Abstract
Superficial sediment samples of the Santos Estuary (SE Brazil) were analyzed for benthic foraminifera (dead fauna), aliphatic hydrocarbons (AHs), polycyclic aromatic hydrocarbons (PAHs), total organic carbon, total nitrogen, and trace metals. The foraminiferal fauna results were related to estuarine hydrodynamics and pollution parameters. The residual circulation of the estuary drives the transport of particles into the Santos channel. Thereby, tidal currents promote an advective transport of the species, including Pararotalia cananeiaensis, Discorbis sp. and Pseudononion japonicum from the Santos Bay to the upper estuary. Additionally, the dredging of the Santos channel leads to the suspension of sediments, favoring the post mortem transport. Enrichment factor and pollution load index for trace metals evidenced a contamination (mainly by Zn) in the Piaçaguera River, and AHs and PAHs analyses revealed a widespread presence of petroleum-related residues and petroleum combustion products in the region of the upper estuary, with values of unresolved complex mixtures ranging from 95.2 to 402.3 µg g−1 and total PAHs concentrations from 1176 to 32,153.3 ng g−1. Although, the data obtained are from dead fauna, the impact of anthropogenic activities is also pointed by the benthic foraminifera, revealing that the pollutants impact are acting for some years in the upper estuary. The ecological indices show lowest values of richness and density, and low values of diversity for benthic foraminifera mainly in the upper estuary. Moreover, high values of dominance were observed due to the high abundance of the opportunistic species Ammonia sp1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Foraminifera are among the most abundant unicellular meiofaunal organisms found on the sediment surface in modern oceans (Murray 2007; Sen Gupta 1999), being present from coastal to deep-sea environments. Benthic foraminifera have been frequently used as bioindicators of environmental conditions (Alve 1991, 1995; Yanko et al. 1994, 1999; Martin 2000; Samir and El-Din 2001; Armynot du Châtelet and Debenay 2010; Frontalini and Coccioni 2011; Martins et al. 2013) because they have a short reproductive cycle and, therefore, their response to environmental changes are rapid (Kramer and Botterweg 1991), being able to provide good information about the environmental quality status of their place of life (Schönfeld et al. 2012).
Environmental assessment and monitoring studies usually analyze living specimens, as they reflect the environmental conditions on the time of their sampling (Schönfeld et al. 2012). However, in cases where the first centimeters of sediment (0–2 cm) are sampled, this situation possibly express the average of the environmental changes that occurred over a few years, and represent the total species that colonized the site during all or part of this period (Scoot and Medioli 1980; Hayward 1992; Leorri et al. 2008). Regarding the dead foraminifera fauna (i.e., empty tests) analysis, studies have shown that this application may give important information about the hydrodynamics of the environment (Debenay et al. 2001; Duleba and Debenay 2003), since the movement of water bodies determines the distribution of living assemblages, and the post mortem transport or accumulation may occur in areas characterized by strong water movement and exposure to different conditions during tidal cycles or in calm environments (Coulbourn and Resig 1975; Murray 1982; Debenay et al. 2001; Duleba and Debenay 2003). Dead fauna can be applied in conjunction with other proxies and knowledge about the sediment accumulation rate in environmental assessment studies.
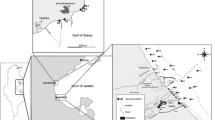
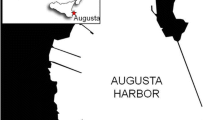
This study focuses on the Santos Estuarine System, located in the Santos Metropolitan Region, State of São Paulo, southeastern Brazil (Fig. 1). It contains the largest port in Latin America and the largest industrial complex in the country, with approximate 1.100 industries, including fertilizer industries, steel industries, refineries, chemical and petrochemical plants (CBH-BS 2011), and a population of around 1.2 million inhabitants (IBGE 2017), being one of the most economically important areas of Brazil. It has been urbanized and industrialized in an environmentally unfriendly way since the 1950s, and its industrial activity made the Santos Estuary a large recipient of toxic wastes and contaminated liquid effluents (CETESB 1978, 2001; Tommasi 1979). During the early 1980s, the region became known as one of the most polluted in the world, due to high levels of organic contaminants in the air and water (Blacksmith Institute 2006). Although nowadays there is a better control in pollutants emission, several contaminants from the urban, industrial and port activities, such as trace metals and petroleum hydrocarbons, may still be introduced into the Estuary.
Trace metals can occur in the environment from natural weathering and sedimentation processes (Hernández-Crespo and Marín 2015) and from anthropic activities, such as industrial activities, dredging operations, domestic sewage and port activities (Fu and Wang 2011; Al Rashdi et al. 2015; Vallejo-Toro et al. 2016). Due to its high mobility, affinity for organic matter and capacity for biomagnification, some trace metals can be harmful to the biota, the entire ecosystem and even to human health (Bargagli et al. 1991; Kim et al. 2016).
Petroleum and petroleum by-product contamination in marine environments can be assessed by the concentrations of polycyclic aromatic hydrocarbons (PAHs) and aliphatic hydrocarbons (AHs), such as n-alkanes, and unresolved complex mixtures (UCM) in sediments (e.g., Silva and Bícego 2010). PAHs can originate from the direct introduction of petroleum and its products on the ambient or from the incomplete combustion of fossil fuels (Lima et al. 2005). Among hydrocarbons, PAHs present the highest toxicity, with some being potentially carcinogenic and mutagenic (Neff 1979; Rengarajan et al. 2015).
In this scenario, benthic foraminifera dead fauna analysis on superficial sediment samples of the Santos Estuary can give valuable information about effects of the estuarine hydrodynamics and pollutants introduction over the meiofauna. Some studies have already described the foraminifera fauna distribution in this estuary (Suguio et al. 1975; Bonetti 2000; Debenay et al. 2001; Geslin et al. 2002; Díaz et al. 2014). However, Debenay et al. (2001) was the only study relating the effect of estuarine hydrodynamics on the distribution of foraminifera species, describing the distribution of the species Pararotalia cananeiaensis along the estuary.
In this context, the main objectives of this study are to investigate the distribution and abundance of benthic foraminifera (dead fauna) on superficial sediments from Santos Estuary, and to assess the effects of estuarine hydrodynamics and pollutants on its distribution, thus contributing to the implementation of benthic foraminifera as bioindicators in environmental monitoring and management in the region by monitoring and regulatory agencies.
1.1 Study area
The Santos Estuary (Fig. 1) is located in the central portion of São Paulo State’s coast (SE Brazil), between latitudes 23.85°S, 46.50°W, 24°S and longitudes 46.10°W. It consists of three main estuarine channels: Santos, São Vicente and Bertioga. Sedimentation accumulation rates in the region range from 1.11 to 1.37 cm year−1 (Martins et al. 2010), and the estuarine system is affected by semidiurnal tides, which propagate simultaneously through the Santos, São Vicente and Bertioga channels. The mean amplitudes vary between 0.27 m during neap tides and 1.23 m during spring tides (Harari and De Camargo 2003). According to Harari et al. (1990), the upper estuary is under the influence of rivers that flow into the region, coming from Serra do Mar highlands, tides and waters coming from the Bertioga channel, which generates a unidirectional flow at the surface waters, propagating towards the Santos Bay. This flow is mainly noticed in the Santos channel.
The Santos channel presents the most intense flows (Roversi et al. 2016). In the region near its mouth, the velocities reach up to 1.4 m s−1 during spring tides and up to 0.7 m s−1 during neap tides. During neap tides, the velocities of the main channels (disregarding the regions near the inlets) reach approximately 0.4 m s−1. During the spring tides, the velocities are more intense and present greater spatial variability, reaching approximately 1.2 m s−1 (Roversi et al. 2016).
The estuary as a whole presents a large temporal variation of salinity, controlled by the tidal cycle and variations in freshwater discharge into the system. According to Harari et al. (2008), salinity varies spatially from 15 to 35 in the Santos estuary; with vertical variations presenting an increase in salinity with depth of up to 4. Similar vertical stratification has been observed in the Piaçaguera channel, located at the upper estuary (Miranda et al. 2012).
2 Materials and methods
2.1 Sampling
During the summer of 2015, a total of 10 superficial sediment samples (0–2 cm) (Fig. 1, Table 1) were collected using stainless steel Van Veen grab in the Santos Estuary, Channel and Bay to obtain a representative area of the estuary. Samples were transferred to pre-cleaned polyethylene containers for benthic foraminifera and trace metals analyzes, and to aluminum foils for organic analyzes. The samples were freeze-dried and homogenized to ensure analytical quality and reliability. All samples (1–10) were analyzed for benthic foraminifera, trace metals, total organic carbon (TOC), total nitrogen (TN), grain size and δ13C (13C/12C). Aliphatic and aromatic hydrocarbon analyses were proceeded on five samples (1, 2, 3, 5, 6) collected on the upper estuary region, as it is historically the most polluted area in the Santos region (Medeiros and Bícego 2004; Bícego et al. 2006; Fontenelle et al. 2019).
2.2 Benthic foraminifera
A volume of 10 cm3 of wet sediment per sample was carefully washed in a 63-μm mesh sieve to separate the sand fraction from the finer fractions (Scoot et al. 2001). Fractions > 63 μm were then dried in an oven at 40 °C. For the removal of the largest portion of terrigenous sediment, a flotation process with ZnCl2 was performed, following the method described by Semensatto and Dias-Brito (2009). To select approximately 300 specimens per sample, a number recommended by Fatela and Taborda (2002), samples with abundant foraminifera were aliquoted with a micro-fossil splitter to obtain the desired number of specimens for counting. Foraminifera picking and identification were done on an Olympus SZX16 stereomicroscope (magnification up to 230×). The residue of the flotation process passed through a quality control, being analyzed for foraminifera tests.
