Abstract
The study of benthic foraminifera in sediment cores provides the opportunity to recognize environmental changes, including those due to the anthropogenic impact. The integration of these data with chemical-physical parameters provides a comprehensive quality assessment. This research was applied to a sediment core collected in the Augusta bay, where a very large commercial and military harbor and one of the largest petrochemical poles in Europe are present. Inside the petrochemical area also operated, from 1958 to 2003, a chlor-alkali plant with mercury cell technology which caused anthropic contamination of surrounding land and marine areas. The sediment core was collected in front of this plant and characterized for grain size and pollutants directly associated to chlor-alkali activity, such as mercury (Hg), barium (Ba), polychlorobiphenyls (PCBs), and polycyclic aromatic hydrocarbons (PAHs). Composition of foraminiferal assemblages and faunal parameters such as specific diversity, faunal density, abundance of abnormal specimens, and foraminiferal size were investigated as potential indicators of environmental status. Statistical analysis indicated a main common origin for Hg, Ba, and PCBs and the influence of pollutants on species distribution and faunal diversity and density. Exceptionally high Hg concentrations (63–680 mg/kg d.w.) were recorded in the whole core, where the geochronological study attributed the most contaminated levels to the period of maximum activity of the chlor-alkali plant, while a decrease of contamination was recorded after the stop of the activity. Distinct foraminiferal assemblages identified different ecozones along the core, which suggested decreasing anthropogenic impact from the bottom to the top.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last decades, benthic foraminifera have been widely recognized as reliable environmental indicators and applied to studies finalized to the assessment of the environmental status (Hallock et al. 2003; Carnahan et al. 2009; Bouchet et al. 2012). In the context of the Water Framework Directive, Barras et al. (2014) developed a biotic index based on the abundance of living stress-tolerant foraminiferal taxa, able to distinguish between natural and anthropogenic eutrophication. Dolven et al. (2013) demonstrated the applicability of conventional ecological classification systems based on diversity indices to fossil foraminiferal data from sediment cores. In marine coastal areas, affected by strong anthropogenic activities, benthic foraminifera showed change in structure and composition of their assemblage; moreover, the modification of faunal density, the increasing morphological abnormalities, and the reduced size of tests were attributed to increasing pollution levels (Cearreta et al. 2000; Burone et al. 2006; Ferraro et al. 2006; Romano et al. 2008, 2009; Cosentino et al. 2013; Martins et al. 2013; Capotondi et al. 2014). Foraminifera offer a considerable advantage with respect to other groups of benthic organisms, because of their hard shell (called “test”) that leaves a record in sediments and their small size that makes them abundant in small sample volumes, providing a solid database for statistical purposes. These characters make foraminifera suitable indicators for environmental studies in sediment cores, offering the possibility to study temporal changes of ecological conditions. Moreover, as the quality of environmental status must be assessed by comparison with reference conditions, sediment cores offer the advantage to recover them in the ancient levels settled before the human impact, instead of searching a pristine area with the same characteristics of the studied one (Alve et al. 2009). This feature is particularly suitable in areas where time series of biological and chemical data are not available. The study of benthic foraminifera in sediment cores, finalized to the identification of the environmental changes due to the anthropic impact along time, has been applied for several years (Alve 1995), even if it is not still a common practice. The effects of different kinds of pollutants, such as heavy metals, polycyclic aromatic hydrocarbons (PAHs), and polychlorobiphenyls (PCBs), deriving from different human activities, mining, industries, domestic effluents, and harbors were investigated (Cearreta et al. 2002, 2008; Elberling et al. 2003; Hayward et al. 2004; Scott et al. 2005; Alve et al. 2009; Debenay and Fernandez 2009; Dolven et al. 2013).
One of the largest European petrochemical poles, which determined a strong pollution of marine sediments, faces on the Augusta bay where a very large commercial and military harbor is also present. Several studies on the marine sediments were carried out in this area. Results showed high concentration of several pollutants such as mercury (Hg), lead (Pb), copper (Cu), heavy hydrocarbons, hexachlorobenzene (HCB), PAHs, and PCBs in the southern part of the bay, close to the industrial pole and chlor-alkali plant (ICRAM 2008; Romano et al. 2009; Sprovieri et al. 2011). In recent times, benthic foraminifera and pollution levels in marine surface sediments (0–2 cm) of the whole bay were studied. The highest concentrations of Hg and PCBs were found in the southern part of the study area, where benthic foraminifera showed evidence of environmental stress, testified by decreasing of diversity and density (Romano et al. 2013).
Taking into account previous results, a sediment core (AU10) was collected and analyzed for benthic foraminifera, grain size, specific metals (barium (Ba) and Hg), and organic compounds (PAHs and PCBs). The aim of this research was to investigate the environmental changes which occurred in the last decades in the southern Augusta bay, by means of an integrated sedimentological, chemical, and faunal approach. The reconstruction of temporal changes of environmental status, with particular attention to the effects of the industrial activities, was supported by a geochronological study which determined sedimentation rate in the AU10 site (Croudace et al. 2015).
Study area
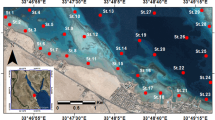
The Augusta area is located in a natural bay, delimited in the northern sector by the town of Augusta and closed to South and East by artificial dams (Fig. 1). Two main inlets allow the connection with the open sea. The coastal area, facing the bay, hosts one of the most important Italian petrochemical centers. Industrial activities started in the late 1950s, and, in the 1960s, they underwent to fast development, becoming the largest industrial petrochemical site in Europe until the 1980s. Successively, some industries were closed, but several oil refineries and petrochemical industries are still active, with strong consequences on the terrestrial and marine environment. Sediment contamination has become the most important element of environmental concern since the 1970s, when high content of pollutants of industrial origin, such as hydrocarbons and heavy metals, was found in the marine sediments (Sciacca and Fallico 1978). Recent studies, finalized to recognize extent of contamination and vertical/spatial distribution for heavy metals, PAHs, and PCBs, highlighted exceptionally high concentrations of Hg (up to 198 mg/kg d.w. in the surface samples and to 728 mg/kg d.w. in the deeper ones), associated to very high levels of Ba, HCB, and mostly PCBs (up to 0.83 mg/kg d.w. in the surface samples and to 14 mg/kg d.w. in the deeper ones) in the southern coastal area (ICRAM 2008; Romano et al. 2009, 2013; Croudace et al. 2015). Such contamination was mainly attributed to the activity of a chlor-alkali plant using a mercury cell technology, which was established in 1958 and it was operative until 2003. Mercury cells were finally removed in 2005. The area was also demonstrated to be an important contributor of Hg in the Mediterranean Sea basin, with an Hg output of 0.162 kmol/year to coastal and offshore waters (Sprovieri et al. 2011).
