Abstract
We aimed to investigate whether quercetin had a therapeutic effect in an experimental rat model of allergic rhinitis. The study was conducted with 35 rats, which were randomly assigned into 4 groups: group 1 (n = 5), sham group; group 2 (quercetin group, n = 10) received 80 mg/kg day quercetin; group 3 (steroid group, n = 10) received steroid (mometasone furoate); and group 4 (control group, n = 10), received ovalbumin alone. Rats were sensitized by administration of ovalbumin on alternate days over 14 days via an intraperitoneal route. On day 15, in addition to ovalbumin via an intranasal route, quercetin and steroid were given over 7 days to the corresponding groups. All rats were then sacrificed and nasal turbinates were evaluated histopathologically, and serum total IgE and ovalbumin (OVA)-specific IgE values were measured before and after treatment. A significant increase in OVA-specific IgE values was detected in all groups except sham group. A significant increase was detected in post-treatment total IgE levels in the control group, while no significant change was detected in the sham, quercetin, and intranasal steroid groups. On histopathological evaluation, it was observed that findings of allergic rhinitis were suppressed in the quercetin group when compared to the control group. In immunohistochemical evaluation, it was detected that COX-2 and VIP expressions were weaker in the quercetin group compared to the control group. Based on these findings, we conclude that quercetin was effective in allergic rhinitis induced by ovalbumin in rats both histopathologically and serologically.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allergic rhinitis is a common disorder characterized by inflammation of nasal mucosa, in which nasal symptoms, including nasal congestion, sneezing, and itching, are prominent [1]. Allergic rhinitis is a chronic IgE-related disorder of the respiratory tract with high prevalence, which progresses to labor loss, impaired quality of life, and comorbid disease [2]. There are many medical treatment modalities used in the treatment of allergic rhinitis, including antihistamines, steroids, montelukast inhibitors, and immunotherapy [3]. However, these therapeutic modalities can fail on some occasions. Thus, numerous animal studies have been conducted to construct allergic rhinitis models and to evaluate the effects of different agents [4].
Flavonoids are compounds with beneficial biological effects that are plentifully present in herbal foods. These compounds are mainly present in fruits, vegetables, tea, and leguminous seeds, and they provide the color of many fruits and flowers. Antioxidant effects are the best-defined characteristics of almost all flavonoid groups [5]. Quercetin is one of the well-defined flavonoids and has potent antioxidant activity when compared to other flavonoids. In addition to antioxidant features, it has many biological benefits, including anti-proliferative, anti-inflammatory, anti-viral, anti-thrombotic, anti-atherosclerotic, anti-tumoral, anti-ototoxic, and anti-allergic effects [6,7,8,9,10,11]. However, its effectiveness in allergic rhinitis is unknown.
In the present study, we aimed to investigate whether quercetin given via an intraperitoneal route had therapeutic activity in an experimental rat model of allergic rhinitis.
Materials and methods
Study plan
The study was approved by the Local Ethics Committee on Experimental Animal Studies of Erciyes University (14/127). The study was conducted with 35 female Sprague–Dawley rats weighing 200–250 g and aged 20–22 weeks at Hakan Çetinsaya Clinical and Experimental Animal Research Center of Erciyes University. Rats were housed under the standard conditions at a temperature of 21 °C, maintaining a 12-h dark-light cycle. All animals were fed with the standard commercial pellet diet and water ad libitum.
Anesthesia and drug administration
In all rats, sedation was achieved by administration of 10 mg/kg xylazine (Rompun, Bayer, Germany) and 40 mg/kg ketamine hydrochloride (Ketalar, Eczacibasi, Turkey) via an intraperitoneal route. Nasal examination was performed before the study in all rats and those with serous secretion were excluded.
