Abstract
Interleukin (IL)-6 plays important roles in autoimmunity and inflammation and is essential for T helper (Th) 2 and Th17 differentiation. However, whether it is involved in the development and function of dendritic cells (DCs) during allergen-induced airway inflammation and airway hyper-reactivity (AHR) remains undefined. In this study, Dermatophagoides pteronyssinus (Der p)-induced airway inflammation and AHR were studied in IL-6 knockout (KO) mice. Der p-loaded bone marrow-derived DCs (BMDCs) from IL-6 KO mice were used to assaying their ability to induce airway inflammation in naïve wild-type mice. Our results showed that IL-6 KO mice showed reduced AHR, significant decreases in inflammatory cell recruitment and Th2 and Th17 cytokine production in the airways, and lowered Der p-specific immunoglobulin G1 after Der p exposure. Further exploration of BMDCs from IL-6 KO mice revealed decreased activity of phagocytosis and reduced expression of MHC class II and CD86 after Der p stimulation. Adoptive transfer of Der p-loaded BMDCs from IL-6 KO mice also showed a functional defect in their inability to induce Th2 and Th17 immune responses and trigger airway inflammation and AHR in recipient mice. Finally, in allergic asthmatics, DCs that differentiated from monocytes treated with anti-IL-6 receptor antibody (tocilizumab) had poor capacity for eliciting Th2 polarization as compared to DCs generated from monocytes without antibody treatment. In conclusion, IL-6 signaling in DCs is essential for their uptake of allergens, maturation, and initiation of Th2/Th17-mediated airway inflammation and AHR in asthma, thus providing a new potential target for treating allergic asthma.
Key messages
-
IL-6 signaling is important for DCs to take up allergens and to initiate Th2/Th17-mediated airway inflammation.
-
DCs from allergic asthmatics treated with anti-IL-6 receptor antibody had poor capacity for eliciting Th2 polarization.
-
Anti-IL-6 treatment may provide a new potential target for treating allergic asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allergic asthma is characterized by eosinophil infiltration in the inflammatory airway induced by interleukin (IL)-4-, IL-5-, and IL-13-producing T helper (Th)2 lymphocytes, which lead to immunoglobulin (Ig)E production, mucus overproduction, and airway hyper-reactivity (AHR) [1–3]. Accumulating evidence indicates that Th17 cells and the main Th17-derived cytokine, IL-17A, which mediate neutrophil-dependent airway inflammation and AHR, are also associated with steroid-resistant asthma [4, 5]. In addition, Th17 cells are capable of enhancing airway eosinophilia by upregulating eotaxin expression [6, 7].
It has been reported that IL-6 suppresses transforming growth factor (TGF)-β-mediated forkhead box protein (Foxp) 3 expression and synergizes with TGF-β to drive naïve CD4+ T cells toward Th17 development [8, 9]. IL-6 secreted from dendritic cells (DCs) also promotes Th2 differentiation by inducing nuclear factor of activated T(NFAT) cells expression and inhibits Th1 polarization by upregulating suppressor of cytokine signaling (SOCS)1 expression [10, 11]. Previous studies showed increased IL-6 levels in bronchial epithelial cells, induced sputum, bronchoalveolar lavage (BAL), and peripheral blood of asthmatic patients [12–15]. Increased IL-6 levels have also been described in mouse models of allergic asthma [16, 17]; however, the role of IL-6 in aeroallergen-induced airway inflammation and AHR remains unclear.
Previous findings suggest that overexpression of human IL-6 in murine airway epithelial cells causes lymphocyte, rather than eosinophil, infiltration into surrounding airways, airway wall thickening, and subepithelial airway fibrosis, but these mice have similar basal airway resistance and are less responsive to methacholine (MCh) compared to wild-type (WT) mice [18, 19]. Although overexpression of IL-6 induces peribronchial inflammatory cell accumulation, IL-6 transgenic mice do not show representative features of allergic asthma, such as eosinophil-mediated airway inflammation and AHR. Therefore, the role of IL-6 in aeroallergen-induced animal models of allergic asthma remains to be defined.