Material and methods
Sampling
Core AU10 was collected in July 2008, in the southern part of the harbor not affected by previous dredging activities, in front of the industrial area, by means of a gravity corer (mod. SW104) at 10 m water depth (Fig. 1). The total sediment recovery was of 127 cm and 20 levels with a thickness of 3 cm were subsampled. A continuous sampling was carried out in the first 30 cm of the core to guarantee the highest resolution of data for more recent times, and one level each 9 cm below this depth was collected to detect the main environmental changes. Sediment samples were analyzed for grain size, Ba, Hg, PCBs, PAHs, and benthic foraminifera. In this paper, samples were labeled progressively from 1 to 20, from core top to the bottom (Table. 1).
Grain size analysis
Each sample was treated twice with hydrogen peroxide (30 %) and distilled water solution in proportion 1:3 for 24/48 h at room temperature and then washed twice with natural water. Then, samples were wet separated into two grain size fractions (>63 and <63 μm) which were oven dried and weighed. The coarser fraction (>63 μm) was dry sieved by means of ASTM series sieves with meshes ranging from −1 to +4 ϕ, and intervals of 0.5 ϕ. The fine fraction (<63 μm) was split into subsamples representative of the whole sample, dispersed in a solution of distilled water and sodium hexametaphosphate (0.05 %) at the rate of 2.5 g of sample for 80 mL of solution, and then analyzed by means of a laser granulometer (mod. Sympatec Helos).
Ba and Hg analysis
Metal dissolution was conducted using microwave-assisted digestion (Milestone MLS Ethos TC high performance microwave digestion unit). About 0.5 g of oven-dried (48 h, 35 °C) sediment was digested in Teflon bombs with 3 mL of HNO3 and 9 mL of HCl superpure (37 %). After cooling, the solution was diluted using distilled water at 50 mL volume. Barium was measured with coupled emission plasma ICP-OES (Liberty AX, Varian). For Hg analysis a direct mercury analyzer (DMA-80, FKV) was used. The DMA-80 is a direct atomic absorption instrument with gold amalgamation for direct mercury determination in solid and liquid samples without the need of sample chemical pre-treatment (EPA Method 7473). The Hg analysis was performed using sediment samples dried at 35 °C for 48 h. To guarantee quality assurance/quality control (QA/QC), quality parameters such as accuracy, quantification limit, and repeatability were estimated. The accuracy for total content of metals was evaluated using certified reference materials (PACS-2 and MESS); Ba and Hg showed a recovery higher than 90 %. Quantification limits were 0.0005 mg/kg for Hg and 1.5 mg/kg for Ba. All of the samples (and reference materials) were run in triplicate.
PCBs and PAH analysis
About 4 g for each sample was weighted into a Falcon tube, and a 5-mL aliquot of solvent mixture (3/2 v/v methanol/acetone) was added and put into an ultrasonic bath (at 30 °C). The vessel was centrifuged for 15 min at 2000 rpm and an aliquot of the supernatant was filtered and transferred into a 2-mL amber glass vial. The 15 PAHs were determined using an Agilent 1100 HPLC-FLD system. A water/acetonitrile chromatographic gradient was employed on a LC-PAH Supelcosil column (Supelco) kept at 30 °C. For quantification of the 15 PAHs, a six-level external standard calibration was realized spanning in the range of 2–500 ng/mL. The results were expressed in milligram per kilogram on the dried weight of the sample. The quantification limit is 0.001 mg/kg. For the determination of PCBs, the extraction was performed by pressurized fluid extraction (PLE) using a Dionex ASE 200 instrument and a 60:40 petroleum ether/dichloromethane solvent mixture. A 5 g sample aliquot was loaded in extraction cells that contained a 2.5 g Florisil bed for performing a preliminary partial purification. Sulfur was removed by elution of the PLE extract on a polypropylene cartridge containing activated copper powder on a sodium sulfate bed. The purified extract was then transferred to a 2 mL autosampler vial. The gas chromatographic analysis was performed on a dual column–dual injector–dual detector system (Agilent 6890 N) with electron capture detection (lECD), an Agilent XLB 25 m 0.20 mm, 0.33 lm, and an Agilent DB-35MS 30 m 0.32, 0.25 lm. The quantification was performed by external standard using a six-point calibration curve (from 1 to 100 ng/mL). The quantification limit is 0.0001 mg/kg. Quality control procedures for PAH and PCB analyses included, in each batch of 20 samples processed, the analysis of a method blank, a spiked blank of two sample replicates, and the recovery check for the surrogates in each sample. A certified reference material was analyzed every five batches of samples.