Five rats were assigned as the sham group (group 1). Subsequently, in the remaining 30 rats, 1 mg ovalbumin (Sigma-Aldrich, St. Louis, MO, USA) plus 10 mg aluminum hydroxide (dissolved in 1 ml normal saline) was given on alternate days via an intraperitoneal route over 14 days to induce allergy. The drugs were given at the same time each day. After sensitization, 30 rats were randomly assigned into three groups. In group 2 (quercetin group, n = 10), 80 mg/kg quercetin (Sigma-Aldrich Chemical Co., St. Lousi, MO, USA) was given via an intraperitoneal route over 7 days [12]. In group 3 (steroid group, n = 10), 0.1 ml mometasone furoate (Nasonex®, Schering Plough, İstanbul, Turkey) was applied to each nostril using a micro-pipette over 7 days. Group 4 was assigned as a control group. In addition, 10 µg/µl ovalbumin (once daily) was applied to each nostril over 7 days using a micro-pipette to trigger allergy between the 15th and 21st days in all rats.
Assessment of nasal symptoms
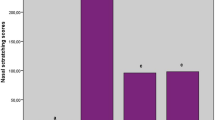
Before all intranasal administrations, nasal examination was performed in all rats and findings were recorded periodically throughout the study. After repeated intranasal ovalbumin administration following intraperitoneal ovalbumin, nasal assessment was performed by observation beginning on day 14. Nasal symptom scores were assessed independently by two blinded observers to the experimental groups. After a 10-min adaptation period, rats were observed over 10 min and nasal symptoms, including sneezing, itching, and rhinorrhea, were rated on a 0–3 point scale (Fig. 1a) [13]. Allergic rhinitis scores were recorded on days 15, 16, 18, 20, 21, and 22 (Fig. 2). The allergic rhinitis model was considered to be successful when the total score exceeded five points.
Measurement of total IgE and ovalbumin-specific IgE levels
In all rats, 1 cc blood samples were drawn via intra-cardiac puncture under anesthesia at baseline and at study completion. Blood samples were centrifuged at 3000 rpm for 20 min. Sera obtained were stored at −80 °C until assayed. Total IgE and ovalbumin-specific IgE levels were measured by an ELISA method (Rat OVA SIgE Antibody ELISA 96-well micro-titer plate kit, Rat Total IgE Antibody ELISA 96-well micro-titer plate, Mybiosource, Inc, San Diego, USA).
At the end of the study, the rats were sacrificed by cervical dislocation under anesthesia. Nasal cavity, nasal septum, paranasal sinus, and conchas were removed by en bloc dissection. Tissue samples were stored in 10% formaldehyde.
Histopathological examination
Tissues removed were fixed in neutral formaldehyde for 24 h. Tissue samples were then washed overnight and dehydration was performed by treating with an alcohol series of increasing concentration. Xylene was used to obtain transparent tissue. Tissue sections (5 µm in thickness) were then mounted on poly-lysine coated slides. Some slides were stained using hematoxylin–eosin (H&E) for histopathological assessment. The remaining slides were used for immunohistochemical evaluations. Slides were incubated at 60 °C overnight and deparaffinized using xylene followed by dehydration with an alcohol series of increasing concentration. For antigen retrieval, slides were placed in citrate buffer (10 mM, pH 6.0) and boiled in a microwave oven for 15 min. To prevent endogenous peroxidase activity, slides were treated with hydrogen peroxide for 15 min. Slides were then incubated with blocking serum for 15 min (Ultra V Block, TP-060-HL, NeoMarker, Fremont, CA, USA). The slides were incubated with primary COX-2 (Thermo, CA, USA), MMP-9 (Scbt, USA) and VIP (Scbt, USA) antibodies at room temperature for 60 min. Antigen–antibody complex was fixed using biotinylated secondary antibody and streptavidin–peroxidase complex. Labeling was achieved using DAB (Labvision, CA, USA). Contrast staining was achieved using Mayer’s hematoxylin and slides were closed with mounting medium. Images were captured by a digital camera attached to an Olympus microscope (Cx31, Germany).