In this study, we first address whether IL-6 plays a role in the pathogenesis of allergic asthma. We hypothesize that IL-6 influences the development and function of DCs and that DCs from IL-6 knockout (KO) mice exhibit defects in inducing airway inflammation and AHR. Our results show that IL-6 KO mice exhibit diminished airway inflammation and AHR in Dermatophagoides pteronyssinus (Der p)-induced allergic asthma. Compared to WT bone marrow-derived DCs (BMDCs), immature BMDCs from IL-6 KO mice display reduced phagocytic activity and markedly lower expression of major histocompatibility complex II (MHC) class II and CD86 after Der p stimulation. Intratracheal transfer of in vitro Der p-loaded BMDCs from IL-6 KO mice into naïve WT mice results in impaired Th2 and Th17 responses and remarkably reduced airway inflammation and AHR after repeated Der p challenge. In patients with allergic asthma, Der p-loaded DCs that differentiated from monocytes treated with anti-IL-6 receptor antibodies have defective Th2 priming capacity. Our findings show that IL-6 signaling enables DCs to capture allergens, mature, and initiate allergen-specific Th2 and/or Th17 effector cell-mediated pulmonary inflammation and AHR and thus may provide a new target for allergic asthma treatment.
Materials and methods
Mice
Six- to 8-week-old female C57BL/6J mice and C57BL/6J-derived IL-6 KO (B6.129S2-Il6 tm1 kopf /J) mice (The Jackson Laboratory, Bar Harbor, ME) were used for our study. All mice were maintained in specific pathogen-free conditions. All experimental protocols were approved by the Institutional Animal Care and Use Committee at National Cheng Kung University.
Induction of allergic airway inflammation
The concentration of bacterial endotoxins in the Der p preparation (Allergon AB, Angelholm, Sweden) was <0.03 EU/ml (Pyrotell Limulus amebocyte lysate test; Associates of Cape Cod, Inc., Falmouth, MA). WT and IL-6 KO mice were intranasally administered 25 μg of Der p or saline daily for 10 days [20]. AHR was measured 48 h after the last saline or Der p exposure. Sera, bronchoalveolar lavage (BAL), and lung tissues were obtained from mice sacrificed 72 h post-exposure. The measurement of AHR, BAL analysis, and Ig quantification is described in the Supporting Information.
Adoptive transfer of Der p-loaded BMDCs
BMDCs were generated using a previously described procedure [21]. On day 6, the CD11c+ fraction of BMDCs was isolated with magnetic beads specific for mouse CD11c (Miltenyi Biotech, Cologne, Germany); the purity of isolated CD11c+ BMDCs as determined by flow cytometry was over 95 %. Der p-loaded isolated CD11c+ BMDCs (1 × 106) from WT or IL-6 KO mice were adoptively transferred intratracheally into naïve WT mice. Ten days later, recipient mice were intranasally challenged with 10 μg of Der p daily for 6 days. AHR was measured 24 h post-challenge, and recipient mice were sacrificed after 48 h.
Generation of human DCs and naïve CD4+ T cell priming
The definition of allergic asthma was based on Genetic Information Nondiscrimination Act guidelines and described in the Supporting Information. Details regarding DC stimulation and T cell cocultures in mice and humans are described in the online supplement.
Statistics
In mouse and human experiments, data are expressed as mean ± standard error of the mean (SEM; n = 6 per group). In the AHR measurement experiment, differences between groups were determined via two-way analysis of variance with a Bonferroni post-test. For the remaining mouse experiments, differences between groups were analyzed using unpaired, two-tailed Student’s t tests. For human studies, differences were tested using paired t tests. Statistical differences were analyzed using GraphPad Prism 5.00 software (GraphPad Software, Inc., La Jolla, CA). P values <0.05 were considered statistically significant.