Identification of genera and species followed the classification made by Loeblich and Tappan (1987), Boltovskoy et al. (1980), Debenay et al. (1998), Bonetti (2000) and Debenay et al. (2001). The names of the species have been updated according to the World Register of Marine Species (WORMS) website (www.marinespecies.org). The number of fragmented and unidentified organisms was also counted, being summed and classified as “Not identified”. Individuals that were fragmented but the identification of the genera was possible were classified as “spp.”.
Photos of the species were taken with a ZEISS SteREO Discovery.V20 stereomicroscope, using ZEN Imaging Software and with a Scanning Electron Microscope Quanta 650 FEG.
2.3 Hydrodynamic model
The hydrodynamic model was elaborated with the Flow module of the Delft3D numerical model (developed by Deltares). Delft3D simulates hydrodynamic processes by solving the Navier–Stokes equations vertically integrated into the water column, including the Coriolis effect, the wind friction, the bottom friction, and the turbulent viscosity. The numerical method is based on finite differences, discretized in curvilinear or rectilinear grids. Delft3D-FLOW is the hydrodynamic module of the program, in which a system of shallow water equations is solved in two- or three-dimensional mode. The FLOW module considers variations in the turbulence and inclusion of wet/dry areas in the domain, thereby including the intertidal mangrove areas of the estuary. The Delft3D numerical model is suitable for applications in studies of shallow seas, coastal areas, estuaries, rivers, lakes, and lagoons. Further details on the formulation of the Delft3D numerical model can be found in Deltares (2011). The Santos model domain covers the whole estuarine complex with its three main channels (Santos, São Vicente and Bertioga) and the adjacent inner shelf. Boundary conditions include offshore water levels and riverine inputs throughout the domain. Numerical simulations have been performed using the model setup calibrated and validated with in situ measured data by Seiler (2019), adapted to the present application with the inclusion of drifters in each new experiment. The model has been used in its two-dimensional mode, with the aim of providing the residual circulation in the system that drives the distribution of particles within the estuary. Results have been analyzed through the use of Lagrangian drifters (drogues) released in the model at different positions inside the Santos channel. The simulation of ~ 16 days covers neap and spring tide conditions and defines the released drifter’s trajectory over this period.
2.4 Analytical procedure for trace metals analyses
A partial digestion technique was performed according to the method SW 846 USEPA 3050B (USEPA 1996), which consists of acid digestion using HNO3, H2O2 and HCl, that dissolves elements that could be environmentally available. A total of eight trace metals (As, Cr, Cu, Ni, Pb, Sc, V and Zn) were investigated using inductively coupled plasma optical spectrometry (ICP-OES). The accuracy and precision of the method were checked using a certified reference material (SS-2) from EnvironMAT™ CRM SPC Science that was subjected to the same analytical procedures. All results were within the recommended range of USEPA (1996), that is, between 75 and 125%.
2.5 Analytical procedure for organic pollutants analyses
Hydrocarbon analysis followed the method described in UNEP (1992), in which 10 g of dry sediment was soxhlet-extracted over 8 h with 80 mL of hexane/dichloromethane mixture (1:1, v:v), after the addition of surrogate standards on all samples, blanks and reference material. Samples then were purified with a chromatographic column packed with 3.2 g of silica and 1.8 g of alumina (both 5% deactivated) and AHs were eluted using 10 mL of n-hexane (F1) and PAHs with 15 mL of dichloromethane/n-hexane (3:7, v:v) (F2). AHs and PAHs were quantitatively analyzed in a gas chromatograph 6890 from Agilent Technologies, coupled with a flame ionization detector (GC–FID) and with a mass spectrometer (GC/MS) 5973N, in selected ion mode (SIM), respectively. For method quality assurance and control, a series of procedural blanks, spiked blank, spiked matrix and reference material were analyzed. All recoveries ranged within the 65–120% approval interval.
2.6 Analytical procedure for grain size, total organic carbon (TOC), total nitrogen (TN) and stable isotopes (δ13C)
The grain size analysis was performed according to the methodology described in Suguio (1973), whereby samples were wet sieved on a 63-μm mesh sieve to quantify the mud fraction (clay + silt; < 63 μm) of the sediments. Total organic carbon (TOC), total nitrogen (TN) and carbon stable isotopes (δ13C) were analyzed on an elemental combustion system (Costech Instruments) coupled to an Isotope Ratio Mass Spectrometer (Thermo Scientific) (EA-IRMS). Before TOC and δ13C analysis, inorganic carbon from the sediment was removed using HCl 1 M.
2.7 Data analyses
2.7.1 Ecological indices
The sedimentation rate in the estuary (1.11–1.37 cm year−1) shows that the first 2 cm of the analyzed sediment is modern, and thus the foraminifera results could be used to calculate the absolute and relative abundances for each species, as well as other ecological indices. Density (d) (n° individuals/10 cm3 of sediment), Richness (S) (number of different species per sample), diversity, dominance, and evenness were determined using Past 3.0 software (Hammer et al. 2009), based on the following formulas: Shannon–Wiener Diversity (Shannon 1948) H′ = sum (pi ln pi), Simpson Index (Simpson 1949) D = sum (ni2/n), Simpson Diversity (Simpson 1949) 1-D and Pielou Evenness (Pielou 1975) J′ = H′/H max, where pi is the proportion of individuals of the taxon in the sample, ln pi is the natural log of pi, ni is the number of individuals from taxon i and H max is ln S.
2.7.2 Environmental quality indices
Trace metals assessment was conducted using the enrichment factor (EF) (Zoller et al. 1974) (Eq. 1):
where M is the concentration of the element of interest and X the normalizer element. EF quantifies the concentration of the element in the sample relative to the background value and minimizes the effect of granulometry with the introduction of a normalizing element in the equation (Luiz-Silva et al. 2006). In this study, background values were taken from Kim et al. (2017) and the concentration of Sc was used as the normalizer element.
Pollution load index (PLI) (Tomlinson et al. 1980) (Eq. 2) was also used:
where n is the number of potentially contaminant elements, i the element and CFi is the concentration factor of the element defined by the Eq. 3:
where [Element i] is the concentration of the studied element i. This index represents the number of times the concentration of metals and semimetals exceeds the base value and gives a general indication of its toxicity in a particular sample (Priju and Narayana 2006).
2.7.3 Statistical analysis
A Q-mode hierarchical cluster analysis (CA) was performed by selecting the enrichment factor of three trace metals (Cu, Pb and Zn), pollution load index results, TOC, percentage of mud, density of benthic foraminifera, Shannon–Wiener diversity index, dominance and Pielou Evenness. Organic pollutants data were not used since there are no data for all the samples. The analyses were based on Ward’s linkage method, which produces dendrograms with exceptionally well-defined clusters (Parker and Arnold 1999), and using the Bray–Curtis similarity index. A standardization of the data was performed to remove the effects of orders of magnitude difference between variables by subtracting the mean value and dividing by the standard deviation. A principal component analysis (PCA) was carried out for the ordination of sample locations based on the same parameters of the CA. PCA reduces large data matrices composed of several variables to a small number of components representing the main modes of variation and helps the interpretation of a large volume of data.
3 Results
3.1 Foraminiferal fauna
The total abundance and relative abundance of benthic foraminifera species present in the study area are summarized in the “Appendix 1”. A total of 25 genera and 32 different species were identified in a total of 6809 individuals screened in the 10 samples. Hyaline and porcelaneous foraminifera were observed in all samples along the Santos channel and in the Santos Bay. No agglutinated foraminifers were found. Photos of the most abundant species are shown in the Figs. 2, 3 and 4. The most abundant species per region of the estuary are represented in Fig. 5.
Stereomicroscope photos of benthic foraminifera. 1, Umbilical side, 2, spiral side, 3 edge view: Ammonia sp1.; 4, umbilical side, 5, spiral side: Ammonia tepida; 6, umbilical side, 7, spiral side: Ammonia parkinsoniana; 8, umbilical side, 9, spiral side: Ammonia sp2.; 11, umbilical side 12, spiral side: Discorbis sp.; 13, umbilical side, 14, spiral side: Cibicidoides lobatulus; 15, umbilical side, 16, spiral side: Pararotalia cananeiaensis
Stereomicroscope photos of benthic foraminifera. 1, 2: Cribroelphidium excavatum; 3, 4: Nonionella opima; 5, 6 Pseudononion japonicum; 7, 8: Cribroelphidium poeyanum; 9, umbilical side, 10, spiral side: Haynesina germanica; 11, 12: Buliminella elegantissima; 13: Bolivina striatula; 14: Bolivina ordinaria; 15, 16: Quinqueloculina seminula; 17, 18: Pseudotriloculina patagonica
In all samples, there was a predominance of the genus Ammonia. Ammonia is among the most abundant benthic foraminifera genera in coastal areas worldwide (Hayward et al. 2004). They have a high morphological variability that may interfere in the taxonomic determination, bringing inconsistency to phylogenetic analysis. Five species/morphotypes were distinguished and identified as Ammonia sp1., Ammonia sp2., Ammonia tepida, Ammonia parkinsoniana and Ammonia rolshauseni. Ammonia sp1. and Ammonia sp2. are species that could not be identified by morphological analysis with previous species described in the region.
The species/morphotype Ammonia sp1. presents a test almost equally biconvex with a deeply excavated umbilical region, a slightly depressed dorsal surface, a rounded peripheral margin and presents 8–9 chambers in the last whorl which differs from A. tepida. Chambers form a pointy end towards the umbilicus. Sutures slightly depressed somewhat limbate. The aperture is umbilical extraumbilical. On the other hand, Ammonia sp2. exhibits an almost equally biconvex test with a deeply excavated umbilical region with a thin umbilical button, a slightly depressed dorsal surface, a rounded peripheral margin and presents 8–9 chambers in the last whorl. The aperture is umbilical extraumbilical. Chambers form a pointy end towards the umbilicus. Sutures somewhat limbate, and the test is thinner and smaller than Ammonia. sp1. Further DNA barcoding analysis should be performed on these species for taxonomy refinement.