In each preparation, bilateral nasal turbinates were assessed and H score was calculated according to intensity and amount of staining in ten randomly selected fields (400×). Immunolabeling scores were evaluated independently by two blinded observers to the experimental groups. The intensity of staining was semi-quantitatively scored as follows: 0, (no staining); 1 (+, weak immunoreactivity); 2 (++, moderate immunoreactivity); and 3 (+++, strong immunoreactivity). Percent staining was calculated as proportion of cells/structure with immunoreactivity to total cells/structure: 1, (0–10%, focal); 2 (11–50%, regional); and 3 (51–100, diffuse). For each field, intensity and amount scores were calculated using the following formula: \(\sum Pi \cdot (i + 1)\) (Pi percent staining; i intensity of staining). Total score was obtained by adding scores of each field. All counts were performed on ten fields from each slide using the image analysis software (Leica Q Win V3 Plus Image, Germany).
Statistical analysis
Data were analyzed using Statistical Package for Social Sciences (SPSS version 16.0; SPSS Inc., IL, USA). Descriptive statistics are given as mean and standard deviation. Distribution of parametric variables was analyzed using Kolmogrov–Smirnov test. One-way ANOVA and post hoc Bonferroni tests were used to compare pre- and post-treatment total IgE and ovalbumin-specific IgE levels between groups. Student’s t test was used to compare pre- and post-treatment values with normal distribution, while Wilcoxon rank test was used to compare pre- and post-treatment values with skewed distribution within each group. In binary comparisons, independent samples t test was used for data with normal distribution, while Mann–Whitney U test was used for data with skewed distribution. In all analyses, a p value <0.05 was considered to be statistically significant.
Results
Nasal symptom findings
All rats underwent nasal examination before the study and those without serous secretion were included in the study. When nasal symptoms were assessed throughout the study, allergic rhinitis development was exclusively observed in rats in the control group. Rats with a symptom score ≥5 were considered to have allergic rhinitis. Although mild-to-moderate serous secretion, sneezing, and itching were seen in quercetin and steroid groups, there was no experimental group rated as ≥5 points. No symptom was observed in the sham group throughout the study (Fig. 1b).
Total IgE and ovalbumin-specific IgE levels
There was no significant difference in baseline total and OVA-specific IgE levels among the groups (p = 0.509). In intra-group comparisons, there was a significant increase in post-treatment serum total IgE levels when compared to baseline in the control group (p < 0.05), while no significant increase was detected in the sham, quercetin, and steroid groups (p > 0.05) (Table 1). Significant increases were detected in serum OVA-specific IgE levels measured at the end of the study when compared to baseline in all groups other than the sham group (p < 0.05) (Table 1).
There were significant differences in serum total and OVA-specific IgE levels measured at the end of the study among the groups (p < 0.001). It was found that the extent of increase was greater in the control group compared to the remaining groups (Table 1).
Histopathological findings
Hematoxylin–eosin staining findings
In the control group, it was observed that there was increased mucosal inflammation, vascular dilatation, and marked dilatation in ducts of serous glands of the nasal turbinate (Fig. 3a). In quercetin and steroid groups, vascular dilatation, mucosal inflammation, and dilatation in ducts of serous glands were markedly decreased when compared to the control group (Fig. 3b, c). It was seen that the normal architecture of the nasal turbinate was preserved in the sham group (Fig. 3d). The number of inflammatory cells was low in connective tissue and no dilatation was detected in secretory ducts. In addition, the eosinophil count in mucosa of the nasal turbinate was significantly higher in the control group when compared to quercetin and steroid groups (p < 0.001).
Immunohistochemical staining findings
In the control group, strong COX-2 expression was observed in chondroblasts, epithelial cells, and connective tissue cells in cartilage of the nasal turbinate (Fig. 4a). In quercetin and steroid groups, it was observed that COX-2 expression was moderate in both chondroblasts and other cells (Fig. 4b, c), while it was weak in cartilage and epithelial tissue in the sham group (Fig. 4d).
MMP-9 expression in nasal mucosa and connective tissue of the nasal turbinate was weak in all groups.
It was found that VIP expression was moderate-to-strong in nasal mucosa in the control group (Fig. 5a), while it was weak-to-moderate in the remaining groups (Fig. 5b–d).