Results
IL-6 is involved in Der p-induced AHR and lung inflammation
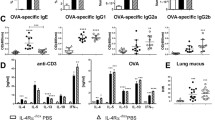
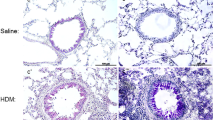
We chose Der p, one of the most common allergens, for the induction of allergic airway inflammation in this study [22, 23]. Mice were administered Der p (25 μg) intranasally daily for 10 days, and the control group received saline. Invasive assessments of respiratory mechanics were performed 48 h after the last Der p exposure. Der p-exposed WT mice developed airway resistance against methacholine (MCh) in a dose-dependent manner, as compared to saline-challenged WT mice. In contrast, Der p-exposed IL-6 KO mice did not show any increase of airway resistance against MCh (Fig. 1a). Total infiltrating cells, eosinophils, neutrophils, and lymphocytes in the BAL of Der p-exposed IL-6 KO mice substantially decreased compared with those in WT mice (Fig. 1b and Fig. S1). Histological examination of hematoxylin and eosin (H&E)-stained lung sections also revealed less inflammatory cell accumulation in the surrounding perivascular and peribronchial regions in Der p-exposed IL-6 KO mice (Fig. 1c). Moreover, the levels of tumor necrosis factor (TNF)-α, IL-4, CCL11, CCL17, and IL-17 in the BAL (Fig. S1) and Der p-specific IgG1 in the serum (Fig. 1d) markedly decreased in Der p-exposed IL-6 KO mice compared with those in WT mice. There were no significant differences in interferon (IFN)-γ (Fig. S1) and IgE levels (Fig. 1d) between Der p-exposed WT and IL-6 KO mice. These results indicate that IL-6 is essential for Der p-induced airway inflammation and AHR.
IL-6 knockout (KO) mice demonstrate less airway hyper-reactivity (AHR) and airway inflammation upon exposure to Dermatophagoides pteronyssinus (Der p). Naïve wild-type (WT) and IL-6 KO mice were intranasally exposed to Der p or saline for 10 consecutive days. a Total airway resistance (Rrs) to increasing dosages of aerosolized methacholine (MCh). b Numbers of total cells, eosinophils, lymphocytes, and neutrophils in the bronchoalveolar lavage fluid (BALF). c Representative hematoxylin and eosin (H&E)-stained lung sections from indicated groups of mice. Magnification = ×200. Images are representative of 6 mice per group from 1 experiment. d Total serum immunoglobulin (Ig)E and Der p-specific IgG1 levels. Data are expressed as mean ± standard error of the mean (SEM; n = 6 mice per group). *P < 0.05, **P < 0.01. Similar results were obtained in 2 additional experiments
The influence of IL-6 on DC development and function
Inflammatory CD11c+MHC class II+ DCs play a key role in the initiation of allergen-induced inflammation [24]. To determine whether endogenous IL-6 affects the development and function of CD11c+ DCs, BMDCs were generated from WT or IL-6 KO mice. The percentage of immature CD11c+ BMDCs from IL-6 KO mice was significantly lower than that from WT mice (Fig. 2a). Moreover, the proportion of CD11c+ BMDCs from WT mice, but not IL-6 KO mice, increased after stimulation with lipopolysaccharide (LPS; 100 ng/ml), TNF-α (1 μg/ml), or increasing doses of Der p (1, 10, and 20 μg/ml). As shown in Fig. 2b, purified CD11c+ BMDCs from WT mice (52.9 %) had higher phagocytic activity than those from IL-6 KO mice (34.0 %), as measured from the uptake of fluorescent latex beads. CD11c+ BMDCs from WT, but not IL-6 KO mice, exhibited upregulated MHC class II and CD86 expression following LPS, TNF-α, and Der p stimulation (Fig. 2c). WT CD11c+ BMDCs secreted significant levels of IL-6 in response to LPS, TNF-α, and Der p stimulation. IL-6 levels in the supernatants collected from IL-6 KO CD11c+ BMDCs were below the detection limit even after stimulation (Fig. 2d). However, no significant differences in TNF-α or IL-10 production were observed between WT and IL-6 KO CD11c+ BMDCs stimulated with LPS, TNF-α, or increasing doses of Der p (Fig. S2). IL-12p70 was not detected in LPS, TNF-α, or Der p-stimulated CD11c+ BMDCs obtained from WT and IL-6 KO mice (data not shown). In the coculture experiment with naïve CD4+ T cells, Der p-loaded WT CD11c+ BMDCs induced Th2 and Th17 differentiation with an increase in IL-4, IL-5, IL-13, and IL-17 cytokine production in the culture supernatants (Fig. S3). In contrast, there was increased IFN-γ production (for Th1 differentiation) in the supernatants when naïve CD4+ T cells were cocultured with IL-6 KO CD11c+ BMDCs primed with Der p (Fig. S3). These data suggest that IL-6 promotes CD11c+ DC generation and confers DCs to have the better capacity for activating naïve CD4+ T cells.