Benthic foraminifera analysis
Samples for foraminiferal study were wet-sieved on 63 and 125 μm sieves to eliminate mud particles and obtain two separate size fractions (63 μm < x < 125 μm; x > 125 μm), and then oven-dried at 40 °C. The quantitative analysis was carried out separately on the two fractions, and all of the foraminifera present in the sample (or in a representative split) were counted and classified. This procedure was applied because the abundance of small-sized specimens was recorded by previous studies (Romano et al. 2009, 2013). A preliminary check of the two fractions confirmed this condition, and, consequently, the count of the >63 μm fraction, obtained by the sum of the counts of the above two fractions, was considered for this study. In order to avoid, as much as possible, to include allochtonous specimens in the count, only well-preserved tests were picked, counted, and classified. Classification at the genus level was made according to Loeblich and Tappan (1987), while species were determined according to several studies relative to the Mediterranean area (Jorissen 1988; Cimerman and Langer 1991; Sgarrella and Moncharmont-Zei 1993) and to the World Modern Foraminifera Database (Hayward et al. 2015). The foraminiferal number (FN) was calculated as the number of specimens per gram of dry sediment (specimens/g). The species diversity was measured considering the Shannon H index (Shannon 1948) and α-index (Fisher et al. 1943), which were calculated by using the statistical package PAlaeontological STatistics—PAST version 2.17 (Hammer et al. 2001; Hammer and Harper 2006). To quantify the abundance of small foraminifera, with a size comprised between 63 and 125 μm with respect to the larger ones, a new index, the foraminiferal size index (FSI), was devised and applied for this study. FSI = N 63–125/N >125 (N 63–125 = number of specimens with a size between 63 and 125 μm; N >125 = number of specimens with a size above 125 μm).
Statistical analysis
Statistical analysis was carried out by means of the statistical package PAlaeontological STatistics—PAST version 2.17 (Hammer et al. 2001; Hammer and Harper 2006). Chemical and sedimentological data were processed by means of a principal component analysis (PCA) as a descriptive method to reduce data variability (Farcomeni and Greco 2015). Due to extremely different range scales of the considered variables, data were standardized by means of z-score method. Hierarchical cluster analysis (HCA) and PCA were applied to a matrix including the relative abundance of species to highlight different species distribution among samples. Only the 18 commonly occurring species (i.e., more abundant than 5 % in at least one sample) were considered to avoid the noise determined by rare species (Samir et al. 2003). For statistical purposes, only samples with more than 50 specimens were considered to offer a statistically solid basis (Barras et al. 2014). The Ward’s linkage method was adopted for HCA because it produces well-separate clusters, with the squared Euclidean distance (Parker and Arnold 1999). Canonical correspondence analysis (CCA) was carried out on a matrix including relative abundance of the commonly occurring species and the abiotic parameters (grain size and chemical data) (Ferraro et al. 2009). Correlation between faunal and abiotic parameters was checked by means of the nonparametric Spearman’s index (ρ). The ρ index describes how well the relationship between two variables can be described by a monotonic, not necessarily linear, function (Spearman 1904).
Results
Grain size
The relative abundance of sediment fractions (sand, silt, and clay) along core depth does not show dramatic changes and, especially clay, appears rather constant along core depth, while moderate variability of sediment texture is mainly due to changes of relative abundance of sand and silt (Fig. 2). Silt resulted, except for one level (90–93 cm), the most abundant fraction in the core with a mean value of 53 % and low variability. Clay and sand show similar mean values (22 and 25 %, respectively) but while the first one has a moderate variability, the second one demonstrates a wide range of values from 5 to 52 %. On the whole, the core shows a coarser sediment in the range between 102 and 81 cm, and at 57 cm; the upper part of the core (0–15 cm) also shows sand percentage above 25 %.
Chemical data
From the analyses of chemical concentration profile (Fig. 3), it is evident that, in general, Hg and Ba are characterized by similar profiles with higher values in the lower part of the core and a decreasing pattern in the upper one, with just a difference in the sector below 100 cm depth. Mercury profile shows several peaks along the core with concentration ranging from 680 mg/kg d.w., in the middle section of the core, to 63 mg/kg d.w. in the top level (Fig. 3). Barium ranges between 5708 and 680 mg/kg d.w. in the top level. PCB and PAH profiles show two important peaks at around 75 cm (22.9 mg/kg d.w.) and 48 cm (83.3 mg/kg d.w.), respectively, while all the other samples show levels ranging from 1.6 to 9.1 mg/kg d.w. for PCBs and between 4.6 and 32.8 mg/kg d.w. for PAH concentrations. The comparison of metal concentration profiles with clay content suggests that no correlation exists between clay percentages and pollutant levels, and then, their changes are attributable to variation of anthropogenic contribution.
Plot along a double scale, core depth, and age of PAH, PCB, Ba, and Hg concentrations and foraminiferal number (FN). The distribution along the core of ecological zones is also given. The chronological scale of the y-axis is obtained applying the sedimentation rate determined by Croudace et al. (2015)
Benthic foraminifera
Benthic foraminifera were found in all of the analyzed samples with the exception of sample 16 which was barren; in the samples 14, 15, and 17, less than 50 specimens were found. For this reason, these four samples were not considered for statistical purposes. A total of 121 taxa were recognized, only 3 of these belonging to the order of Textulariida, 37 to Miliolida, and 81 to Rotaliida. On the whole, taking into account the absolute abundance, the dominant species resulted to be Miliolinella subrotunda (675 specimens), followed by Bolivina seminuda (385 specimens) and Lobatula lobatula (324 specimens). Other abundant taxa are Rosalina bradyi and R. floridana (241 and 225 specimens, respectively), Quinqueloculina stelligera (236 specimens), and Cornuspira involvens (223 specimens). Morphological abnormalities were extremely rare, never exceeding the natural threshold of 1 % for unpolluted environments (Stouff et al. 1999; Morvan et al. 2004). For this reason, the abundance of abnormal specimens was not discussed in this paper and not included in statistical analysis.
In order to recognize groups of samples with similar faunal content, a Q-mode HCA on the relative abundance of the 18 commonly occurring species was carried out (Fig. 4). It identified three distinct clusters, each one including samples with similar foraminiferal content, regarded as an assemblage, which are described below. The median values of species percentages and faunal parameters for each cluster were calculated and reported in Table 2 and Fig. 5.