Ammonia sp1. was predominant in samples 1, 2, 3, 5 and 6. In samples 3 and 5, the relative abundance of this species was higher than 60%. Samples 4 and 7 showed the predominance of the species Pararotalia cananeiaensis, with an approximate abundance of 25%. These samples also presented a high abundance of the species A. tepida, with values of 25% and 12%, respectively. Samples 8, 9 and 10 showed a predominance of A. tepida, with an abundance of approximately 31% in samples 8 and 9 and approximately 23% in sample 10. It is important to note the high abundance of P. cananeiaensis in these three samples, with approximate values of 17%, 20% and 19%, respectively.
The relative abundance of Cribroelphidium species such as Cribroelphidium excavatum, Cribroelphidium poeyanum and Cribroelphidium spp. was also high, being present in all samples except for sample 3. The species Pseudononion japonicum appears with consistency from sample 4 to the Santos Bay, being present along the whole Santos channel. This species presents significant abundance, reaching values up to 11.8%. Species of the genus Bolivina such as Bolivina striatula and Bolivina ordinaria were also present in almost all samples, except for samples 1 and 2, located in the Piaçaguera River. Other species such as Haynesina germanica, Pseudotriloculina patagonica and Buliminella elegantissima were present in the samples along the Santos channel and in the upper estuary, but with lower abundance. H. germanica presented relative abundance from 0.4 to 2%, while P. patagonica presented relative abundance from 0.4 to 3.4% and B. elegantissima from 0.45 to 3.5%. The species Cibicidoides lobatulus, Discorbis sp. and Globocassidulina sp. occur throughout the Santos channel, from the Santos Bay upwards the region nearby Bertioga channel. However, only the species Globocassidulina sp. occurs in the uppermost region of the estuary (sample 1), presenting relatively high abundance (7.9%).
3.2 Ecological indices
Results of the ecological indices are summarized in Table 1 and Fig. 6. A decrease in richness from the open marine regions towards the upper estuary is observed. The highest richness value (S = 30) was observed in Santos Bay (sample 10) and the lowest value (S = 5) was found in the Piaçaguera River (sample 3). The density also follows the same decrease pattern, with the highest value (1798 ind./10 cm3) and lowest value (131 ind./10 cm3) present in the Santos Bay (sample 10) and in the Cubatão Industrial Complex (sample 1), respectively.
A trend of increasing of Simpson Dominance index (D) values is observed towards the upper estuary. The lowest value obtained was D = 0.12 in the Santos channel (sample 7) and the highest value D = 0.48 in the Piaçaguera River (sample 3). The Simpson (1-D) index and Shannon (H′) Diversity index showed similar results, corroborating the Simpson’s Dominance index. They presented their highest values in sample 7 (1-D = 0,88 and H’ = 2.46) and the lowest values in sample 3 (1-D = 0.52 and H’ = 0.90).
The Pielou evenness (J’) values also indicated a decreasing trend, showing higher values in Santos Bay (J’ = 81, sample 7) and lower values in the upper estuary (J’ = 0.54, sample 5).
3.3 Hydrodynamic model
Based on the numerical model application, the residual transport in the estuary can be seen through the drifters released at different positions inside the Santos estuarine channel (Fig. 7). The trajectories and the displacement time of the drifters provide a better understanding of the residual transport inside the Santos channel, helping the assessment of the distribution of foraminiferal species that depend on the advective transport generated by the tidal currents. Results show the residual transport for particles in the water column for the period of ~ 16 days that aims to cover the different tide conditions which control the estuarine dynamics. For all four drifters released inside the channel, residual transport drives materials into the estuarine system, resulting in net landward transport (Fig. 7). In general, drifters released at different positions in the channel are transported to the upper estuary, where areas of lower dynamics may result in their deposition. Deposition of particles is not represented by the simulated Lagrangian drifters, with their trajectories covering the whole ~ 16-day period. However, advected particles, mainly larger particles, tend to be deposited when they reach areas of lower dynamics in the upper estuary, where they are retained and unavailable for transport back to the estuarine channel.
3.4 Environmental quality indices
The EF results are shown in Table 2 and Fig. 8. Sample 1, located at the Piaçaguera River, presented the highest value of enrichment for Zn and Cu among all samples, with values of 10.340 and 2.862, respectively. Sample 2, also located in the Piaçaguera River, presented a moderate enrichment for Zn, with EF = 2.315, while samples 7 and 9, located along the Santos channel, showed a moderate enrichment of Pb (EF equals to 2.16 and 3.70, respectively). No sample showed significant As, Cr or Ni enrichment. Sample 4, located at the upper estuary, presented the lowest level of metal enrichment, showing the lowest EF values for Cu, Ni, Pb and Zn (0.43, 0.89, 0.69 and 0.56, respectively).
The PLI results varied between 0.66 and 2.31 (Table 2, Fig. 9), with the highest value found at the Cubatão Industrial Complex (sample 1) and the lowest one at the upper estuary (sample 4).
3.5 Organic pollution indices
Aliphatic and aromatic hydrocarbons results are summarized in Table 3 and Fig. 10. Resolved alkanes and UCM concentrations varied between 19.7 and 75 µg g−1 and 95.2 and 402.3 µg g−1, respectively. UCM is a clear indicative of oil introduction on sediments, as they are a mixture of unresolved hydrocarbons that cannot be separated within chromatographic technics. UCM was identified in all samples, with higher concentrations and UCM/Resolved Alkanes values closer to the Cubatão Industrial Complex (sample 1) and decreasing towards the Santos channel (sample 5 and 6). Total polycyclic aromatic hydrocarbons concentrations ranged within 1176 and 32,153.3 ng g−1, also presenting higher concentrations closer to Cubatão Industrial Complex, and lower ones closer to the Santos channel.
3.6 Grain size, total organic carbon (TOC), total nitrogen (TN) and stable isotopes (δ13C)
The percentage of fine fraction in the sediments does not show a clear pattern of distribution in the study area. The fine fraction is higher than 50% in most of the samples, except for sample 4 (upper estuary) and 9 (Santos channel), with values of 38.5% and 39.6%, respectively. The highest percentages of fine sediments were observed in Santos Bay (97.5%, sample 10) and in the Piaçaguera River (93.69%, sample 2) (Tab. 4) (Fig. 11).
A large range of the TOC values is observed along the estuary. The highest values (6.02% and 4.06%) were recorded in Cubatão Industrial Complex (samples 1 and 2, respectively) and the lowest (0.26%) in the upper estuary (sample 4). Most of the samples recorded TOC values below 3% (Table 4, Fig. 11).
The highest value of TN (0.32%) was observed in the Santos Bay (sample 10), and the lowest (0.08%) at the upper estuary (sample 4). In the other samples, TN values oscillated between 0.12 and 0.20% (Table 4, Fig. 11).
Stable isotopes δ13C presents a pattern of increase from the upper estuary towards the Santos Bay. The lowest value (− 27.08) and the highest value (− 23.5) were observed in the samples 2 and 9, respectively (Table 4).
Values of C/N ratio can be analyzed in Table 4 and Fig. 11. Smaller values of the C/N ratio indicate the presence of particles of marine origin in the organic matter (Meyers 1997). Samples 1 and 2, located at the Cubatão Industrial Complex, presented the highest values (> 20), indicating a continental origin for the organic matter in the sediment; while sample 4, situated at the upper estuary, showed the lowest value (3.2), indicating a marine origin for the organic matter encountered. The other samples showed values ranging from 8.4 to 15.4, indicating mixed sources.
The scatter plot between δ13C and C/N ratio of samples, according to the diagram established by Lamb et al. (2006), provides additional information on the contributions of organic matter sources to the sediment (Fig. 12). The Santos Bay (sample 10) has an exclusive marine DOC contribution, while the Santos channel (samples 5, 7 and 8) has an overlap of sources, influenced by C3 terrestrial plants and marine DOC. The uppermost part of the Piaçaguera River (sample 1) was the only one with exclusive origin of C3 terrestrial plants. These C3 plants may be representing mangroves, which are very abundant in the region. Samples 2 and 3 still located in the Piaçaguera River presented an overlapping of sources, with influence of C3 terrestrial plants and Dissolved Organic Carbon (DOC).
Scatter plot between the δ13C and C/N ratios obtained for the samples. The different fields in the δ13C versus C/N plot correspond to the ranges for organic matter inputs to coastal environments (according to Lamb et al. 2006)
3.7 Statistical analysis
Two distinct clusters (1 and 2) can be recognized (Fig. 13). Furthermore, the cluster 2 can be divided into two subclusters (2a and 2b) (Fig. 13). Cluster 1 (samples 1, 2, 3 and 5), Cluster 2a (samples 4, 6, 7 and 8) and Cluster 2b (samples 9 and 10). Cluster 1 includes samples located in the uppermost part of the estuary, whereas Cluster 2 comprises samples located in the outermost part of the study area. The PCA was used to relate samples to ecological indices, trace metals and environmental data to better understand the clusters. Two principal components (factor) have been identified (eigenvalue > 1), explaining together 73.8% of the total variance. The first and the second factors explain 46.8% and 27.0%, respectively, of the total variance. The first factor was plotted against the second in Fig. 14.
Dendrogram classification of stations produced by Q-mode cluster analysis using Ward’s linkage method and the Bray–Curtis similarity index based on the enrichment factor of Cu, Pb and Zn, pollution load index results, total organic carbon, percentage of mud, density of benthic foraminifera, Shannon–Wiener diversity index, dominance and Pielou Evennes
The PCA analysis bunched stations according to their environmental conditions, in approximately the same groups obtained with the Q-mode cluster analysis. Accordingly, the stations in Cluster 1 can be considered polluted by trace metals, where samples are related to the enrichment factor of Cu, Pb and Zn, PLI and higher dominance. On the other hand, stations in Cluster 2 cannot be considered polluted, once they are related with higher diversity, density and equitability, and negatively correlated with trace metals.