Development and function of bone marrow-derived dendritic cells (BMDCs) are modulated by IL-6. a Percentages of CD11c+ BMDCs. Bone marrow cells from WT or IL-6 KO mice were cultured with recombinant murine granulocyte–macrophage colony-stimulating factor (GM-CSF) for 6 days. Non-adherent cells were harvested, counted, incubated in medium alone (C) or stimulated with lipopolysaccharide (LPS), tumor necrosis factor (TNF)-α, or Der p for 24 h, and stained for CD11c. Data are expressed as mean ± SEM (n = 6 mice per group). *P < 0.05. b Phagocytic activity of CD11c+ BMDCs. Isolated CD11c+ BMDCs were incubated with fluorescent carboxylate-modified polystyrene latex beads, washed, and analyzed by flow cytometry. Representative histogram plots of WT CD11c+ BMDCs (left panel) and IL-6 KO CD11c+ BMDCs (right panel) are representative of 6 mice per group from 1 experiment. The black line indicates that CD11c+ BMDCs from WT and IL-6 KO mice were not incubated with fluorescent beads. c Major histocompatibility complex (MHC) class II and CD86 expression and d IL-6 release in the culture supernatants of purified CD11c+ BMDCs from WT or IL-6 KO mice were incubated in medium alone or stimulated with LPS, TNF-α, or Der p for 24 h. Data are expressed as mean ± SEM (n = 30 mice per group) pooled from 5 experiments. *P < 0.05. Similar results were obtained in 2 additional experiments. BD below detection
IL-6 is required for DC-induced airway inflammation and AHR
To further investigate the role of IL-6 in DC-induced allergic airway inflammation, Der p-loaded CD11c+ BMDCs from WT or IL-6 KO mice were adoptively transferred intratracheally into naïve WT mice. Ten days after adoptive transfer, allergic airway inflammation was triggered by repeated intranasal instillations of Der p (Fig. 3a). Naïve WT mice receiving saline-loaded WT CD11c+ BMDCs exhibited no elevation in Der p-specific IgE and IgG1 after Der p challenge (data not shown). The assessment of AHR (Fig. 3b), total and differential BAL cell counts (Fig. 3c), lung histological analysis, and periodic acid-Schiff staining (Fig. 3d) showed that CD11c+ BMDCs from IL-6 KO mice could not induce allergic airway inflammation and AHR in naïve WT mice compared with Der p-loaded WT CD11c+ BMDCs. In contrast to mice that received WT CD11c+ BMDCs, those that received IL-6 KO CD11c+ BMDCs had no increase in serum Der p-specific IgE and IgG1 levels despite intranasal instillation of Der p (Fig. 3e). In another experiment, BMDCs from wild-type mice have been adoptively transferred into IL-6 KO mice which were able to induced AHR in recipient IL-6 KO mice, but to a lesser extent than wild-type BMDCs into wild-type mice (Fig S4). In contrast, wild-type BMDCs were ablated for IL-6 gene by IL-6 siRNA treatment failed to induce AHR in recipient IL-6 KO mice (Fig. S4). Decreased levels of CCL17 and IL-17 were also found in the BAL of mice that received IL-6 KO CD11c+ BMDCs (Fig. S4). However, the IFN-γ and IL-10 concentrations in the BAL were markedly higher in mice that received IL-6 KO CD11c+ BMDCs than those in mice that received WT CD11c+ BMDCs (Fig. S4).
