases (Abelson et al. 2015). TNF-α is a proinflammatory cytokine secreted from mast cells, which is considered as an initiator of cytokine-associated inflammatory states. During the induction of inflammatory response, TNF-α facilitates leukocyte infiltration and tissue fibrosis. Previously, the blockade of TNF-α was reported to mitigate the pathological inflammation in AR guinea pigs (Guo-Zhu et al. 2015). IL-6 is a pleiotropic cytokine mainly produced by macrophages, T lymphocytes, and B lymphocytes. When the body undergoes an inflammatory reaction, viruses, endotoxins, and many kinds of cytokines can induce the production of IL-6. Intranasal challenge with IL-6 was shown to enhance the production of nasal mucus (Diamant et al. 2010). A previous study disclosed that IL-6 knockout mice exhibited lower levels of allergen-specific IgG1, decreased Th2/Th17 cytokine production, and attenuated inflammatory cell recruitment after allergen sensitization (Lin et al. 2016). IL-1β is a pro-inflammatory cytokine that is present in the cytoplasm as an inactive zymogen (pro-IL-1β). Pro-IL-1β can be hydrolyzed to IL-1β when IgE mediates mast cell activation. IL-1β can stimulate monocytes and facilitate eosinophil recruitment. Additionally, anti-IL-1β IgY, the inhibitor of IL-1β, was demonstrated to alleviate pathological allergic inflammation in AR guinea pigs (Wei-xu et al. 2014). Consistently, our data showed that OVA stimulation increased histamine, IgE, IgG, TNF-α, IL-6, and IL-1β levels in AR mice. Nevertheless, the administration of diosmetin remarkably reduced the levels of the above mediators.
The Th1/Th2 cytokine imbalance is the key factor of allergic disorders. The exposure of the body to allergens induces the differentiation of Th0 cells into Th2 cells, resulting in the decrease of Th1 cells, increase of Th2 cells, and decrease of Th1/Th2 ratio (Th1/Th2 imbalance) and further leading to AR (Asayama et al. 2020). Cytokines produced by Th1 cells include IL-2 and IFN-γ, which are associated with cellular immunity of virus clearance and delayed allergic reaction. Cytokines released by Th2 cells mainly include IL-4, IL-5, and IL-13, which participate in allergic inflammatory reactions. IL-5 is linked with eosinophilic inflammation and infiltration into the airway. IL-4 and IL-13 regulate IgE synthesis and are associated with Th2 cell differentiation. The increase of eosinophils in tissues and blood is one major characteristic of allergic inflammation in humans. The eosinophil recruitment during allergen-induced rhinitis is related to the expression of cytokines released by Th2 cells. To evaluate the effects of diosmetin on Th1/Th2 imbalance in OVA-induced AR mice, the expression of Th1/Th2-related cytokines was detected. The results showed that AR mice displayed decreased expression of IFN-γ and IL-12 and increased expression of IL-4, IL-5, and IL-13. However, diosmetin administration reversed the changes in the expression of these cytokines in AR mice. The histopathologic findings further supported these results. The infiltration of mast cells and eosinophils in the nasal mucosa tissues was reduced after administration of diosmetin. This indicated that diosmetin exerted its anti-allergic effect in AR by modulating the Th1/Th2 imbalance.