4 Discussion
Although the dead assemblages of benthic foraminifera represent modern fauna, since the sedimentation rates are less than 1.5 cm per year, the assemblages may include allochthonous specimens due to post mortem transport (Alve and Murray 1994). Thus, we should carefully discuss the results aiming to understand the parameters that are controlling the fauna distribution in the estuary, and the possible effects of the estuarine hydrodynamics on post mortem transport.
4.1 Foraminifera and the hydrodynamics
Analyzing the dead fauna of benthic foraminifera and the hydrodynamic numerical model, it was possible to verify the effect of the estuarine hydrodynamics along the Santos channel, over the distribution of two different species and one genus: Pararotalia cananeiaensis, Pseudononion japonicum and Discorbis sp. These species and genus are considered marine dwellers (Eichler et al. 2001) and occur in the Santos Bay, where the environmental conditions are exclusively marine and suitable for their reproduction and growth.
Residual flows shown by the hydrodynamic numerical model (Fig. 7) in the Santos channel are landwards, pumping drifting materials into the estuary, since the released drifters are all retained in the estuary during the ~ 16-day simulation. Even drifters released close to the channel mouth were gradually pumped into the estuary and remained in the upper estuarine portion during the simulated period. This result evidences the post mortem transport from the Santos Bay to the upper estuary. The dredging of the Santos channel, which remobilizes the sediment raising them in suspension, can also be favoring the transport of the tests to the uppermost part of the estuary.
Pararotalia cananeiaensis distribution extended from the Santos Bay (sample 10) along the Santos channel, until the upper estuary (sample 4), where it presents the highest abundance. According to Debenay et al. (2001), the Santos Bay is favorable for the colonization of this species because it is open to the ocean but protected from strong currents. Bonetti (2000) and Debenay et al. (2001) highlighted an important presence of this species near Cubatão, inside the Santos channel, which is often dredged and where the influence of tidal currents is strong.
Discorbis sp. is typically marine and has a heavy test, having a less efficient transport by the currents (Eichler et al. 2001). This genus was present in the samples in the Santos Bay and along the Santos channel, regions with a greater marine influence. Due to their higher weight, they may be deposited earlier as flows decrease, not reaching the region of the upper estuary (sample 4). Pseudononion japonicum also appears to be related to the regions of greater marine influence. Their distribution resembled the one described by Díaz et al. (2014) in the Santos Estuary, and also appears to be transported upstream by tidal currents.
Similar to P. cananeiaensis, P. japonicum presented the highest abundance in sample 4 in the upper estuary. The presence of these species in higher abundance in the upper estuary rather in the Santos Bay is possibly due to two reasons. Firstly, as the numerical model evidenced, there is a retention of particles transported by tidal currents in the upper estuary, meaning that the empty foraminiferal tests from the Santos Bay are accumulating more in this region. Secondly, there is a probable condition of life for these species in the upper estuary, as well as in the Santos channel, since there is a great marine influence in the region. According to Debenay et al. (2001), the abundance of P. cananeiaensis presents a negative correlation with the C/N ratio, and smaller values of the C/N ratio indicate the presence of particles of marine origin (Meyers 1997). Values are lower than 15 in the Santos Bay and along the Santos channel, where the P. cananeiaensis, Discorbis sp. and P. japonicum are found in greater abundance. The ratio decreases drastically in sample 4, presenting its lowest value and, consequently, the highest values of P. cananeiaensis and P. japonicum abundances. This evidences that particles in residual flows from the Santos Bay are accumulating in the upper estuary, and the great marine influence possibly makes the environment suitable for living individuals.
This scenario is corroborated by the scatter plot between δ13C and C/N ratio of samples, according to the diagram established by Lamb et al. (2006), which provides additional information on the contributions of organic matter sources to the sediment (Fig. 12). The Santos Bay has an exclusive marine DOC contribution, while the Santos channel has an overlap of sources, influenced by C3 terrestrial plants and marine DOC, also indicating that these regions have suitable conditions for living individuals of P. cananeiaensis, Discorbis sp. and P. japonicum.
As the Piaçaguera River, located in the uppermost part of the estuary, presented an exclusive terrestrial influence for the organic matter when analyzing the C/N ratio and the scatter plot between δ13C and C/N ratio of samples, according to the diagram established by Lamb et al. (2006), the species P. cananeiaensis, Discorbis sp. and P. japonicum were not found in this region.
4.2 Foraminifera and the pollution
Numerous studies dealing with the relationships between foraminifera and pollution have considered trace metals and hydrocarbons to be an important parameter acting on ecosystems and affecting benthic foraminifera distribution (e.g. Alve 1991, 1995; Yanko et al. 1994, 1998; Coccioni 2000; Samir and El-Din 2001; Armynot du Châtelet et al. 2004, 2011; Frontalini et al. 2009; Martins et al. 2013). When analyzing the values obtained by EF and utilizing the Sutherland (2000) classification of EF, it is observed that the most contaminated sample by metals is located in the uppermost part of Piaçaguera River, in the Cubatão Industrial Complex (sample 1). It has a moderate enrichment of Pb and Cu, and a significant enrichment of Zn. The middle part of the Piaçaguera River (sample 2) presented a moderate enrichment of Zn. The Santos channel (samples 7 and 9) showed a moderate enrichment of Pb, and the upper estuary (sample 4) presented the lowest level of metal enrichment, showing the lowest EF values for Cu, Ni, Pb and Zn. When analyzing the PLI results and utilizing Tomlinson et al. (1980) classification of PLI, it is observed that the upper Piaçaguera River, next to the Cubatão Industrial Complex (sample 1), was the most deteriorated region. The upper estuary (sample 4) was the only region that presented no deterioration by metals. The result of the PLI corroborates the result obtained by the EF.
In comparison with previous studies conducted on the Santos Estuary, results of UCM and Total PAHs found on this study are similar to ones published by Nishigima et al. (2001) (PAHs: < 42,300 ng g−1), Medeiros and Bícego (2004) (UCM: < 59.18 µg g−1; PAHs: < 15,400 ng g−1) and Bícego et al. (2006) (UCM: < 2015 µg g−1; PAHs: < 68,100 ng g−1). These studies also indicated a similar concentration pattern, with higher values on samples from the Piçaguara River, closer to the Cubatão Industrial Complex, and decreasing to the Santos channel. Compared to other estuarine regions over the world (Table 5), the Total PAHs sum found on this study are higher than values found on other estuaries from Brazil (Zanardi et al. 1999; da Silva et al. 2007), but still 10 times lower than other heavily industrialized areas as the Boston Harbor USA (Wang et al. 2001). The presence of pollution by oil products is a common feature in some Brazilian coastal systems (e.g., Taniguchi et al. 2016; Martins et al. 2018a; Souza et al. 2018; Andrade et al. 2019; Almeida et al. 2018; Santos et al. 2019).
Most of the studies carried out in polluted environments have shown that a lowering in density and diversity can be viewed as a measure of environmental stress on benthic foraminiferal communities caused by pollution (e.g., Schafer 1973; Yanko et al. 1998, Samir and El-Din 2001; Armynot du Châtelet et al. 2004, 2011; Frontalini et al. 2009; Dimiza et al. 2016). Increased pollution has also been reported as being the cause of a high number of individuals belonging to a few opportunistic species (Murray 1973). According to the PCA, low density, diversity and equitability values were found in stations more affected by trace metals and pollution, where the indices show negative correlation with trace metals (Cu, Pb, Zn and the PLI), as well as negative correlation with % mud and TOC. These indices are correlated with the samples in the Santos channel and Santos Bay. On the other hand, dominance was correlated with trace metals in the first component, as well as TOC and % mud. Dominance is well correlated with the Piaçaguera River and upper estuary samples. The positive correlation of trace metals with TOC and % mud is explained by the adsorption of trace metals in organic matter and Fe/Mn oxides, since finer sediments have larger contact surfaces and more mineralogical particles for adsorption of trace metals (Ho et al. 2010; Song et al. 2010; Suzuki et al. 2016).
A close examination of the ecological indices shows a better understanding of the pollution effects on the benthic fauna. Regarding the richness and density indices, the samples located in the Piaçaguera River, closer to the Cubatão Industrial Complex, presented the lowest values (S = 8 and density = 131 ind./10 cm3) of the whole estuary, characterizing these results as typical of polluted regions (Schafer 1973; Alve 1991; Armynot du Châtelet et al. 2004, 2011; Frontalini et al. 2009; Díaz et al. 2014; Martins et al. 2016a). This region also presented highest value of UCM/Resolved Alkanes and highest enrichment of metals and PLI value, pointing out a pollution scenario. The highest values of TOC in the whole estuary were also encountered in this region, and the high amount of organic matter might be also affecting the fauna in a negative way. On the other hand, the regions towards the Santos Bay presented an opposite scenario, with greater richness and densities (S = 30 and density = 1798 ind./10 cm3), indicating better ecological conditions. The diversity index had the lowest value in the Piaçaguera River, and highest value towards the Santos Bay, probably not only due to a higher influence of freshwater, which is an unfavorable condition for most of foraminifera species (Boltovskoy et al. 1991; de Rijk 1995; Martins et al. 2016a; Dong et al. 2019; Sreenivasulu et al. 2019), but also may indicate that the pollution scenario present in the Piaçaguera River is affecting the meiofaunal organisms.