IL-6 is a key factor in the initiation of DC-induced airway inflammation and AHR. a Der p-loaded CD11c+ BMDCs from WT or IL-6 KO mice were adoptively transferred intratracheally into naïve WT mice. Ten days later, recipient mice were intranasally challenged with Der p daily for 6 days. b Rrs to increasing dosages of aerosolized MCh. c Numbers of total cells (Total), macrophages (Mac), lymphocytes (Lymph), neutrophils (Neu), and eosinophils (Eos) in the BAL. d Representative examination of airway inflammation and airway mucus production (dark purple) on lung sections stained with H&E (upper panels) and periodic acid-Schiff (PAS) (lower panels). Magnification = ×200. Images are representative of 6 mice per group from 1 experiment. e Serum Der p-specific IgE and IgG1 levels. Data are expressed as mean ± SEM (n = 6 mice per group). *P < 0.05, **P < 0.01. Similar results were obtained in 2 additional experiments
Immunohistochemical staining revealed that CD4+, GATA binding protein (GATA)-3+, and retinoic acid-related orphan receptor (ROR)γt+ cells were predominantly distributed in lung sections of mice that received WT, but not IL-6 KO CD11c+, BMDCs (Fig. 4a). When the cells from lung-draining lymph nodes (LLNs) were stimulated with Der p in vitro for 72 h, significant increases in the levels of IL-4, IL-5, IL-13, and IL-17 were detected in mice that received WT CD11c+ BMDCs (Fig. 4b). Elevated IFN-γ production was observed in mice that received IL-6 KO CD11c+ BMDCs (Fig. 4b). These data show that IL-6 is essential for DC-induced airway inflammation and AHR. Moreover, IL-6 KO CD11c+ BMDCs fail to trigger pathogenic Th2 and Th17 responses to Der p allergen in vivo.
IL-6-competent DCs are necessary to induce pathogenic Th2 and Th17 responses. Mice were sensitized and challenged as described in Fig. 3. a Representative photographs of immunohistochemistry for CD4 (red), GATA-binding protein (GATA)-3 (brown), and retinoic acid-related orphan receptor (ROR)γt (brown) in lung sections. Magnification = ×400. Arrows indicate cells positive for CD4 (upper panel), GATA-3 (middle panel), and RORγt (lower panel). Images are representative of 6 mice per group from 1 experiment. b Interferon (IFN)-γ, IL-4, IL-5, IL-13, and IL-17 levels in the culture supernatants collected from single cells of lung-draining lymph nodes incubated in medium alone or re-stimulated with Der p in vitro. Data are expressed as mean ± SEM (n = 30 mice per group) pooled from 5 experiments. *P < 0.05, **P < 0.01. Similar results were obtained in 2 additional experiments
The effect of IL-6 signaling blockade on human monocyte-derived DC function
Because our data in mice described above suggested that endogenous IL-6 regulates the development and function of DCs, we further investigated whether the inhibition of IL-6 signaling also influences human DC development and DC-induced naïve CD4+ T cell polarization. Tocilizumab, a humanized monoclonal antibody that binds to IL-6 receptor-α to neutralize IL-6, is Food and Drug Administration-approved for the treatment of rheumatoid arthritis [25]. Peripheral blood monocytes, collected from Der p-sensitive, allergic asthmatics and non-atopic controls, were differentiated into DCs by addition of recombinant human (rh) granulocyte–macrophage colony-stimulating factor (GM-CSF) and rhIL-4 in culture. During this differentiation phase, monocytes were also treated in the absence (medium-DCs) or presence of tocilizumab (tocilizumab-DCs). We found that the expression of human histocompatibility leukocyte antigen (HLA)-DR and CD86 was reduced in Der p-loaded tocilizumab-DCs compared with that in Der p-loaded medium-DCs in allergic asthmatics (Fig. 5a). In non-atopic controls and allergic asthmatics, decreased HLA-DR expression was also observed in immature tocilizumab-DCs as compared to that in immature medium DCs (Fig. 5a). Moreover, in allergic asthmatics, IL-5 levels were decreased in the supernatants of naïve CD4+ T cells cocultured with Der p-loaded tocilizumab-DCs relative to Der p-loaded medium-DCs (Fig. 5b). These data further show that Der p-loaded DCs that differentiated from monocytes with IL-6 receptor-α blockade have lower HLA-DR and CD86 expression and poor capacity for eliciting Th2 development.