Box plot (maximum, minimum, median, and 25th and 75th percentile values) of faunal parameters in the three clusters (A–C): species diversity (α-index and H index), foraminiferal number (FN) and foraminiferal size index (FSI). A clear increasing trend is recognizable from cluster A to cluster C only for H-index and FN
Cluster A—Rosalina spp., M. subrotunda, and L. lobatula assemblage
This assemblage was recognized in all of the samples below the barren interval (samples 18, 19, and 20) and in three samples above it (samples 11, 12, and 13). Rosalina bradyi and R. floridana, with a total median abundance of 10.6 %, M. subrotunda with 9.4 %, and L. lobatula with 7.3 % strongly characterize this assemblage.
These species are typically epiphytic but may also live in detritic sediment, which is very common in the Augusta bay, having an epifaunal lifestyle (Spezzaferri et al. 2000; Murray 2006). In particular, Celia Magno et al. (2012) found in the Augusta bay a specific preference for medium sand to gravel fractions of L. lobatula and R. bradyi. An ubiquitous behavior was found for M. subrotunda, by Sgarrella and Moncharmont Zei (1993), who found it from very shallow to bathyal environment.
This assemblage is characterized by rather high diversity, with a median of 14.9 and 3.3 for α and H, respectively. Conversely, foraminiferal density, represented by the FN, may be considered very low, with a median of 186 specimens/g. The median FSI of 3.7 indicates the strong prevalence of small-sized foraminifera.
Cluster B—M. subrotunda and Spirillina vivipara assemblage
This assemblage was recognized in the intermediate part of the core, in the samples from 6 to 10, and also in sample 3. The assemblage is strongly characterized by M. subrotunda (14.5 %) associated with S. vivipara (10.0 %). The last one is an epifaunal taxon clinging hard substrates and preferring detritic bottom (Murray 2006).
Other common species are B. seminuda (6.3 %) and C. involvens (5.1 %). The first one is an infaunal taxon which was rather common in the Adriatic Sea, in correspondence of the fine sediments due to the contributions of the Po River, where it demonstrates a rather high degree of opportunism (Barmawidjaja et al. 1995). However, Jorissen (1988) found that it benefits of high food supply but does not tolerate disoxia. The second one is an infaunal ubiquitous taxon recorded from shallow waters down to bathyal depth (Sgarrella and Moncharmont-Zei 1993; Murray 2003). It demonstrated to be a stress-tolerant species in hydrocarbon seep environment, because it was found abundant in bacterial mat and particularly in one sample as dominant species of the living assemblage (Lobegeier and Sen Gupta 2008). In recent surface sediment samples of the Augusta bay, this species was classified as a pollution-tolerant indicator because it showed positive correlation with Hg, PAH, and PCB concentrations (Romano et al. 2009).
Species diversity is very similar to that recorded in cluster A, with median α and H of 14.4 and 3.4, respectively, while the median FN is 483 specimens/g, showing a significant increase with respect to cluster A. The highest rate of small-sized (63–125 μm) foraminifera, indicated by a median FSI of 7.2, was recorded in this assemblage.
Cluster C—B. seminuda and M. subrotunda assemblage
This assemblage was recognized in all of the samples of the surface sector of the core (samples 1, 2, 4, and 5), with the exception of sample 3 which was included in cluster B. It is not very dissimilar from the assemblage corresponding to cluster B about species composition, but it shows significant differences as regards the structure. The two main species show similar abundance with median of 9.2 % for B. seminuda and 8.8 % for M. subrotunda. Other common taxa are Ammonia tepida (5.2 %), nearly absent in the other assemblages, and L. lobatula (5.2 %).
Ammonia tepida is a typical euryhaline and stress-tolerant taxon, very common in lagoonal environmental setting, also in case of heavy metal pollution (Carboni et al. 2009; Coccioni et al. 2009; Frontalini et al. 2009).
Species diversity appears slightly higher than in other assemblages, with median α and H of 16.5 and 3.5, respectively. In this assemblage, the median FSI is 2.9 to indicate that, although the prevalence of small-sized foraminifera is still very clear, a significant increase of foraminifera larger than 125 μm is observed. Moreover, a high foraminiferal density (median FN of 1774 specimens/g) characterizes this assemblage.
Discussion
To assess the extent of contamination in the sediment core, the mean crustal concentrations for Hg and Ba (0.08 and 570 mg/kg, respectively) were considered as reference values (Li and Schoonmaker 2005) and also used for determination of enrichment factors (EF = Metalsample/Metalbackground). They range from 786 to 8501 for Hg and from 1.2 to 10 for Ba, highlighting severe levels of contamination, especially for the first one.
Considering the reference values and taking into account the concentrations at the core bottom, it is clear that core AU10 does not include levels of pre-industrial age (earlier than 1950s). A sedimentation rate of 26 mm/year was attributed to the core taking into account the geochronological study based on 137Cs profiles by Croudace et al. (2015). The authors carried out core dating using both 137Cs and 210Pb, but the complex interplay between sediment composition and activity made 210Pb dating straightforward application somewhat difficult. The 137Cs dating method uses a set of indicator features, viz the first appearance of 137Cs (~1954) and the 1963 “bomb” maximum (Ritchie and McHenry 1990; Appleby and Oldfield 1992). The possible downward migration or mixing of 137Cs has a potential impact when using the first appearance criterion only. In some parts of Europe, an additional peak corresponding to the Chernobyl event (1986) is also seen (e.g., Miller et al. 2014), but the impact of Chernobyl is generally claimed to be low in Sicily, but is not necessarily absent (Cundy et al. 1998).