Regarding the species dominance index, the high value that was observed in the Piaçaguera River can be explained by the colonization of the environment by opportunistic species (Murray 1973). Opportunistic species colonize restricted environments under conditions of environmental stress and are considered tolerant to pollution by trace metals and hydrocarbons. Some species of the genus Ammonia, such as Ammonia tepida, are globally known as the main opportunistic species (Yanko et al. 1994; Alve 1995; Coccioni 2000; Armynot du Châtelet et al. 2004; Ferraro et al. 2006), and is one of the most common and dominant species in other impacted Brazilian coastal systems (e.g., Delavy et al. 2016; Duleba et al. 2018; Pregnolato et al. 2018; Martins et al. 2019a). The species/morphotypes Ammonia sp1. and Ammonia sp2. were the most opportunistic in the most polluted regions by metals and hydrocarbons, being found in greater abundance and being responsible for the high values of dominance obtained in the Piaçaguera River. These species do not appear to be affected by the hydrodynamics; however, this is still an open question.
The above-mentioned findings are well visualized in the Q-mode cluster, which evidences three scenarios in the Estuary. Cluster 1 represents the region most affected by pollutants and has the greatest terrestrial influence and the highest values of TOC. In this region, benthic foraminifera fauna is dominated by Ammonia, with lower diversity, density and richness. Cluster 2a represents a transitional zone with terrestrial and marine influences, sheltering species suitable for life in these two environments, with an enrichment of the marine species, such as P. cananeiaensis, Discorbis sp. and P. japonicum, due to post mortem transport, which has been studied in several places (e.g., Martins et al. 2016b, c, 2018b, 2019b, c; Belart et al. 2018; Hohenegger et al. 2018). The cluster 2b represents the most marine regions, with greater density, and where the pollution is not affecting the fauna composition. Although the physicochemical field measurements were not obtained, the decrease of salinity in the upper estuary where the influence of the fluvial processes prevails to the oceanic processes can be also affecting the fauna distribution of the regions in Cluster 1.
To better understand the benthic foraminifera distribution on the Santos estuary, further physicochemical measurements should be analyzed in conjunction of living benthic foraminifera (e.g., salinity, temperature and oxygenation of the substrate), as rapid changes in these parameters can be responsible for morphological change in the foraminiferal tests, and would generally be viewed as unfavorable. However, many morphological changes are species specific and often opposite among different species for the same parameter (Boltovskoy et al. 1991); thus, the ecology of species in the region should be better studied to understand the effect of physicochemical parameters on the fauna.
5 Conclusion
In this study, 10 superficial sediment samples of the Santos Estuary were analyzed for benthic foraminifera, molecular markers and pollutants, and a hydrodynamic numerical model was generated, to verify the effect of the estuarine circulation and pollution on the foraminifera dead fauna.
Benthic foraminifera tracked well the estuarine residual circulation shown by the hydrodynamic model, especially with the distribution of species Pararotalia cananeiaensis, Discorbis sp. and Pseudononion japonicum. These species appear to be transported from the Santos Bay towards the upper estuary by tidal currents, and the dredging of the Santos channel appears to be complementing this process by resuspending the bottom sediment and favoring the post mortem transport. In the upper estuary, there is a greater influence of fresh water, which is unfavorable for most foraminifera species. It may be noted that weaker hydrodynamic conditions in some areas of the upper estuary may favor the retention of particles, including foraminifera tests, carried from the ocean.
Highest concentrations of trace metals were found near the Cubatão Industrial Complex, at the northernmost part of the Piaçaguera River, with moderate enrichment values for Cu and Pb, and significant enrichment values for Zn. PLI indicated a progressive deterioration of the area. Hydrocarbons analysis showed a widespread contamination on the estuary (∑PAHs > 1176 ng g−1 and UCM > 95.2 µg g−1), with higher concentrations in the Piçaguera River, decreasing towards the Santos channel. Benthic foraminifera appear to be a good bioindicator of pollution in the estuary, when analyzing the ecological indices. The richness, density and diversity indices decreased landward. Ammonia sp.1 showed to be opportunistic, with highest dominance in the most polluted regions of the estuary.
This study serves as an initial survey for environmental assessment along this estuary; nevertheless, new studies analyzing living fauna in conjunction of physicochemical measurements should be performed to assess the exact current environmental quality of the Santos Estuary.
6 Fauna list
-
Foraminiferal species observed in this study are listed by genus and in alphabetic order.
-
Ammonia parkinsoniana (d’Orbigny, 1839)
-
Ammonia rolshauseni (Cushman & Bermúdez, 1946)
-
Ammonia sp1.
-
Ammonia sp2.
-
Ammonia spp. (Brünnich, 1771)
-
Ammonia tepida (Cushman, 1926)
-
Amphicoryna scalaris (Batsch, 1791)
-
Bolivina ordinaria (Phleger & Parker, 1952)
-
Bolivina pulchella (d’Orbigny, 1839)
-
Bolivina striatula (Cushman, 1922)
-
Bulimina aculeata (d’Orbigny, 1826)
-
Buliminella elegantissima (d’Orbigny, 1839)
-
Cibicidoides lobatulus (Walker & Jacob, 1798)
-
Cornuspira planorbis (Schultze, 1853)
-
Cribroelphidium excavatum (Terquem, 1875)
-
Cribroelphidium poyanum (d’Orbigny, 1839)
-
Cribroelphidium spp. (Cushman & Brönnimann, 1948)
-
Discorbis sp. (Lamarck, 1804)
-
Elphidium galvestonensis (Kornfeld, 1931)
-
Favulina melo (d’Orbigny, 1839)
-
Fissurina lucida (Williamson, 1848)
-
Fursenkoina pontoni (Cushman, 1932)
-
Globocassidulina sp. (Voloshinova, 1960)
-
Haynesina germanica (Ehrenberg, 1840)
-
Hopkinsina pacifica (Cushman, 1933)
-
Lagena sp. (Walker & Jacob, 1798)
-
Miliolinella subrotunda (Montagu, 1803)
-
Nonionella opima (Cushman, 1947)
-
Nonionella sp. (Cushman, 1926)
-
Pararotalia cananeiaensis (Debenay, Duleba, Bonetti De Melo e Souza & Eichler, 2001)
-
Pseudononion japonicum (Asano, 1936)
-
Pseudononion spp. (Asano, 1936)
-
Pseudotriloculina patagonica (d’Orbigny, 1839)
-
Quinqueloculina seminula (Linnaeus, 1758)
-
Quinqueloculina spp. (d’Orbigny, 1826)
-
Reussoolina laevis (Montagu, 1803)
-
Rosalina bradyi (Cushman, 1915)
-
Rosalina williamsoni (Chapman & Parr, 1932)
-
Sphaeroidina bulloides (d’Orbigny in Deshayes, 1828)
-
Triloculina sp. (d’Orbigny, 1826)
References
Al Rashdi, S., Arabi, A. A., Howari, F. M., & Siad, A. (2015). Distribution of heavy metals in the coastal area of Abu Dhabi in the United Arab Emirates. Marine Pollution Bulletin,97(1–2), 494–498. https://doi.org/10.1016/j.marpolbul.2015.05.052.
Almeida, M., Nascimento, D. V., Mafalda, P. O., Jr., Patire, V. F., & Albergaria-Barbosa, A. C. R. (2018). Distribution and sources of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of a Tropical Bay influenced by anthropogenic activities (Todos os Santos Bay, BA, Brazil). Marine Pollution Bulletin,137, 399–407. https://doi.org/10.1016/j.marpolbul.2018.10.040.
Alve, E. (1991). Benthic foraminifera in sediment cores reflecting heavy metal pollution in Sorfjord, Western Norway. Journal of Foraminiferal Research,21(1), 1–19.
Alve, E. (1995). Benthic foraminifera response to estuarine pollution: A review. Journal of Foraminiferal. Research,25(3), 190–203.
Alve, E., & Murray, J. W. (1994). Ecology and taphonomy of benthic foraminifera in temperate mesotidal inlets. Journal of Foraminiferal Research,24, 18–27.
Andrade, M. V. F., Santos, F. R., Oliveira, A. H. B., Nascimento, R. F., & Cavalcante, R. M. (2019). Influence of sediment parameters on the distribution and fate of PAHs in an estuarine tropical region located in the Brazilian semi-arid (Jaguaribe River, Ceará coast). Marine Pollution Bulletin,146, 703–710. https://doi.org/10.1016/j.marpolbul.2019.07.027.
Armynot du Châtelet, E., & Debenay, J.-P. (2010). The anthropogenic impact on the Western French coasts as revealed by foraminifera: A review. Revue de Micropaléontologie,53(3), 129–137. https://doi.org/10.1016/j.revmic.2009.11.002.
Armynot du Châtelet, E., Debenay, J.-P., & Soulard, R. (2004). Foraminiferal proxies for pollution monitoring in moderately polluted harbors. Environmental Pollution,127, 27–40. https://doi.org/10.1016/S0269-7491(03)00256-2.
Armynot du Châtelet, E., Gebhardt, K., & Langer, M. R. (2011). Coastal pollution monitoring: Foraminifera as tracers of environmental perturbation in the port of Boulogne-sur-Mer (Northern France). Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen,262(1), 91–116. https://doi.org/10.1127/0077-7749/2011/0187.
Bargagli, R., Monaci, F., Sanchez-Hernandez, J. C., & Cateni, D. (1991). Biomagnification of mercury in an Antartic marine coastal food web. Marine Ecology Progress Series,169, 65–76. https://doi.org/10.3354/meps169065.
Belart, P., Clemente, I., Raposo, D., Habib, R., Volino, E. K., Villar, A., et al. (2018). Living and dead Foraminifera as bioindicators in Saquarema Lagoon System, Brazil. Latin American Journal of Aquatic Research,46(5), 1055–1072. https://doi.org/10.3856/vol46-issue5-fulltext-18.