As reported, SIRT1 influences a wide range of cellular processes, including cell cycle, mitochondrial biosynthesis, and energy homeostasis, and macroscopically regulates aging, apoptosis, and inflammatory responses. Recent research has confirmed that high expression of SIRT1 helps ameliorate allergic symptoms and mitigate inflammatory response in murine models of AR. In addition, SIRT1 was proved to directly inactivate NF-κB signaling through deacetylating the p65 subunit of NF-κB complex. Xu et al. (2022) demonstrated that inhibition of histone deacetylase 4 mitigates inflammatory response and mucus production in IL-13-treated nasal epithelial cells in AR by activating SIRT1/NF-κB signaling. NF-κB is a crucial transcription factor that participates in regulating inflammatory response. Activated NF-κB induces the upregulation in the expression of Th2 cytokines and plays a crucial role in the pathogenesis of AR. Numerous studies have elucidated that the inhibition of NF-κB pathway ameliorates AR development via mediating Th1/Th2 imbalance. For example, mangiferin ameliorates nasal mucosa inflammation and reduces epithelial disruption and inflammatory cell infiltration in lung tissues of AR mice by inhibiting NF-κB pathway (Piao et al. 2020a). Luteolin plays an anti-allergic role in AR rats via improving the Th1/Th2 imbalance by repressing TLR4/NF-κB pathway (Dong et al. 2021). Importantly, diosmetin was previously demonstrated to play an anti-inflammatory role in human diseases by inhibiting the NF-κB signaling pathway. Diosmetin exhibits anti-proliferative and anti-inflammatory effects in rheumatoid arthritis fibroblast–like synoviocytes MH7A cells by suppressing the Akt and NF-κB pathways (Chen et al. 2020b). Diosmetin ameliorates neuroinflammation and neuronal apoptosis in a rat model of Streptococcus pneumoniae meningitis by repressing the PI3K/AKT/NF-κB signaling pathway (Zhang et al. 2019). In addition, diosmetin markedly decreases the acetylation of NF-κB p65 and inhibits NF-κB signaling pathway by activating the circ-Sirt1/Sirt1 axis, thereby alleviating colon inflammation and oxidative damage in the mouse model of colitis (Li et al. 2022), suggesting the regulation of diosmetin on the SIRT1/NF-κB pathway. Herein, we discovered that treatment of diosmetin counteracted the reduction in ace-NF-κB p65, p-NF-κB p65, and p-IκBα levels and elevation in SIRT1 and IκBα levels in AR mice, implying that diosmetin activated SIRT1 and thereby suppressed NF-κB signaling in AR mice. Moreover, treatment with EX527, the inhibitor of SIRT1, reversed the effects of diosmetin on the SIRT1/NF-κB signaling pathway, Th1/Th2 cytokine imbalance, and pro-inflammatory cytokine expression in AR mice.
Conclusion
In summary, diosmetin has a protective effect on OVA-challenged AR mice through alleviating rhinitis symptoms and repressing the production of inflammatory mediators. Mechanically, diosmetin displays an anti-inflammatory role by improving Th1/Th2 cytokine imbalance via activating SIRT1 and inhibiting NF-κB pathway (Fig. 8). Further studies into the dose, safety, efficacy, and bioavailability are required to support the application of diosmetin in the treatment of AR and other human allergic diseases.
Schematic diagram displaying the mechanism by which diosmetin (1) acts in allergic rhinitis. Exposure to allergens facilitates the differentiation of Th0 cells into Th2 cells and stimulates B cells to produce IgE. IgE binds to mast cells and triggers them to release pro-inflammatory cytokines, which together with cytokines released by Th2 cells induce the recruitment and activation of leukocytes, resulting in symptoms of allergic airway inflammation. NF-κB pathway is activated to further exacerbate the allergic inflammation. Diosmetin exerts its anti-allergic inflammatory effect by improving the Th1/Th2-released cytokines imbalance through the activation of SIRT1 and the inhibition of NF-κB signaling