Applying this sedimentation rate to the concentration profiles, it may be observed that Hg and Ba maintain very high levels through the 1960s, 1970s, and 1980s; only from the late 1980s a moderate decrease occurred (Fig. 3). But only at the beginning of the twenty-first century, in correspondence of the closing of the chlor-alkali plant in 2003, the most significant decrease was recorded (Croudace et al. 2015). The difference between Ba and Hg is relative to different concentrations found in the upper part of the core, which in the first one are close to the reference values, while in the second one are still extremely high. For this reason, Hg may be clearly considered as an element of high environmental concern in the study area. As regards organic compounds, concentrations exceeding Environmental Quality Standards (EQS) for PAHs and PCBs, which are 8 and 800 μg/kg d.w., respectively (Italian Decree 56/2009), were recorded along all the core. Because EQS are recognized taking into account also the ecotoxicological responses, representing the threshold below which adverse biotic effects are negligible, the very high recorded concentrations are expected to influence negatively also benthic foraminifera.
A PCA applied to grain size and chemical data highlights the high correlation of Hg, Ba, and PCBs among themselves and to the levels of the lower part of the core (Fig. 6) showing a common source for these contaminants. Also PAHs appear correlated to Hg, Ba, and PCBs and particularly characterize samples 12 and 14. All these pollutants are not correlated to fine fractions (silt and clay). The scatter plot clearly shows up the most superficial samples as those less affected by contamination. These results confirm the findings of Romano et al. (2013), who studied geochemistry and benthic foraminifera in surface sediments (0–2 cm) of the whole Augusta Harbor, taking into account the distributional patterns of Hg and PCBs. They identified a main source of these pollutants located along the coast of the southern part of the harbor, which was found to be the most contaminated sector in the area. Contamination due to Hg, Ba, PCBs, and PAHs, which is commonly linked to chlor-alkali plant (Kannan et al. 1997; Sprovieri et al. 2011; Brinkmann et al. 2014), was attributed to the one present in the Augusta Harbor using Hg cathodes, which operated for more than four decades. In these sites, the Hg contamination is generally due to the atmospheric deposition, as well as to the historical disposal of graphite sludge deriving from the use of graphite anodes. Hg contamination can persist in sediments over a long period after the end of the industrial activity, with the risk of transformation in the methylated form, methyl-mercury (MeHg), which accumulates in biota and is biomagnified in aquatic food webs (Ullrich et al. 2007). Methyl-mercury was generally recorded close to chlor-alkali plants (Piani et al. 2005; Raldúa et al. 2007; Covelli et al. 2010), and, in particular, its presence in the Augusta Harbor was confirmed by Ausili et al. (2008) who found high concentrations in mussels and fishes. Also Ba enters in the chlor-alkali industrial process because Ba salts are used in the process of brine purification with consequent production of Ba sulfate waste (Brinkmann et al. 2014).
The foraminiferal data are used to assess the environmental status by means of quantitative parameters which can be modified by the anthropogenic impact. The presence of diversified foraminiferal assemblages in such contaminated sediments confirms their nature of suitable environmental indicators in heavily polluted areas. The high tolerance of foraminifera to polluted environments is due to their effective biological defense mechanisms against pollutants (Yanko et al. 1994a).
The homogeneous distribution of cluster assemblages along the core and the absence of foraminifera in correspondence of the most contaminated levels indicate that benthic foraminifera are not affected by significant transport. Consequently, the different assemblages are reliable descriptors of different environmental characteristics along the core. A PCA, carried out on relative abundance of foraminiferal species, led to the same clear grouping obtained by cluster analysis (Fig. 7). Samples belonging to cluster A show, respectively, negative values for the first component and positive for the second one; they are associated to the epifaunal/epiphytic species L. lobatula, Rosalina spp., and Asterigerinata mamilla. Samples of cluster B load on the positive side of the first component and show positive values for the second one; they are strongly characterized by epiphytic/epifaunal species such as S. vivipara and M. subrotunda and by the infaunal C. involvens. Finally, samples of cluster C load on the negative side of the second axis and are characterized by infaunal species such as B. seminuda, Bulimina marginata, Haynesina depressula, and Ammonia spp.
PCA applied to the relative abundance of the 18 commonly occurring foraminiferal species. Components 1 and 2 account for the 30 and 27 % of variance, respectively. In the following, full species names are reported. A_park: Ammonia parkinsoniana; A_tepi: Ammonia tepida; A_mami: Asterigerinata mamilla; B_aena: Bolivina aenariensis; B_earl: Bolivina earlandi; B_marg: Bulimina marginata; C_invo: Cornuspira involvens; E_vitr: Epistominella vitrea; H_depr: Haynesina depressula; L_loba: Lobatula lobatula; M_subr: Miliolinella subrotunda; P_corr: Patellina corrugata; Q_lata: Quinqueloculina lata; Q_stel: Quinqueloculina stelligera; Ros_spp.; Rosalina spp.; S_cost: Sigmoilinita costata; S_vivi: Spirillina vivipara
The components 1 and 2 of PCA, which account for 29 and 25 % of variance, respectively, could be interpreted as the environmental factors determining distribution of species. The Spearman’s index showed that no significant correlation exists between PC1 and the analyzed abiotic parameters. The two groups of species on the two sides of component 1 may be distinguished for the higher degree of opportunism which characterizes species of cluster B. However, taking into account that abiotic parameters influencing foraminifera distribution, such as oxygen or nutrients, were not considered in the present research, a confident interpretation of PC1 is not possible. Differently, the plot of PCA clearly showed that samples with the highest contaminant concentrations, together with Rosalina spp., L. lobatula, and A. mamilla, load on the positive side of the second axis, while samples with the lowest contamination levels, together with B. seminuda, A. tepida, and H. depressula, load on the negative side. Therefore, the component 2 is attributable to contamination level and the taxa attributable to less contaminated sediments. To confirm this, PC2 was found to be positively correlated to Ba, Hg, PCBs, and PAHs (ρ 0.882, 0.891, 0.768, and 0.856, respectively). This means that contamination, on the whole, is one of the main factors determining species distribution, and, consequently, it strongly conditions assemblage composition.