Bícego, M. C., Taniguchi, S., Yogui, G. T., Montone, R. C., Silva, D. A. M., Lourenço, R. A., et al. (2006). Assessment of contamination by polychlorinated biphenyls and aliphatic and aromatic hydrocarbons in sediments of the Santos and São Vicente Estuary System, São Paulo, Brazil. Marine Pollution Bulletin,52, 1784–1832. https://doi.org/10.1016/j.marpolbul.2006.09.011.
Blacksmith Institute. (2006). The world’s worst polluted places. The top ten. http://www.blacksmithinstitute.org/top10/worst35d.html#s14.
Boltovskoy, E., Giussani, G., Watanabe, S., & Wright, R. (1980). Atlas of Benthic Shelf Foraminifera of the Southwestern Atlantic. The Hague: Dr. W. Junk Publishers.
Boltovskoy, E., Scott, D. B., & Medioli, F. S. (1991). Morphological variations of benthic foraminiferal tests in response to changes in ecological parameters: A review. Journal of Paleontology,65(2), 175–185.
Bonetti, V. D. H. C. C. (2000). Foraminíferos como bioindicadores do gradiente de estresse ecológico em ambientes costeiros poluídos. Estudo aplicado ao Sistema Estuarino de Santos-São Vicente [Tese de doutorado]. São Paulo: Universidade de São Paulo, Instituto Oceanográfico.
CETESB—Companhia de Tecnologia de Saneamento Ambiental. (1978). Poluição das águas no estuário e Baía de Santos. Relatório Técnico CETESB.
CETESB—Companhia de Tecnologia de Saneamento Ambiental. (2001). Sistema Estuarino de Santos e São Vicente. Relatório Técnico CETESB, São Paulo.
Coccioni, R. (2000). Benthic foraminifera as bioindicators of heavy metal pollution. In R. E. Martin (Ed.), Environmental micropaleontology. Topics in geobiology (Vol. 15). Boston, MA: Springer. https://doi.org/10.1007/978-1-4615-4167-7_4.
Comitê da Bacia Hidrográfica da Baixada Santista (CBH-BS). (2011) Relatório de situação: recursos hídricos da Baixada Santista. http://www.sigrh.sp.gov.br/public/uploads/documents/7315/rs-2011-cbh-bs-versao-final.pdf.
Coulbourn, W. T., & Resig, J. M. (1975). On the use of benthic foraminifera as sediment tracers in a Hawaiian bay. Pacific Science,29(1), 99–115.
Da Silva, T., Azevedo, D., & Neto, F. (2007). Distribution of polycyclic aromatic hydrocarbons in surface sediments and waters from Guanabara Bay, Rio de Janeiro, Brazil. Journal of the Brazilian Chemical Society,18(3), 628–637. https://doi.org/10.1590/S0103-50532007000300021.
De Rijk, S. (1995). Salinity control on the distribution of salt marsh foraminifera (Great Marshes, Massachusetts). Journal of Foraminiferal Research,25, 156–166. https://doi.org/10.2113/gsjfr.25.2.156.
Debenay, J. P., Duleba, W., Bonetti, C., Souza, S. H. M., & Eichler, B. B. (2001). Pararotalia cananeiaensis n. sp.: Indicator of marine influence and water circulation in Brazilian coastal and paralic environments. Journal of Foraminiferal Research,31(2), 152–163. https://doi.org/10.2113/0310152.
Debenay, J.-P., Eichler, B., Duleba, W., Bonetti, C., & Eichler, P. (1998). Water stratification in coastal lagoons: Its influence on foraminiferal assemblages in two Brazilian lagoons. Marine Micropaleontology,35(1), 67–89. https://doi.org/10.1016/S0377-8398(98)00011-5.
Delavy, F. P., Figueiredo, A. G., Jr., Martins, M. V. A., Rodrigues, R., Pereira, E., Brito, M. A. R. C., et al. (2016). High-resolution acoustic mapping of gas charged sediments and living benthic foraminifera assemblages from the NE region of the Guanabara Bay (RJ, Brazil). Journal of Sedimentary Environments,1(3), 360–384. https://doi.org/10.12957/jse.2016.26281.
Deltares. (2011). Delft3D-FLOW user manual. Netherlands: Deltares.
Díaz, T. L., Rodrigues, A. R., & Eichler B. B. (2014). Distribution of foraminifera in a subtropical Brazilian estuarine system. The Journal of Foraminiferal Research44(2), 90–108.
Dimiza, M. D., Triantaphyllou, M. V., Koukousioura, O., Hallock, P., Simboura, N., Karageorgis, A. P., et al. (2016). The Foram Stress Index: A new tool for environmental assessment of soft-bottom environments using benthic foraminifera. A case study from the Saronikos Gulf, Greece, Eastern Mediterranean. Ecological Indicators,60, 611–621. https://doi.org/10.1016/j.ecolind.2015.07.030.
Dong, S., Lei, Y., Li, T., & Jian, Z. (2019). Responses of benthic foraminifera to changes of temperature and salinity: Results from a laboratory culture experiment. Science of China Earth Sciences,62(2), 459–472. https://doi.org/10.1007/s11430-017-9269-3.
Duleba, W., & Debenay, J.-P. (2003). Hydrodynamic circulation in the estuaries of Estação Ecológica Juréia-Itatins, Brazil, inferred from foraminífera and thecamoebian assemblages. Journal of Foraminiferal Research,33(1), 62–93. https://doi.org/10.2113/0330062.
Duleba, W., Teodoro, A. C., Debenay, J.-P., Alves Martins, M. V., Gubitoso, S., Pregnolato, L. A., et al. (2018). Environmental impact of the largest petroleum terminal in SE Brazil: A multiproxy analysis based on sediment geochemistry and living benthic foraminifera. PLoS ONE,13(2), e0191446. https://doi.org/10.1371/journal.pone.0191446/.
Eichler, B. B., Eichler, P. P. B., Miranda, L. B., Bérgamo, A. L., Bernardes, M. E. C., Pereira, E. R. M., et al. (2001). Utilização de foraminíferos como bioindicadores da influência marinha na Baía de Guanabara, (RJ, Brasil). Pesquisas em Geociências,28(2), 251–282. https://doi.org/10.22456/1807-9806.20300.
Fatela, F., & Taborda, R. (2002). Confidence limits of species proportions in microfossil assemblages. Marine Micropaleontology,45(2), 169–174. https://doi.org/10.1016/S0377-8398(02)00021-X.
Ferraro, L., Sprovieri, M., Alberico, I., Lirer, F., Prevedello, L., & Marsella, E. (2006). Benthic foraminifera and heavy metals distribution: A case study from the Naples Harbour (Tyrrhenian Sea, Southern Italy). Environmental Pollution,142(2), 274–287. https://doi.org/10.1016/j.envpol.2005.10.026.
Fontenelle, F. R., Taniguchi, S., da Silva, J., & Lourenço, R. A. (2019). Environmental quality survey of an industrialized estuary and an atlantic forest biosphere reserve through a comparative appraisal of organic pollutants. Environmental Pollution,248, 339–348. https://doi.org/10.1016/j.envpol.2019.02.023.
Frontalini, F., Buosi, C., Da Pelo, S., Coccioni, R., Cherchi, A., & Bucci, C. (2009). Benthic foraminifera as bio-indicators of trace element pollution in the heavily contaminated Santa Gilla lagoon (Cagliari, Italy). Marine Pollution Bulletin,58(6), 858–877. https://doi.org/10.1016/j.marpolbul.2009.01.015.
Frontalini, F., & Coccioni, R. (2011). Benthic foraminifera as bioindicators of pollution: A review of Italian research over the last three decades. Revue de Micropaléontologie,54(2), 115–127. https://doi.org/10.1016/j.revmic.2011.03.001.
Fu, F., & Wang, Q. (2011). Removal of heavy metals ions from wastewaters: A review. Journal of Environmental Management,92(3), 407–418. https://doi.org/10.1016/j.jenvman.2010.11.011.
Geslin, E., Debenay, J.-P., Duleba, W., & Bonetti, C. (2002). Morphological abnormalities of foraminiferal tests in Brazilian environments: Comparison between polluted and non-polluted areas. Marine Micropaleontology,45(2), 151–168. https://doi.org/10.1016/S0377-8398(01)00042-1.
Goswami, P., Ohura, T., Guruge, K. S., Yoshioka, M., Yamanaka, N., Akiba, M., et al. (2016). Spatio-temporal distribution, source, and genotoxic potential of polycyclic aromatic hydrocarbons in estuarine and riverine sediments from southern India. Ecotoxicology and Environmental Safety,130, 113–123. https://doi.org/10.1016/j.ecoenv.2016.04.016.
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2009). PAlaeontological STatistics, p. 92 ver. 1.9 Users 501 manual. http://folk.uio.no/ohammer/past/past.pdf.
Harari, J., & De Camargo, R. (2003). Numerical simulation of the tidal propagation in the coastal region of Santos (Brazil, 24°S 46°W). Continental Shelf Research,23(16), 1597–1613. https://doi.org/10.1016/S0278-4343(03)00143-2.
Harari, J., França, C. A. S., & Camargo, R. (2008). Climatology and hydrography of Santos Estuary. In R. Neves, J. Baretta, & M. Mateus (Eds.), Perspectives on integrated coastal zone management in South America (Vol. 1, pp. 147–160). Lisboa: Instituto Superior Técnico.
Harari, J., Mesquita, A. R., Marone, E., França, C. A. S., Camargo, R., Pereira, J. E. R., et al. (1990). Tehnical report of the Project Flow measurement in the Bay of Santos. São Paulo: FUNDESPA.
Hayward, B. W. (1992). Associations of benthic foraminifera (Protozoa Sarcodina) of inner shelf sediments around Cavalli Islands, north-east New Zealand. New Zealand Journal of Marine and Freshwater Research,16, 27–56. https://doi.org/10.1080/00288330.1982.9515945.