Der p-loaded DCs differentiated from monocytes with IL-6 receptor-α blockade are defective in inducing Th2 responses. To determine the effect of IL-6 signaling on DC development and function, in addition to incubation with recombinant human (rh) GM-CSF and rhIL-4, some monocyte cultures were supplemented with tocilizumab to neutralize IL-6. DCs differentiated from monocytes treated with tocilizumab or controls were stimulated with Der p for 48 h. a Expression of human histocompatibility leukocyte antigen (HLA)-DR and CD86 on immature and Der p-loaded monocyte-derived DCs was assayed by flow cytometry. b IFN-γ, IL-5, and IL-13 levels in the supernatants collected from naïve CD4+ T cells cocultured with immature or Der p-loaded DCs. Data are expressed as mean ± SEM (n = 6 individuals per group). *P < 0.05, # P < 0.05, † P < 0.05
Discussion
In the present study, we found that IL-6 was necessary for the development of Der p-induced airway inflammation and AHR. Former observations suggested that IL-6 is not related to the development or severity of AHR [18, 19, 26]. However, our data imply that the development or exacerbation of AHR is due to IL-6-triggered Th2/Th17 expansion in Der p-induced airway inflammation, which is in agreement with previous studies showing that IL-13 or IL-17 can promote AHR [5, 27]. A previous study showed that IL-6 was not required for airway eosinophilia because IL-6 deficiency did not impair the production of CCL11, an important eosinophil chemoattractant, in response to inhaled extracts of Aspergillus fumigatus [28]. However, we found that the levels of IL-5 (data not shown), IL-17, and CCL11 significantly decreased in Der p-exposed IL-6 KO mice accompanied by markedly reduced airway eosinophilia. These results are reasonable, as IL-6 can directly upregulate CCL11 expression [29] or indirectly by increasing IL-17 expression [6]. Moreover, IL-6-deficient mice display enhanced Th2 responses and eosinophilia in the chronic mouse model of ovalbumin (OVA)-induced allergic asthma [30]. Therefore, the different roles of IL-6 in mouse models of allergic asthma may depend on the duration of allergen exposure.
DCs play an important role in the initiation of adaptive immune responses and induction of peripheral tolerance, including T cell anergy and/or regulatory T cell (Treg) induction. The factors that program DCs toward inflammatory or tolerogenic phenotypes remain inconclusive. We found that CD11c+ BMDCs from IL-6 KO mice were functionally defective because their reduced phagocytic activity and expression of MHC class II and CD86 were not upregulated after antigen stimulation. CD11c+ BMDCs tended to exhibit an immature or tolerogenic phenotype when IL-6 signaling was deficient in the bone marrow. Moreover, we found that IL-6 KO mice generated fewer CD11c+ BMDCs than WT mice. Even following stimulation with LPS, TNF-α, and Der p, the percentages of CD11c+ BMDCs from IL-6 KO mice did not increase as compared to those from WT mice, implying that IL-6 can promote DC generation. In a previous study, IL-6 levels were also positively correlated with DC yield in CD34+ progenitor cell culture [31]. Our results appear to contrast the report of Bleier et al. [32], which shows that IL-6 KO BMs generate more CD11c+ BMDCs than WT BMs. This discrepancy may be due to the timing of harvest and culture of BMDCs from IL-6 KO mice, which requires that need further confirmation in a future study.
Our in vitro BMDC-naïve CD4+ T cell coculture experiments revealed that Der p-loaded CD11c+ BMDCs from IL-6 KO mice induced fewer Th2 and Th17 cells and more Th1 cells than did CD11c+ BMDCs from WT mice. This finding is supported by the former observation that IL-6 KO BMDCs induce less allogeneic T cell proliferation as compared to WT BMDCs [32]. The impaired capability of IL-6 KO BMDCs to stimulate T cell proliferation is partially due to their inability to produce IL-6 [32]. In addition, we found that after repeated Der p challenge, lower GATA-3 and RORγt expression was observed in the lungs of mice that received IL-6 KO CD11c+ BMDCs. Higher levels of IFN-γ and IL-10 and lower Th2 cytokine expression were observed in the BAL of mice that received IL-6 KO CD11c+ BMDCs. Our data imply that CD11c+ DCs, generated in a microenvironment lacking endogenous IL-6, convert pathogenic Th2 and Th17 responses to harmless Th1 responses or induce tolerance in response to allergen sensitization and challenge.