Discussion
Several allergens, such as ovalbumin, pollen from Cryptomeria japonica (Sugi tree), toluene 2-4-diisocyanate (TDI), Schistosoma mansoni egg antigen (SEA), and Ascaris suum extract antigen (ASA), have been used to develop animal models of allergic rhinitis [14,15,16,17]. For immunization with antigen, ovalbumin has been given with an adjuvant such as aluminum via an intraperitoneal route followed by an intranasal route at different doses and times, and increased inflammatory cells and eosinophils were observed in the nasal mucosa of subjects given ovalbumin in histopathological evaluation [18, 19]. In our study, histopathological findings of allergic rhinitis, such as increased inflammation, eosinophil count, vasodilatation, dilatation of secretory ducts of serous glands, and edema, were detected in the control group receiving ovalbumin alone in histopathological evaluations.
Wen and Avinçsal developed a qualitative scale by scoring nasal symptoms in rats [13, 19]. The authors rated sneezing, itching, and rhinorrhea symptoms on a 0–3 point scale (Fig. 1a). In our study, allergic rhinitis scores were recorded on days 1, 14, 15, 16, 18, 20, 21, and 22 in rats sensitized with ovalbumin. It was seen that allergic rhinitis developed in control rats only. On physical examination, there was no group rated with ≥5 points in total score, although mild-to-moderate rhinorrhea, sneezing, and itching were observed in the quercetin and steroid groups. It was seen that allergic rhinitis symptoms were suppressed in the quercetin and steroid groups.
IgE production is increased as a response to environmental allergens in atopic diseases, such as atopic dermatitis, allergic rhinitis, or bronchial asthma. Serum total IgE and OVA-specific IgE levels are increased in animals in which an experimental model of allergic rhinitis is induced by ovalbumin [20, 21]. In our study, we also measured pre- and post-treatment total and OVA-specific IgE by an ELISA method. It was seen that there was a significant increase in total and OVA-specific IgE levels at the end of the study when compared to baseline in the control group. However, no significant increase was detected in total IgE levels at the end of the study in the steroid and quercetin groups, while a significant increase was detected in OVA-specific IgE levels in all groups other than the sham group. The extent of increase in OVA-specific IgE levels was greater in the control group than in the quercetin and steroid groups. Based on these findings, quercetin was found to be effective in allergic rhinitis in a serological manner with values similar to the steroid group.
In agreement with our study, it was shown that serum total and specific IgE levels were decreased by local steroid use in some studies. Ohrui et al. reported that inhaled corticosteroid therapy at a dose of 800 mcg reduced serum total and allergen-specific IgE levels in patients with atopic asthma [22]. Naclerio et al. found that intranasal steroid use suppressed serum ragweed-specific IgE elevation in patients with allergic rhinitis [23]. Most probable explanation which decreases in IgE levels can be associated with additional effects of corticosteroid. It is known that serum IgE level is decreased when exposure to an allergen is reduced. As a result, any therapeutic intervention decreasing inflammation in the airway can result in decreased serum IgE level by decreasing airway permeability.
Immunohistochemical studies evaluating pathological changes in allergic rhinitis demonstrated accumulation of eosinophils and other inflammatory cells within the lamina propria and the epithelium. It was also shown that there were structural changes, such as hypertrophic glands, goblet cell hyperplasia, vascular dilatation, and hypertrophic chondrocytes in patients with allergic rhinitis [24]. In our study, increased inflammatory cells and eosinophil infiltration, edema, vascular dilatation, and dilated secretory ducts of serous glands were detected in mucosa of nasal turbinates in rats in which an experimental model of allergic rhinitis was induced.