Hayward, B., Holzmann, M., Grenfell, H., Pawlowski, J., & Triggs, C. (2004). Morphological distinction of molecular types in Ammonia towards a taxonomic revision of the world’s most commonly misidentified foraminifera. Marine Micropaleontology,50(3–4), 237–271. https://doi.org/10.1016/S0377-8398(03)00074-4.
Hernández-Crespo, C., & Marín, M. (2015). Determination of background levels and pollution assessment for seven metals (Cd, Cu, Ni, Pb, Zn, Fe, Mn) in sediments of a Mediterranean coastal lagoon. CATENA,133, 206–214. https://doi.org/10.1016/j.catena.2015.05.013.
Ho, H. H., Swennen, R., & Van Damme, A. (2010). Distribution and contamination status of heavy metals in estuarine sediments near cua ong harbor, Ha Long Bay Vietnam. Geologica Belgica,13(1–2), 37–47.
Hohenegger, J., Martins, M. V. A., & Frontalini, F. (2018). Methods relieving comparison of living and death assemblages. Micropaleontology,64(4), 255–267.
IBGE. (2017). https://cidades.ibge.gov.br/v4/brasil/sp/cubatao/panorama. Retrieved September 2019.
Kim, B. S. M., Angeli, J. L. F., Ferreira, P. A. L., Sartoretto, J. R., Miyoshi, C., Mahiques, M. M., et al. (2017). Use of a chemometric tool to establish the regional background and assess trace metal enrichment at Baixada Santista: Southeastern Brazil. Chemosphere,166, 372–379. https://doi.org/10.1016/j.chemosphere.2016.09.132.
Kim, B. S. M., Salaroli, A. B., Ferreira, P. A., Sartoretto, J. R., Mahiques, M. M., & Figueira, R. C. L. (2016). Spatial distribution and enrichment assessments of heavy metals in surface sediments from Baixada Santista, Southern Brazil. Marine Pollution Bulletin,103(1–2), 333–338. https://doi.org/10.1016/j.marpolbul.2015.12.041.
Kramer, K., & Botterweg, J. (1991). Aquatic, biological early warning systems: An overview. In D. Jeffrey & N. Madden (Eds.), Bioindicators and environmental management (pp. 95–126). London: Academic Press.
Lamb, A. L., Wilson, G. P., & Leng, M. J. (2006). A review of coastal palaeoclimate and relative sea-level reconstructions using δ13C and C/N ratios in organic material. Earth-Science Reviews,75(1–4), 29–57. https://doi.org/10.1016/j.earscirev.2005.10.003.
Leorri, E., Cearreta, A., Irabien, M. J., & Yusta, I. (2008). Geochemical and microfaunal proxies to assess environmental quality conditions during the recovery process of a heavily polluted estuary: the Bilbao estuary case (N. Spain). Science of Total Environment,396, 12–27. https://doi.org/10.1016/j.scitotenv.2008.02.009.
Lima, A. L. C., Farrington, J. W., & Reddy, C. M. (2005). Combustion-derived polycyclic aromatic hydrocarbons in the environment: A review. Environmental Forensics,6, 109–131. https://doi.org/10.1080/15275920590952739.
Loeblich, A. R., Jr., & Tappan, H. (1987). Foraminiferal genera and their classification. New York: Van Reinhold.
Luiz-Silva, W., Matos, R. H. R., Kristosch, G. C., & Machado, W. (2006). Variabilidade espacial e sazonal da concentração de elementos-traço em sedimentos do sistema estuarino de Santos-Cubatão (SP). Química Nova,29(2), 256–263. https://doi.org/10.1590/S0100-40422006000200016.
Martin, R. (2000). Environmental micropaleontology. New York: Kluwer Academic/Plenum Publishers.
Martins, C. C., Bícego, M. C., Mahiques, M. M., Figueira, R. C. L., Tessler, M. G., & Montone, R. C. (2010). Depositional history of sedimentar linear alkylbenzenes (LABs) in a large South American industrial coastal area (Santos Estuary, Southeastern Brazil). Environmental Pollution,158(11), 3355–3364. https://doi.org/10.1016/j.envpol.2010.07.040.
Martins, V. A., Frontalini, F., Tramonte, K. M., Figueira, R. C. L., Miranda, P., Sequeira, C., et al. (2013). Assessment of health quality of Ria de Aveiro (Portugal): Heavy metals and benthic foraminifera. Marine Pollution Bulletin,70(1–2), 18–33. https://doi.org/10.1016/j.marpolbul.2013.02.003.
Martins, M. V. A., Hohenegger, J., Frontalini, F., Alveirinho Dias, J. M., Geraldes, M. C., & Rocha, F. (2019a). Dissimilarity between living and dead benthic foraminiferal assemblages in the Aveiro Continental Shelf (Portugal). PLoS ONE,14(1), e0209066. https://doi.org/10.1371/journal.pone.0209066.
Martins, M. V. A., Hohenegger, J., Frontalini, F., Laut, L., Miranda, P., Rodrigues, M. A., et al. (2018a). Heterogeneity of sedimentary environments in the Aveiro Lagoon mouth (Portugal): Comparison between the dead and living benthic foraminiferal assemblages. Estuarine, Coastal and Shelf Science,213, 199–216. https://doi.org/10.1016/j.ecss.2018.08.018/.
Martins, M. V. A., Hohenegger, J., Frontalini, F., Miranda, P., Rodrigues, M. A. C., & Dias, J. M. A. (2016a). Comparison between the dead and living benthic foraminiferal assemblages in Aveiro Lagoon (Portugal). Palaeogeography, Palaeoclimatology, Palaeoecology,455(1), 16–32. https://doi.org/10.1016/j.palaeo.2016.05.003.
Martins, M. V. A., Hohenegger, J., Frontalini, F., Sequeira, C., Miranda, P., Rodrigues, M. A. C., et al. (2019b). Foraminifera check list and the main species distribution in the Aveiro Lagoon and Adjacent Continental Shelf (Portugal). Journal of Sedimentary Environments,4(1), 1–52. https://doi.org/10.12957/jse.2019.39308.
Martins, M. V. A., Pereira, E., Figueira, R. C. L., Oliveira, T., Pinto Simon, A. F. S., Terroso, D., et al. (2019c). Impact of eutrophication on benthic foraminifera in Sepetiba Bay (Rio de Janeiro State, SE Brazil). Journal of Sedimentary Environments,4(4), 480–500. https://doi.org/10.12957/jse.2019.47327.
Martins, M. V. A., Pinto, A. F. S., Frontalini, F., Fonseca, M. C. M., Terroso, D. L., Laut, L. L. M., et al. (2016b). Can benthic foraminifera be used as bio-indicators of pollution in areas with a wide range of physicochemical variability? Estuarine, Coastal and Shelf Science,182, 211–225. https://doi.org/10.1016/j.ecss.2016.10.011.
Martins, M. V. A., Rodrigues, R., Pereira, E., Miranda, P., Laut, L., Belart, P., et al. (2018b). Organic pollution in the NE Sector of Guanabara Bay (SE, Brazil). Journal of Sedimentary Environments,3(3), 138–154. https://doi.org/10.12957/jse.2018.37826.
Martins, M. V. A., Soares-Gomes, A., Yamashita, C., Pinto, A. F. S., Frontalini, F., Sequeira, C., et al. (2016c). Similarity between the dead and living foraminiferal assemblages of the NE sector of Guanabara Bay (Brazil). Journal of Sedimentary Environments,1(4), 411–431. https://doi.org/10.12957/jse.2016.26875.
Medeiros, P. M., & Bícego, M. C. (2004). Investigation of natural and anthropogenic hydrocarbon inputs in sediments using geochemical markers. I. Santos, SP—Brazil. Marine Pollution Bulletin,49(9–10), 761–769. https://doi.org/10.1016/j.marpolbul.2004.06.001.
Meyers, P. A. (1997). Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Organic Geochemistry,27, 213–250. https://doi.org/10.1016/S0146-6380(97)00049-1.
Miranda, L. B., Olle, E. D., Bérgamo, A. L., Silva, L. S., & Andutta, F. P. (2012). Circulation and salt intrusion in the Piaçaguera Channel, Santos (SP). Brazilian Journal of Oceanography,60(1), 11–23. https://doi.org/10.1590/S1679-87592012000100002.
Murray, J. W. (1973). Distribution and ecology of living benthonic foraminifrids. London: Heinmann Educational Books.
Murray, J. W. (1982). Benthic foraminifera: The validity of living, dead or total assemblages for the interpretation of palaeoecology. Journal of Micropalaeontology,1, 137–140. https://doi.org/10.1144/jm.1.1.137.
Murray, J. W. (2007). Biodiversity of living benthic foraminifera: how many species are there? Marine Micropaleontology,64(3–4), 163–176. https://doi.org/10.1016/j.marmicro.2007.04.002.
Neff, J. M. (1979). Polycyclic aromatic hydrocarbons in the aquatic environment, sources, fates, and biological effects. London: Applied Science.
Nishigima, F. N., Weber, R. R., & Bícego, M. C. (2001). Aliphatic and aromatic hydrocarbons in sediments of Santos and Cananéia, SP, Brazil. Marine Pollution Bulletin,42(11), 1064–1072. https://doi.org/10.1016/S0025-326X(01)00072-8.
Oen, A. M. P., Cornelissen, G., & Breedveld, G. D. (2006). Relation between PAH and black carbon contents in size fractions of Norwegian harbor sediments. Environmental Pollution,141(2), 370–380. https://doi.org/10.1016/j.envpol.2005.08.033.
Parker, W. C., & Arnold, A. J. (1999). Quantitative methods of data analysis. In B. Sen Gupta (Ed.), Foraminiferal ecology, modern foraminifera (pp. 71–89). Boston: Kluwer Academic.
Pielou, E. C. (1975). Ecological diversity (p. 165). New York: Wiley.