In order to investigate the effect of the studied abiotic sediment parameters (grain size, Ba, Hg, PCBs, and PAHs) on composition of benthic assemblages, a CCA was carried out (Fig. 8). Species more abundant in cluster A, such as Rosalina spp., L. lobatula, and A. mamilla, plot on the negative side of the first axis, showing positive correlation with Hg, Ba, PCBs, and PAHs. Consequently, because they have been found in a highly polluted environment, such as Augusta bay was, they may be considered as tolerant species with respect to environmental stress due to industrial contamination. Conversely, species more abundant in cluster C, such as B. seminuda, B. marginata, and A. tepida, load on the positive side of the first axis demonstrating negative correlation with chemical parameters and revealing a pollution-sensitive character. Differently from the present results, A. tepida was usually considered as a resistant opportunistic species in large, polluted harbors (Vilela et al. 2004; Burone et al. 2006; Ferraro et al. 2006). Moreover, species more abundant in cluster B, such as M. subrotunda, C. involvens, and S. vivipara, plot along the second axis showing to be independent from contaminant levels. Although M. subrotunda does not show positive correlation with contaminant concentrations, the high abundance of this species in the contaminated core sediments indicates a particular ability of this species to adapt to stressed conditions due to chemical pollution. This adaptive ability is not surprising because it is a typical characteristic of ubiquitous taxa. Miliolinella subrotunda was abundant in front of the Bagnoli industrial area (Naples), in heavy metal-polluted muddy sands, showing the ability to tolerate anthropogenic stress (Bergamin et al. 2003; Romano et al. 2008; 2009). Although rotaliids were mostly recognized as stress-tolerant, Martins et al. (2011) found that an epifaunal miliolid, such as Quinqueloculina seminulum, showed more tolerance to bioavailable heavy metals with respect to the infaunal rotaliids, Haynesina germanica or A. tepida, which are traditionally considered as pollution-tolerant taxa.
CCA applied on a matrix including relative abundance of commonly occurring species, faunal parameter, and abiotic sediment parameters (grain size fractions, Hg, Ba, PCBs, and PAHs). In the following, full species names are reported. A_park: Ammonia parkinsoniana; A_tepi: Ammonia tepida; A_mami: Asterigerinata mamilla; B_aena: Bolivina aenariensis; B_earl: Bolivina earlandi; B_marg: Bulimina marginata; C_invo: Cornuspira involvens; E_vitr: Epistominella vitrea; H_depr: Haynesina depressula; L_loba: Lobatula lobatula; M_subr: Miliolinella subrotunda; P_corr: Patellina corrugata; Q_lata: Quinqueloculina lata; Q_stel: Quinqueloculina stelligera; Ros_spp.; Rosalina spp.; S_cost: Sigmoilinita costata; S_vivi: Spirillina vivipara
The determination of Spearman’s ρ revealed the negative correlation of FN with Ba, Hg, PCBs, and PAHs (ρ −0.835, −0.935, −0.771, and 0.847, respectively). The H index was also found to be negatively correlated to Hg (ρ −0.632), while the other considered diversity index, α-Fisher index, did not show any correlation. From these results, it may be deduced that both diversity and density are negatively conditioned by increasing contamination levels. This deduction is enforced by the fact that in samples 14, 15, 16, and 17, affected by the highest contamination of Hg and PCBs, foraminifera are totally or nearly absent. Consequently, FN may be considered as a reliable descriptor of environmental status. As regards the H index, in spite of the negative correlation with Hg concentration, the scarce value variability recorded in the whole core (3.1–3.5) does not allow to recognize notable indications of changes of contamination levels. This may be due to the lack of reference conditions in the core, and, although there is the considerable decrease of contaminant concentrations in the upper 20 cm, Hg levels are still very high. Dolven et al. (2013) found that, regardless of the index chosen as metrics for assessment of quality status, it is important to consider the temporal pattern and scale of change of the diversity index within a core. In the study of benthic foraminifera from surface sediments, collected in the Augusta Harbor, the FN was found negatively correlated to contaminants, but also the α-index showed this characteristic (Romano et al. 2013). From the present study, it may be deduced that the use of more than one diversity index, having different characteristics, could be useful where diversity may be a potential indicator of environmental status.
The presence of small-sized foraminiferal faunas was recorded in several areas, affected by chemical pollution due to high anthropogenic impact (Yanko et al. 1994b; Samir and El Din 2001; Romano et al. 2008), and this feature was also recognized by earlier studies on the Augusta Harbor (Romano et al. 2009).
In the present study, the strong prevalence of foraminifera smaller than 125 μm is testified by FSI values up to 13 and for this reason, the fraction >63 μm was used for paleoenvironmental assessment. However, FSI was not found to be correlated to contaminant concentrations; consequently, the issue of foraminiferal size in relation to contamination levels should be better investigated in the future.
The integration of faunal and chemical data, carried out by means of CCA (Fig. 8), allows to identify three core intervals with homogeneous abiotic and biotic features, interpreted as ecozone. The first one (ecozone A) includes samples from the core bottom to 100 cm and from 36 to 57 cm, across an interval totally or nearly barren (Fig. 3). On the whole, this core interval corresponds to the period ranging from 1960s to early 1990s and is characterized by the highest contamination levels. The foraminiferal assemblage shows extremely low foraminiferal density and the lowest species diversity, to indicate high environmental stress. Epifaunal/epiphytic taxa more abundant in these samples, and considered as pollution-tolerant indicators, may survive in spite of such adverse conditions because of several defense mechanisms recognized by Bresler and Yanko (1995). The barren interval may be attributable to a period, from early 1970s and early 1980s, during which the highest production rates without any remediation policy were achieved by the industrial site and, particularly, by the chlor-alkali plant. The re-appearance of foraminifera above this interval corresponds to the late 1980s, probably due to the reduction of the most polluting activities.
Ecozone B is recognized in the core interval ranging from 15 to 30 cm and corresponds to the last decade of activity of the chlor-alkali plant (from late 1990s to early 2000s), when epifaunal ubiquitous taxa tend to be more abundant than below. The most remarkable character of this ecozone is a strong increase of the foraminiferal density, associated to a significant decrease of contaminant concentrations, to indicate a certain amelioration of the ecological status.