Cyclooxygenase (COX) enzymes (COX-1 and COX-2) account for the generation of eicosanoids from arachidonic acid and they are an important component and marker of an inflammatory response [25]. The matrix metalloproteinase (MMP) family is a member of the extracellular proteinases. The most important function of the MMP family is degradation of the extracellular matrix. It is thought that an uncontrolled increase of MMP activity plays a role in acute and chronic disorders through ECM degradation. Vasoactive intestinal polypeptide (VIP) acts as a neurotransmitter in postganglionic parasympathetic neurons. In postganglionic neurons, VIP regulates glandular secretion and vasomotor activity of nasal mucosa in concert with methionine, histidine, and acetylcholine, while it induces inflammation through its effects on target cells, such as immune, mast, and vascular smooth cells [26]. In our study, we found that COX-2 and VIP expressions were weaker in quercetin and steroid groups compared to controls. However, MMP-9 expression was found to be weak in nasal mucosa epithelium and connective tissue of nasal turbinates in all groups. These findings suggest that quercetin may decrease symptoms of allergic rhinitis by suppressing inflammatory pathways and may provide results comparable to steroids, which are potent anti-inflammatory agents.
Besides avoiding allergens, use of antihistamines, leukotriene antagonists, systemic and local steroids, cromolyn sodium, and decongestants can be insufficient in the treatment of allergic rhinitis [2, 27]. Numerous experimental studies have been performed in attempt to seek for treatment, and novel agents have been studied. For this purpose, many substances, including cyclosporine, cyclophosphamide, botulinum toxin, doxycycline, N-acetylcysteine, Tong Qiao, vitamin C, and CTLA4-Ig, have been used in animal models at different doses and times [13, 18,19,20,21, 28, 29]. To the best of our knowledge, there has been no study on the use of quercetin in experimental models of allergic rhinitis.
Quercetin is a member of the flavonoid family, which has several biological activities, such as antioxidant, anti-proliferative, anti-inflammatory, anti-viral, anti-thrombotic, anti-atherosclerotic, anti-tumoral, and anti-allergic activities [6, 10]. In previous studies, it was shown that quercetin inhibited mast cell activation, inflammatory cytokine production, and histamine release after immunological stimulation [30–32]. In a study using an animal model of asthma, it was shown that quercetin caused a decrease in interleukin-5 level and eosinophil count in bronchoalveolar lavage fluid and pulmonary tissue [32]. In an experimental model of asthma, it was reported that quercetin given at a single dose of 10 mg/kg over 5 days suppressed elevation in eosinophil count in peripheral blood and bronchoalveolar lavage fluid [33]. In an in vitro study by Sakai et al., it was reported that quercetin inhibited eosinophil activation following SCF stimulation. Moreover, it was also suggested that oral quercetin (over 21 days) did not suppress eosinophilia in peripheral blood or IgE production [34].
Allergic rhinitis is characterized by chronic inflammation of nasal mucosa. The chronic inflammation results in epithelial injury, subepithelial fibrosis, epithelial metaplasia, decreased nasal ciliary activity, and cilia loss [35, 36]. In a study by Zhang et al., it was found that quercetin enhanced CFTR-related Cl− transport and ciliary beat frequency in murine nasal septal and human sinonasal epithelial cell cultures [37]. In our study, quercetin might have suppressed allergic rhinitis in the rats by increasing Cl− transport and ciliary beat frequency.
From the present study, we concluded that quercetin was effective in the treatment of allergic rhinitis based on the findings that total and ovalbumin-specific IgE levels were lower and that vascular dilatation, inflammation, dilatation in secretory glands of serous glands, eosinophil infiltration, and COX-2 and VIP expressions were lower in the quercetin group compared to the control group. In our study, quercetin could have suppressed findings of allergic rhinitis by a cumulative activity, including anti-inflammatory effect via inhibition of COX-2 and VIP expressions and anti-allergic effect via suppression of total and specific IgE expressions.
It is clear that our study has some limitations. First, quercetin was exclusively given via systemic route in our study. However, it may be appropriate to give quercetin via intranasal route to assess local effectiveness and to compare local steroid administration. In addition, this may enable to compare therapeutic effectiveness of systemic and local quercetin administrations. Second, quercetin was used at a single dose level (80 mg/kg). Thus, therapeutic effectiveness of quercetin at different doses can be investigated using quercetin in a dose-dependent manner.