Data Availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abelson M, Shetty S, Korchak M, Butrus S, Smith L (2015) Advances in pharmacotherapy for allergic conjunctivitis. Expert Opin Pharmacother 16:1219–1231. https://doi.org/10.1517/14656566.2015.1040760
Asayama K, Kobayashi T, D’Alessandro-Gabazza C, Toda M, Yasuma T, Fujimoto H, Okano T, Saiki H, Takeshita A, Fujiwara K, Fridman D’Alessandro V, Nishihama K, Totoki T, Inoue R, Takei Y, Gabazza E (2020) Protein S protects against allergic bronchial asthma by modulating Th1/Th2 balance. Allergy 75:2267–2278. https://doi.org/10.1111/all.14261
Chen M, Chen Z, Huang D, Sun C, Xie J, Chen T, Zhao X, Huang Y, Li D, Wu B, Wu D (2020a) Myricetin inhibits TNF-α-induced inflammation in A549 cells via the SIRT1/NF-κB pathway. Pulm Pharmacol Ther 65:102000. https://doi.org/10.1016/j.pupt.2021.102000
Chen Y, Wang Y, Liu M, Zhou B, Yang G (2020b) Diosmetin exhibits anti-proliferative and anti-inflammatory effects on TNF-α-stimulated human rheumatoid arthritis fibroblast-like synoviocytes through regulating the Akt and NF-κB signaling pathways. Phytother Res 34:1310–1319. https://doi.org/10.1002/ptr.6596
Diamant Z, Boot J, Mantzouranis E, Flohr R, Sterk P, Gerth van Wijk R (2010) Biomarkers in asthma and allergic rhinitis. Pulm Pharmacol Ther 23:468–481. https://doi.org/10.1016/j.pupt.2010.06.006
Dong J, Xu O, Wang J, Shan C, Ren X (2021) Luteolin ameliorates inflammation and Th1/Th2 imbalance via regulating the TLR4/NF-κB pathway in allergic rhinitis rats. Immunopharmacol Immunotoxicol 43:319–327. https://doi.org/10.1080/08923973.2021.1905659
Fang Y, Nicholl MB (2014) A dual role for sirtuin 1 in tumorigenesis. Curr Pharm Des 20:2634–2636. https://doi.org/10.2174/13816128113199990488
Ge A, Liu Y, Zeng X, Kong H, Ma Y, Zhang J, Bai F, Huang M (2015) Effect of diosmetin on airway remodeling in a murine model of chronic asthma. Acta Biochim Biophys Sin 47:604–611. https://doi.org/10.1093/abbs/gmv052
Guo-Zhu H, Xi-Ling Z, Zhu W, Li-Hua W, Dan H, Xiao-Mu W, Wen-Yun Z, Wei-Xu H (2015) Therapeutic potential of combined anti-IL-1β IgY and anti-TNF-α IgY in guinea pigs with allergic rhinitis induced by ovalbumin. Int Immunopharmacol 25:155–161. https://doi.org/10.1016/j.intimp.2014.12.002
Hemmati S, Rahimi N, Dabiri S, Alaeddini M, Etemad-Moghadam S, Dehpour A (2019) Inhibition of ovalbumin-induced allergic rhinitis by sumatriptan through the nitric oxide pathway in mice. Life Sci 236:116901. https://doi.org/10.1016/j.lfs.2019.116901
Hoyte F, Nelson H (2018) Recent advances in allergic rhinitis. F1000Res 7:1333. https://doi.org/10.12688/f1000research.15367.1
Huang D, Sun C, Chen M, Bai S, Zhao X, Wang W, Geng K, Huang W, Zhao T, Wu B, Zhang G, Wu D, Xu Y (2022) Bergenin ameliorates airway inflammation and remodeling in asthma by activating SIRT1 in macrophages to regulate the NF-κB pathway. Front Pharmacol 13:994878. https://doi.org/10.3389/fphar.2022.994878
Hyun J, Woo Y, Hwang DS, Jo G, Eom S, Lee Y, Park JC, Lim Y (2010) Relationships between structures of hydroxyflavones and their antioxidative effects. Bioorg Med Chem Lett 20:5510–5513. https://doi.org/10.1016/j.bmcl.2010.07.068
Lee D, Park J, Choi J, Jang H, Seol J (2020) Anti-inflammatory effects of natural flavonoid diosmetin in IL-4 and LPS-induced macrophage activation and atopic dermatitis model. Int Immunopharmacol 89:107046. https://doi.org/10.1016/j.intimp.2020.107046
Li H, Wei Y, Li X, Zhang S, Zhang R, Li J, Ma B, Shao S, Lv Z, Ruan H, Zhou H, Yang C (2022) Diosmetin has therapeutic efficacy in colitis regulating gut microbiota, inflammation, and oxidative stress via the circ-Sirt1/Sirt1 axis. Acta Pharmacol Sin 43:919–932. https://doi.org/10.1038/s41401-021-00726-0