Finally, ecozone C corresponds to the upper 15 cm of the core, and it is attributable to the years from 2002 to 2008. In these levels, the further decrease of contamination levels is recorded, due to the enclosure of chlor-alkali plant and removal of Hg cathodes, where foraminiferal density increases again. The infaunal taxa that characterize these sediments may be considered as pollution sensitive because they negatively correlated to contaminant concentrations. However, they may not be considered as markers of unpolluted sites because these sediments still have high Hg concentration (63 mg/kg d.w.) corresponding to an EF of 786.
Conclusions
Benthic foraminifera are good proxies for environmental assessment of heavily polluted areas because of their defense mechanisms against contaminants which allow their presence where other benthic groups may not live for adverse conditions. For this reason, they were used as environmental indicators in the integrated study of faunal, textural, and chemical characteristics of a sediment core collected in the Augusta bay, close to a chlor-alkali plant which was operative from 1958 to 2003. The aim of the study was to reconstruct the evolution of the environmental status along time to assess the impact of industrial activities on biota. Exceptionally high contamination levels mostly due to Hg, but also to Ba and PCBs, were recorded in all of the levels of the sediment core. Sedimentation rates obtained by a previous geochronological study allowed to attribute changes in contamination levels to known significant events of the industrial development. A good correspondence was found between changes of contamination levels and changes of composition and structure of foraminiferal assemblages. Faunal density and species diversity were found inversely correlated to contaminant concentration and were considered to be reliable descriptors of environmental status, although the first one was found to be a more sensitive tool to detect changes of anthropogenic impact. Statistical analysis indicated Hg concentration as a main factor determining changes in faunal composition. Benthic foraminifera allowed to identify three distinct ecozones affected by different decreasing degrees of environmental impact, from the core bottom to the top. Very poor foraminiferal assemblage, dominated by epifaunal taxa, was associated with the most contaminated sediments to identify a bad environmental status in earlier times. In particular, an azoic interval was associated to the most contaminated levels corresponding to the decade from early 1970s to early 1980s. Successively, the site was re-populated by the same foraminiferal assemblage present before the azoic interval due to a slight decrease of contamination levels. An environmental amelioration (from late 1990s to early 2000s) was marked by a foraminifera assemblage more rich in terms of absolute abundance and characterized by higher abundance of opportunistic taxa. Finally, a rich and more diversified assemblage was found in less contaminated levels from early 2000s, indicating a remarkable environmental amelioration attributable to the enclosure of the chlor-alkali plant and the removal of Hg cathodes. From this research emerges that the study of benthic foraminifera in sediment cores is a reliable and powerful tool for the reconstruction of past environmental status of areas affected by the legacy of industrial contamination.
References
Alve A (1995) Benthic foraminifera in sediment cores reflecting heavy metal pollution in Sørfjord, Western Norway. J Foraminifer Res 21(1):1–19
Alve A, Lepland A, Magnusson J, Backer-Owe K (2009) Monitoring strategies for re-establishment of ecological reference conditions: possibilities and limitations. Mar Pollut Bull 59:297–310
Appleby P, Oldfield F (1992) Application of lead-210 to sedimentation studies. In: Ivanovich M, Harmon RS (eds) Uranium-series disequilibrium: applications to earth, marine, and environmental science. Oxford University Press, pp 731–738
Ausili A, Gabellini M, Cammarata G, Fattorini D, Benedetti M, Pisanelli B, Gorbi S, Regoli F (2008) Ecotoxicological and human health risk in a petrochemical district of southern Italy. Mar Environ Res 66:215–217
Barmawidjaja DM, van der Zwaan GJ, Jorissen FJ, Puskaric S (1995) 150 years of eutrophication in the northern Adriatic Sea: evidence from a benthic foraminiferal record. Mar Geol 122:367–384
Barras C, Jorissen FJ, Labrune C, Andral B, Boissery P (2014) Live benthic foraminiferal faunas from the French Mediterranean coast: towards a new biotic index of environmental quality. Ecol Indic 36:719–743
Bergamin L, Romano E, Gabellini M, Ausili A, Carboni MG (2003) Chemical-physical and ecological characterisation in the environmental project of a polluted coastal area: the Bagnoli case study. Mediterranean Marine Science 4(2):5–20
Bouchet VMP, Alve E, Rygg B, Telford RJ (2012) Benthic foraminifera provide a promising tool for ecological quality assessment of marine waters. Ecol Indic 23:66–75
Bresler V, Yanko V (1995) Chemical ecology: a new approach to the study of living benthic epiphytic foraminifera. J Foraminif Res 25(3):267–279
Brinkmann T, Santonja GG, Schorcht F, Roudier S, Sancho LD (2014) Best Available Techniques (BAT) reference document for the production of chlor-alkali. Industrial Emissions Directive 2010/75/EU Integrated Pollution Prevention and control Report EUR 26844 EN. doi:10.2791/13138.