However, DCs from BMs or lungs may exhibit different functional characteristics, and a different in vivo role of IL-6 in the differentiation of DCs has been reported [33, 34]. However, our previous study showed that increased IL-6 produced by Der p-loaded monocyte-derived DCs from allergic asthmatics than normal controls is positively related to enhanced DC-induced Th2 polarization [35]. Moreover, in this study, we identified that in addition to reduced HLA-DR and CD86 expression, DCs that differentiated from monocytes treated with tocilizumab failed to effectively polarize naïve CD4+ T cells toward a Th2 phenotype in allergic asthmatics, emphasizing the importance of IL-6 signaling in DC differentiation and function.
Our results clearly show that IL-6 signaling confers DCs to capture allergens, mature, and initiate allergen-specific Th2 and/or Th17 effector cell-mediated pulmonary inflammation and AHR, demonstrating that IL-6 plays an essential role in the development of allergic asthma. Blockade of the IL-6 signaling pathway may represent a novel therapeutic strategy for the prevention or control of allergic asthma.
References
Gould HJ, Sutton BJ (2008) IgE in allergy and asthma today. Nat Rev Immunol 8:205–217
Kay AB (2001) Allergy and allergic diseases. First of two parts. N Engl J Med 344:30–37
Wills-Karp M (2004) Interleukin-13 in asthma pathogenesis. Immunol Rev 202:175–190
McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A et al (2008) TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol 181:4089–4097
Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN (2009) Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med 180:720–730
Rahman MS, Yamasaki A, Yang J, Shan L, Halayko AJ, Gounni AS (2006) IL-17A induces eotaxin-1/CC chemokine ligand 11 expression in human airway smooth muscle cells: role of MAPK (Erk1/2, JNK, and p38) pathways. J Immunol 177:4064–4071
Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y et al (2008) IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med 178:1023–1032
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238
Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B (2006) TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24:179–189
Diehl S, Anguita J, Hoffmeyer A, Zapton T, Ihle JN, Fikrig E, Rincón M (2000) Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity 13:805–815
Diehl S, Chow CW, Weiss L, Palmetshofer A, Twardzik T, Rounds L, Serfling E, Davis RJ, Anguita J, Rincón M (2002) Induction of NFATc2 expression by interleukin 6 promotes T helper type 2 differentiation. J Exp Med 196:39–49
Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI (1992) Cytokines in symptomatic asthma airways. J Allergy Clin Immunol 89:958–967
Konno S, Gonokami Y, Kurokawa M, Kawazu K, Asano K, Okamoto K, Adachi M (1996) Cytokine concentrations in sputum of asthmatic patients. Int Arch Allergy Immunol 109:73–78
Marini M, Vittori E, Hollemborg J, Mattoli S (1992) Expression of the potent inflammatory cytokines, granulocyte-macrophage-colony-stimulating factor and interleukin-6 and interleukin-8, in bronchial epithelial cells of patients with asthma. J Allergy Clin Immunol 89:1001–1009
Yokoyama A, Kohno N, Fujino S, Hamada H, Inoue Y, Fujioka S (1995) Circulating interleukin-6 levels in patients with bronchial asthma. Am J Respir Crit Care Med 151:1354–1358
Chen CL, Wang SD, Zeng ZY, Lin KJ, Kao ST, Tani T, Yu CK, Wang JY (2006) Serine protease inhibitors nafamostat mesilate and gabexate mesilate attenuate allergen-induced airway inflammation and eosinophilia in a murine model of asthma. J Allergy Clin Immunol 118:105–112
Kushwah R, Oliver JR, Wu J, Chang Z, Hu J (2011) Elf3 regulates allergic airway inflammation by controlling dendritic cell-driven T cell differentiation. J Immunol 187:4639–4653