Conclusion
This is the first study conducted showing the effectiveness of quercetin for symptoms of allergic rhinitis in an experimental rat model of allergic rhinitis. It was found that serum total IgE and ovalbumin-specific IgE levels were lower and symptoms of allergic rhinitis were suppressed in the quercetin group when compared to the control group. Serological and histopathological results in the quercetin group were similar to those in the steroid group. In conclusion, quercetin was effective in an experimental rat model of allergic rhinitis, similar to steroid therapy. Based on our findings, further experimental and clinical trials are needed on quercetin use in the treatment of allergic rhinitis.
References
De S, Fenton JE, Jones AS (2005) Matrix metalloproteinases and their inhibitors in non-neoplastic otorhinolaryngological disease. J Laryngol Otol 119:436–442
Nagai H, Teremachi H, Tuchiya T (2006) Recent advances in the development of anti-allergic drugs. Allergol Int 55:35–42
Greiner AN, Meltzer EO (2011) Overview of the treatment of allergic rhinitis and nonallergic rhinopathy. Proc Am Thorac Soc 8:121–131
Kayasuga R, Iba Y, Hossen M, Watanabe T, Kamei C (2003) The role of chemical mediators in eosinophil infiltration in allergic rhinitis in mice. Int Immunopharmacol 3:469–473
Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, Van Norren K, Van Leeuwen PA (2001) Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 74:418–425
Kleemann R, Verschuren L, Morrison M, Zadelaar S, van Erk MJ, Wielinga PY, Kooistra T (2011) Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis 218:44–52
Boots AW, Haenen GR, Bast A (2008) Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 585:325–337
Bischoff SC (2008) Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care 11:733–740
Hirpara KV, Aggarwal P, Mukherjee AJ, Joshi N, Burman AC (2009) Quercetin and its derivatives: synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anticancer Agents Med Chem 9:138–161
Murakami A, Ashida H, Terao J (2008) Multitargeted cancer prevention by quercetin. Cancer Lett 269:315–325
Sagit M, Korkmaz F, Gürgen SG, Gundogdu R, Akcadag A, Ozcan I (2015) Quercetine attenuates the gentamicin-induced ototoxicity in a rat model. Int J Pediatr Otorhinolaryngol 79:2109–2114
Guardia T, Rotelli AE, Juarez AO, Pelzer LE (2001) Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. II Farmaco 56:683–687
Avincsal MO, Ozbal S, Ikiz AO, Pekcetin C, Güneri EA (2014) Effects of topical intranasal doxycycline treatment in the rat allergic rhinitis model. Clin Exp Otorhinolaryngol 7:106–111
Wang LF, Xu LJ, Guo FH, Wang LN, Shen XH (2010) Effect of antiallergic herbal agents on chloride channel-3 and immune microenvironment in nasal mucosal epithelia of allergic rhinitis rabbits. Chin Med J 123:1034–1038
Nabe T, Shimizu K, Mizutani N, Saeki Y, Yamamura H, Takenaka H, Kohno S (1997) A new model of experimental allergic rhinitis using Japanese cedar pollen in guinea pigs. Jpn J Pharmacol 75:243–251
Kawase M, He F, Kubota A, Hata JY, Kobayakawa SI, Hiramatsu M (2006) Inhibitory effect of Lactobacillus gasseri TMC0356 and Lactobacillus GG on enhanced vascular permeability of nasal mucosa in experimental allergic rhinitis of rats. Biosci Biotechnol Biochem 70:3025–3030
Ishida M, Amesara R, Ukai K, Sakakura Y (1994) Antigen (DNP-AS)-induced allergic rhinitis model in guinea pigs. Ann Allergy 72:240–244
Guibas GV, Spandou E, Meditskou S, Vyzantiadis TA, Priftis KN, Anogianakis G (2013) N-acetylcysteine exerts therapeutic action in a rat model of allergic rhinitis. Int Forum Allergy Rhinol 7:543–549
Wei WD, Yuan F, Wang JL, Hou YP (2006) Botulinum toxin therapy in the ovalbumin-sensitized rat. Neuroimmunomodulation 14:78–83