Lin Y, Chen S, Wang J (2016) Critical role of IL-6 in dendritic cell-induced allergic inflammation of asthma. J Mol Med 94:51–59. https://doi.org/10.1007/s00109-015-1325-8
Liu S, Shen H, Li J, Gong Y, Bao H, Zhang J, Hu L, Wang Z, Gong J (2020) Loganin inhibits macrophage M1 polarization and modulates sirt1/NF-κB signaling pathway to attenuate ulcerative colitis. Bioengineered 11:628–639. https://doi.org/10.1080/21655979.2020.1774992
Meltzer E, Bukstein D (2011) The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allerg Asthma Im 106:S12–S16. https://doi.org/10.1016/j.anai.2010.10.014
Piao CH, Fan YJ, Nguyen TV, Song CH, Chai OH (2020a) Mangiferin alleviates ovalbumin-induced allergic rhinitis via Nrf2/HO-1/NF-κB signaling pathways. Int J Mol Sci 21:3415. https://doi.org/10.3390/ijms21103415
Piao CH, Song CH, Lee EJ, Chai OH (2020b) Saikosaponin A ameliorates nasal inflammation by suppressing IL-6/ROR-γt/STAT3/IL-17/NF-κB pathway in OVA-induced allergic rhinitis. Chem Biol Interact 315:108874. https://doi.org/10.1016/j.cbi.2019.108874
Sordon S, Popłoński J, Milczarek M, Stachowicz M, Tronina T, Kucharska AZ, Wietrzyk J, Huszcza E (2019) Structure-antioxidant-antiproliferative activity relationships of natural C7 and C7–C8 hydroxylated flavones and flavanones. Antioxidants 8:210. https://doi.org/10.3390/antiox8070210
Wei-Xu H, Qin X, Zhu W, Yuan-Yi C, Li-Feng Z, Zhi-Yong L, Dan H, Xiao-Mu W, Guo-Zhu H (2014) Therapeutic potential of anti-IL-1β IgY in guinea pigs with allergic asthma induced by ovalbumin. Mol Immunol 58:139–149. https://doi.org/10.1016/j.molimm.2013.11.006
Xu H, Wang L, Chen H, Cai H (2022) HDAC4 depletion ameliorates IL-13-triggered inflammatory response and mucus production in nasal epithelial cells via activation of SIRT1/NF-κB signaling. Immun Inflamm Dis 10:e692. https://doi.org/10.1002/iid3.692
Xu S, Kong Y, Jiao W, Yang R, Qiao Y, Xu Y, Tao Z, Chen S (2019) Tangeretin promotes regulatory T cell differentiation by inhibiting Notch1/Jagged1 signaling in allergic rhinitis. Int Immunopharmacol 72:402–412. https://doi.org/10.1016/j.intimp.2019.04.039
Zhang Y, Jiang Y, Lu D (2019) Diosmetin suppresses neuronal apoptosis and inflammation by modulating the phosphoinositide 3-kinase (PI3K)/AKT/nuclear Factor-κB (NF-κB) signaling pathway in a rat model of pneumococcal meningitis. Med Sci Monit 25:2238–2245
Zhang Y, Lan F, Zhang L (2021) Advances and highlights in allergic rhinitis. Allergy 76:3383–3389. https://doi.org/10.1111/all.15044
Zhou Y, Wang H, Sui H, Li L, Zhou C, Huang J (2016) Inhibitory effect of baicalin on allergic response in ovalbumin-induced allergic rhinitis guinea pigs and lipopolysaccharide-stimulated human mast cells. Inflam Res 65:603–612. https://doi.org/10.1007/s00011-016-0943-0
Zou B, Fu Y, Cao C, Pan D, Wang W, Kong L (2021) Gentiopicroside ameliorates ovalbumin-induced airway inflammation in a mouse model of allergic asthma via regulating SIRT1/NF-κB signaling pathway. Pulm Pharmacol Ther 68:102034. https://doi.org/10.1016/j.pupt.2021.102034
Acknowledgements
The authors appreciate all the participants providing supports for this study.
Author information
Authors and Affiliations
Contributions
QH conceived and designed the experiments. QH and LP carried out the experiments, analyzed the data, and drafted the manuscript. All authors agreed to be accountable for all aspects of the work. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The Institutional Animal Care and Use Committee of Hubei Provincial Hospital of TCM (Wuhan, China) approved all experimental protocols.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, Q., Peng, L. Diosmetin Alleviates Ovalbumin-Induced Nasal Inflammation by Regulating the SIRT1/NF-κB Signaling in Mouse Models of Allergic Rhinitis. Rev. Bras. Farmacogn. 33, 1232–1242 (2023). https://doi.org/10.1007/s43450-023-00448-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-023-00448-w