DiCosmo BF, Geba GP, Picarella D, Elias JA, Rankin JA, Stripp BR, Whitsett JA, Flavell RA (1994) Airway epithelial cell expression of interleukin-6 in transgenic mice. Uncoupling of airway inflammation and bronchial hyperreactivity. J Clin Invest 94:2028–2035
Kuhn C 3rd, Homer RJ, Zhu Z, Ward N, Flavell RA, Geba GP, Elias JA (2000) Airway hyperresponsiveness and airway obstruction in transgenic mice. Morphologic correlates in mice overexpressing interleukin (IL)-11 and IL-6 in the lung. Am J Respir Cell Mol Biol 22:289–295
Cates EC, Fattouh R, Wattie J, Inman MD, Goncharova S, Coyle AJ, Gutierrez-Ramos JC, Jordana M (2004) Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol 173:6384–6392
Krishnamoorthy N, Oriss TB, Paglia M, Fei M, Yarlagadda M, Vanhaesebroeck B (2008) Activation of c-Kit in dendritic cells regulates T helper cell differentiation and allergic asthma. Nat Med 14:565–573
Sporik R, Chapman MD, Platts-Mills TA (1992) House dust mite exposure as a cause of asthma. Clin Exp Allergy 22:897–906
Thomas WR, Hales BJ, Smith WA (2010) House dust mite allergens in asthma and allergy. Trends Mol Med 16:321–328
Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN (2010) Inflammatory dendritic cells—not basophils—are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med 207:2097–2111
Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, Woodworth T, Gomez-Reino JJ (2008) Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 58:2968–2980
Kurashima K, Tamura J, Fujimura M, Qiu Z, Nakao S, Mukaida N (2005) Reduced serum antibody production and acute airway inflammation in interleukin 6-deficient mice challenged with ovalbumin. Allergol Int 54:331–338
Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ (2002) Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 8:885–889
Neveu WA, Allard JB, Dienz O, Wargo MJ, Ciliberto G, Whittaker LA, Rincon M (2009) IL-6 is required for airway mucus production induced by inhaled fungal allergens. J Immunol 183:1732–1738
Langdon C, Kerr C, Tong L, Richards CD (2003) Oncostatin M regulates eotaxin expression in fibroblasts and eosinophilic inflammation in C57BL/6 mice. J Immunol 170:548–555
Qiu Z, Fujimura M, Kurashima K, Nakao S, Mukaida N (2004) Enhanced airway inflammation and decreased subepithelial fibrosis in interleukin 6-deficient mice following chronic exposure to aerosolized antigen. Clin Exp Allergy 34:1321–1328
Santiago-Schwarz F, Tucci J, Carsons SE (1996) Endogenously produced interleukin 6 is an accessory cytokine for dendritic cell hematopoiesis. Stem Cells 14:225–231
Bleier JI, Pillarisetty VG, Shah AB, DeMatteo RP (2004) Increased and long-term generation of dendritic cells with reduced function from IL-6-deficient bone marrow. J Immunol 172:7408–7416
Lewkowich IP, Lajoie S, Clark JR, Herman NS, Sproles AA, Wills-Karp M (2008) Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS One 3, e3879
Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, Kamimura D, Ueda N, Iwakura Y, Ishihara K et al (2004) IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol 173:3844–3854
Huang HJ, Lin YL, Liu CF, Kao HF, Wang JY (2011) Mite allergen decreases DC-SIGN expression and modulates human dendritic cell differentiation and function in allergic asthma. Mucosal Immunol 4:519–527
Acknowledgments
This work was supported by the Ministry of Science and Technology (MOST), Taiwan, under grant number NSC 96-2314-B-006-027-MY3.
Author contributions
YLL designed the research, performed the studies, analyzed the data, interpreted the results, and wrote the manuscript. SHC designed the research and revised the manuscript. JYW designed the research, interpreted the results, and revised the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 365 kb)
Rights and permissions
About this article
Cite this article
Lin, YL., Chen, SH. & Wang, JY. Critical role of IL-6 in dendritic cell-induced allergic inflammation of asthma. J Mol Med 94, 51–59 (2016). https://doi.org/10.1007/s00109-015-1325-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-015-1325-8