Sato J, Asakura K, Murakamı M, Uede T, Kataura A (1999) Suppressive effects of CTLA4-Ig on nasal allergic reactions in presensitized murine model. Life Sci 64:785–795
Xu YY, Liu X, Dai LB, Zhou SH (2012) Effect of tong Qiao drops on the expression of eotaxin, IL-13 in the nasal mucosa of rats with allergic rhinitis. J Chin Med Assoc 75:524–529
Ohrui T, Funayama T, Sekizawa K, Yamaya M, Sasaki H (1999) Effects of inhaled beclomethasone dipropionate on serum IgE levels and clinical symptoms in atopic asthma. Clin Exp Allergy 29:357–361
Naclerio RM, Adkinson NF, Creticos PS, Baroody FM, Hamilton RG, Norman PS (1993) Intranasal steroids inhibit seasonal increases in ragweed-specific immunoglobulin IE antibodies. J Allergy Clin İmmunol 92:717–721
Nakaya M, Dohi M, Okunishi K, Nakagome K, Tanaka R, Imamura M et al (2007) Prolonged allergen challenge in murine nasal allergic rhinitis: nasal airway remodeling and adaptation of nasal airway responsiveness. Laryngoscope 117:881–885
Shaida A, Kenyon G, Devalia J (2001) Matrix metalloproteinases and their inhibitors in the nasal mucosa of patients with perennial allergic rhinitis. J Allergy Clin Immunol 108:791–796
Raap U, Braunstahl GJ (2010) The role of neurotrophins in the pathophysiology of allergic rhinitis. Curr Opin Allergy Clin Immunol 10:8–13
Canonica GW, Tarantini F, Compalati E, Penagos M (2007) Efficacy of desloratadine in the treatment of allergic rhinitis: a meta-analysis of randomized, double-blind, controlled trials. Allergy 62:359–366
Iris K, Joachim H, Guenther W et al (2006) Association of carotenoids, tocopherols and vitamin C in plasma with allergic rhinitis and allergic sensitisation in adults. Public Health Nutr 9:472–479
Ercan I, Cakir BÖ, Başak T et al (2006) Effects of topical application of methotrexate on nasal mucosa in rats: a preclinical assessment study. Otolaryngol Head Neck Surg 134:751–755
Middleton E Jr (1998) Effect of plant flavonoids on immune and inflammatory cell function. Adv Exp Med Biol 439:175–182
Min YD, Choi CH, Bark H et al (2007) Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and p38 MAPK in HMC-1 human mast cell line. Inflamm Res 56:210–215
Dorsch W, Bittinger M, Kaas A et al (1992) Antiasthmatic effects of Galphimia glauca, gallic acid, and related compounds prevent allergen- and platelet-activating factor-induced bronchial obstruction as well as bronchial hyperreactivity in guinea pigs. Int Arch Allergy Immunol 97:1–7
Rogerio AP, Kanashiro A, Fontanari C et al (2007) Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm Res 56:402–408
Sakaı-KM Asano K (2013) Inhibitory action of quercetin on eosinophil activation in vitro. Evid Based Complement Altern Med 2013:127105. doi:10.1155/2013/127105
Ohashi Y, Nakai Y, Kihara S, Ikeoka H, Takano H, Imoto T (1985) Ciliary activity in patients with nasal allergies. Arch Otorhinolaryngol 242:141–147
Skoner DP (2001) Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol 108:2–8
Zhang S, Smith N, Schuster D et al (2011) Quercetin increases CFTR mediated chloride transport and ciliary beat frequency: therapeutic implications for chronic rhinosinusitis. Am J Rhinol Allergy 25:307–312
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no funding, financial relationships, or conflicts of interest to disclose. The authors otherwise have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Human and animal rights’ statement
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. The study was approved by the Local Ethics Committee on Experimental Animal Studies of Erciyes University (14/127)].
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Sagit, M., Polat, H., Gurgen, S.G. et al. Effectiveness of quercetin in an experimental rat model of allergic rhinitis. Eur Arch Otorhinolaryngol 274, 3087–3095 (2017). https://doi.org/10.1007/s00405-017-4602-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-017-4602-z