Pregnolato, L. A., Viana, R. A., Passos, C. C., Misailidis, M. L., & Duleba, W. (2018). Ammonia-Elphidium index as a proxy for marine pollution assessment, Northeast Brazil. Journal of Sedimentary Environments,3(3), 176–186. https://doi.org/10.12957/jse.2018.38001.
Priju, C. P., & Narayana, A. C. (2006). Spatial and temporal variability of trace element concentrations in a tropical lagoon, southwest coast of India: Environmental implications. Journal of Coastal Research,39, 1053–1057.
Rengarajan, T., Rajendran, P., Nandakumar, N., Lokeshkumar, B., Rajendran, P., & Nishigaki, I. (2015). Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pacific Journal of Tropical Biomedicine,5(3), 182–189. https://doi.org/10.1016/S2221-1691(15)30003-4.
Roversi, F., Rosman, P. C. C., & Harari, J. (2016). Análise da renovação de águas do Sistema Estuarino de Santos usando modelagem computacional. Ambiente & Água—An Interdisciplinary Journal of Applied Science,11(3), 566–585. https://doi.org/10.4136/ambi-agua.1770.
Samir, A. M., & El-Din, A. B. (2001). Benthic foraminiferal assemblages and morphological abnormalities as pollution proxies in two Egyptian bays. Marine Micropaleontology,41(3–4), 193–227. https://doi.org/10.1016/S0377-8398(00)00061-X.
Santos, F. R., Martins, D. A., Morais, P. C. V., Oliveira, A. H. B., Gama, A. F., Nascimento, R. F., et al. (2019). Influence of anthropogenic activities and risk assessment on protected mangrove forest using traditional and emerging molecular markers (Ceará coast, northeastern Brazil). Science of the Total Environment,656, 877–888. https://doi.org/10.1016/j.scitotenv.2018.11.380.
Schafer, C. T. (1973). Distribution of foraminifera near pollution sources in Chaleur Bay. Water, Air, and Soil Pollution,2, 219–233. https://doi.org/10.1007/BF00655698.
Schönfeld, J., Alve, E., Geslin, E., Jorissen, F., Korsun, S., Spezzaferri, S., et al. (2012). The FOBIMO (FOraminiferal BIo-MOnitoring) initiative: Towards a formalised protocol for benthic foraminiferal monitoring studies. Marine Micropaleontology,94–95, 1–13. https://doi.org/10.1016/j.marmicro.2012.06.001.
Scoot, D. B., & Medioli, F. S. (1980). Living versus total foraminiferal populations: Their relative usefullness in paleoecology. Journal of Paleontology,54, 814–831.
Scoot, D. B., Medioli, F. S., & Schafer, C. T. (2001). Monitoring coastal environments using foraminifera and thecamoebian indicators (p. 177). New York: Cambridge University Press.
Seiler, L. M. N. (2019). Hidrodinâmica e comportamento de variáveis indicadoras da qualidade da água no complexo estuarino de Santos-São Vicente-Bertioga: uma abordagem numérica. Tese de doutorado. Programa de Pós-Graduação em Oceanografia/USP.
Semensatto, D. L., & Dias-Brito, D. (2009). Alternative saline solutions to float foraminiferal tests. Journal of Foraminiferal Research,37(3), 265–269. https://doi.org/10.2113/gsjfr.37.3.265.
Sen Gupta, B. K. (1999). Modern foraminifera. Dordrecht: Kluwer Academic Publisher.
Shannon, C. E. (1948). A mathematical theory of communication. Bell System Technical Journal,27, 379–423.
Silva, D. A. M., & Bícego, M. C. (2010). Polycyclic aromatic hydrocarbons and petroleum biomarkers in São Sebastião Channel, Brazil: Assessment of petroleum contamination. Marine Environmental Research,69(5), 277–286. https://doi.org/10.1016/j.marenvres.2009.11.007.
Simpson, E. H. (1949). Measurement of diversity. Nature,163, 688. https://doi.org/10.1038/163688a0.
Song, Y., Choi, M. S., Lee, J. Y., & Jang, D. J. (2010). Regional background concentrations of heavy metals (Cr Co, Ni, Cu, Zn, Pb) in coastal sediments of the South Sea of Korea. The Science of the total environment,482–483(1), 80–91. https://doi.org/10.1016/j.scitotenv.2014.02.068.
Souza, A. C., Taniguchi, S., Figueira, R. C. L., Montone, R. C., Bícego, M. C., & Martins, C. C. (2018). Historical records and spatial distribution of high hazard PCBs levels in sediments around a large South American industrial coastal area (Santos Estuary, Brazil). Journal of Hazardous Materials,360, 428–435. https://doi.org/10.1016/j.jhazmat.2018.08.041.
Sreenivasulu, G., Praseetha, B. S., Daud, N. R., Varghese, T. I., Prakash, T. N., & Jayaraju, N. (2019). Benthic foraminifera as potential ecological proxies for environmental monitoring in coastal regions: A study on the Beypore estuary, Southwest coast of India. Marine Pollution Bulletin,138, 341–351. https://doi.org/10.1016/j.marpolbul.2018.11.058.
Suguio, K. (1973). Introdução a Sedimentologia. Ed. Edgard Blucher, EDUSP, São Paulo.
Suguio, K., Vieira, E. M., & Barcelos, J. H. (1975). Ecological interpretation of the Foraminifera from the Santos estuary zone, State of São Paulo, Brazil. Anais de Academia Brasileira de Ciências,47, 277–286.
Sutherland, R. A. (2000). Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environmental Geology,39(6), 611–627. https://doi.org/10.1007/s002540050473.
Suzuki, K. N., Sondermann, N. N., Machado, E. C., Machado, W., Bellido, A. V. B., Bellido, L. F., et al. (2016). Metal sorption by sediments from a mangrove reforestation area in Guanabara Bay (SE Brazil) revealed by using radiotracers. Journal of Sedimentary Environments,1(3), 292–296. https://doi.org/10.12957/jse.2016.23707.
Taniguchi, S., Colabuono, F. I., Dias, P. S., Oliveira, R., Fisner, M., Turra, A., et al. (2016). Spatial variability in persistent organic pollutants and polycyclic aromatic hydrocarbons found in beach-stranded pellets along the coast of the state of São Paulo, southeastern Brazil. Marine Pollution Bulletin,106(1–2), 87–94. https://doi.org/10.1016/j.marpolbul.2016.03.024.
Tomlinson, D. L., et al. (1980). Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgolander Meeresuntersuchungen,33, 566–575. https://doi.org/10.1007/BF02414780.
Tommasi, L. R. (1979). Considerações ecológicas sobre o Sistema Estuarino de Santos, São Paulo. Tese de Livre Docência. Universidade de São Paulo, São Paulo.
UNEP. (1992). Determinations of petroleum hydrocarbons in sediments, reference methods for marine pollution studies 20.
USEPA. (1996). Method 3050B. Acid digestion of sediments, sludges, and soils. Revision 2, December 1996.
Vallejo-Toro, P. P., Vásquez Bedoya, L. F., Correa, I. D., Bernal Franco, G. R., Alcántara-Carrió, J., & Palacio Baena, J. A. (2016). Impact of terrestrial mining and intensive agriculture in pollution of estuarine surface sediments: spatial distribution of trace metals in the Gulf of Urabá, Colombia. Marine Pollution Bulletin,111(1–2), 311–320. https://doi.org/10.1016/j.marpolbul.2016.06.093.
Wang, X. C., Zhang, Y. X., & Chen, R. F. (2001). Distribution and partitioning of polycyclic aromatic hydrocarbons (PAHs) in different size fractions in sediments from Boston Harbor, United States. Marine Pollution Bulletin,42(11), 1139–1149. https://doi.org/10.1016/S0025-326X(01)00129-1.
Yanko, V., Ahmad, M., & Kaminski, M. (1998). Morphological deformities of benthic foraminiferal tests in response to pollution by heavy metals: Implications for pollution monitoring. Journal of Foraminiferal Research,28(3), 177–200.
Yanko, V., Arnold, A., & Parker, W. (1999). Effect of marine pollution on benthic foraminifera. In B. K. SenGupta (Ed.), Modern foraminifera (pp. 217–235). Boston: Kluwer Academic.
Yanko, V., Kronfeld, J., & Flexer, A. (1994). Response of benthic foraminifera to various pollution sources: implications for pollution monitoring. Journal of Foraminiferal Research,24, 1–17.
Zanardi, E., Bícego, M. C., Miranda, L. B., & Weber, R. R. (1999). Distribution and origin of hydrocarbons in water and sediment in São Sebastião, SP, Brazil. Marine Pollution Bulletin,38(4), 261–267. https://doi.org/10.1016/S0025-326X(98)90143-6.
Zoller, W. H., Gladney, E. S., Duce, & R. A. (1974). Atmospheric concentrations and sources of trace metals at the south pole. Science183(4121), 198–200.
Acknowledgements
The authors would like to thank FAPESP (São Paulo Research Foundation) for a research grant (2009/01211-0, 2011/50581-4 and 2018/10232-0), the Oceanographic Institute of the University of São Paulo (IOUSP), Laboratory of Environmental Bioindicators (LaBi), Laboratory of Marine Organic Chemistry (LabQOM), Laboratory of Inorganic Marine Chemistry (LaQiMar) and the Coastal Dynamics Laboratory (LDC). The authors would also like to acknowledge the journal editor and an anonymous reviewer for their evaluations and their constructive comments that improved the quality of the manuscript. S.H.M.S, M.C.B, E.S., R.C.L.F. are CNPq research fellows.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. V. Martins
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Damasio, B.V., Timoszczuk, C.T., Kim, B.S.M. et al. Impacts of hydrodynamics and pollutants on foraminiferal fauna distribution in the Santos Estuary (SE Brazil). J. Sediment. Environ. 5, 61–86 (2020). https://doi.org/10.1007/s43217-020-00003-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43217-020-00003-w