Burone L, Venturini N, Sprechmann P, Valente P, Muniz P (2006) Foraminiferal responses to polluted sediments in the Montevideo coastal zone, Uruguay. Mar Pollut Bull 52:61–73
Capotondi L, Bergami C, Orsini G, Ravaioli M, Colantoni P, Galeotti S (2014) Benthic foraminifera for environmental monitoring: a case study in the central Adriatic continental shelf. Environ Sci Pollut Res 22:6034–6049
Carboni MG, Succi MC, Bergamin L, Di Bella L, Frezza V, Landini B (2009) Benthic foraminifera from two coastal lakes of southern Latium (Italy). Preliminary evaluation of environmental quality. Mar Pollut Bull 59:268–280
Carnahan EA, Hoare AM, Hallock P, Lidz BH, Reich CD (2009) Foraminiferal assemblages in Biscayne Bay, Florida, USA: responses to urban and agricultural influence in a subtropical estuary. Mar Pollut Bull 59:221–233
Cearreta A, Irabien MJ, Leorri E, Yusta I, Croudace IW, Cundy AB (2000) Recent anthropogenic impacts on the Bilbao Estuary, Northern Spain: geochemical and microfaunal evidence. Estuar Coast Shelf Sci 50:571–592
Cearreta A, Irabien MJ, Leorri E, Yusta I, Quintanilla A, Zabaleta A (2002) Environmental transformation of the Bilbao Estuary, N. Spain: microfaunal and geochemical proxies in the recent sedimentary record. Mar Pollut Bull 44:487–503
Cearreta A, Alday M, Irabien MJ, Etxebarria N, Soto J (2008) Modern conditions and recent environmental development of the Muskiz estuary: historical disturbance by the largest oil refinery of Spain. J Iber Geol 34:191–213
Celia Magno M, Bergamin L, Finoia MG, Pierfranceschi G, Venti F, Romano E (2012) Correlation between textural characteristics of marine sediments and benthic foraminifera in highly anthropogenically-altered coastal areas. Mar Geol 315–318:143–161
Cimerman F, Langer M (1991) Mediterranean Foraminifera. Slovenska Akademija Znanosti in Umetnosti, Academia Scientiarum Artium Slovenica, Classis IV, Historia Naturalia 30, Ljubliana.
Coccioni R, Frontalini F, Marsili A, Mana D (2009) Benthic foraminifera and trace element distribution: a case-study from the heavily polluted lagoon of Venice (Italy). Mar Pollut Bull 59:257–267
Cosentino C, Pepe F, Scopelliti G, Calabrò M, Caruso A (2013) Benthic foraminiferal response to trace element pollution—the case study of the Gulf of Milazzo, NE Sicily (central Mediterranean Sea). Environ Monit Assess 185(10):8777–8802
Covelli S, Emili A, Acquavita A, Dinelli E, Koron N, Faganeli J (2010) Benthic fluxes measurement of mercury and methylmercury in Pialassa Baiona (Ravenna, Italy). Int J Environ Qual (EQA) 3:33–40
Croudace IW, Romano E, Ausili A, Bergamin L, Rothwell G (2015) X-ray core scanners as an environmental forensics tool: a case study of polluted harbour sediment (Augusta Bay, Sicily). In: Croudace, I.W., Rothwell, G. (Eds.). Micro-XRF studies of sediment cores, chapter 15, Springer Developments in Environmental Research (Series Editor - J.P. Smol). Springer, Berlin, in press.
Cundy AB, Collins PEF, Turner SD, Croudace IWC, Horne D (1998) 100 years of environmental change in a coastal wetland. Augusta Bay, southeast Sicily: evidence from geochemical and palaeoecological studies. In: Black KS, Paterson DM, Cramp A (Eds.) Sedimentary process in the intertidal zone. Geological Society, London, Special Publications 139: 243-254.
Debenay J-P, Fernandez J-M (2009) Benthic foraminifera records of complex anthropogenic environmental changes combined with geochemical data in a tropical bay of New Caledonia (SW Pacific). Mar Pollut Bull 59:311–322
Dolven JK, Alve E, Rygg B, Magnusson J (2013) Defining past ecological status and in situ reference conditions using benthic foraminifera: a case study from the Oslofjord, Norway. Ecol Indic 29:219–233
Elberling B, Knudsen KL, Kristensen PH, Asmund G (2003) Applying foraminiferal stratigraphy as a biomarker for heavy metal contamination and mining impact in a fiord in West Greenland. Mar Environ Res 55:235–256
Farcomeni A, Greco L (2015) Robust methods for data reduction. CRC press, Boca Raton, FL
Ferraro L, Sprovieri M, Alberico I, Lirer F, Prevedello L, Marsella E (2006) Benthic foraminifera and heavy metals distribution: a case study from the Naples Harbour (Tyrrhenian Sea, Southern Italy). Environ Pollut 142:274–287
Ferraro L, Sammartino S, Feo ML, Rumolo P, Salvagio Manta D, Marsella E, Sprovieri M (2009) Utility of benthic foraminifera for biomonitoring of contamination in marine sediments: a case study from the Naples Harbour (Southern Italy). J Environ Monit 11:1226–1235
Fisher RA, Corbet AS, Williams CB (1943) The relationship between the number of species and the number of individuals in a random sample of an animal population. J Anim Ecol 12:42–58
Frontalini F, Buosi C, Da Pelo S, Coccioni R, Cherchi A, Bucci C (2009) Benthic foraminifera as bio-indicators of trace element pollution in the heavily contaminated Santa Gilla lagoon (Cagliari, Italy). Mar Pollut Bull 58:858–877
Hallock P, Lidz BH, Cockey-Burhard EM, Donnelly KB (2003) Foraminiferal as bioindicators in coral-reef assessment and monitoring: the FORAM Index. Environ Monit Assess 81:221–238
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistic software package for education and data analysis. Palaeontol. Electron., 4(1), art. 4: 9pp., 178kb, http://palaeo-electronica.org/2001_1/past/issue1_01.htm.
Hammer Ø, Harper DAT (2006) Paleontological data analysis. Blackwell Publishing, Oxford
Hayward BW, Grenfell HR, Nicholson K, Parker R, Wilmhurst J, Mark Horrocks M, Swales A, Sabaa AT (2004) Foraminiferal record of human impact on intertidal estuarine environments in New Zealand’s largest city. Mar Micropaleontol 53:37–66
Hayward BW, Cedhagen T, Kaminski M, Gross O (2015) World Foraminifera Database. Accessed at http://www.marinespecies.org/foraminifera on 2015-04-24.
ICRAM (2008) Progetto preliminare di bonifica della rada di Augusta inclusa nel sito di bonifica di interesse nazionale di Priolo Fase I e Fase II. Report. BoI-Pr-SI-PR-Rada di Augusta-03.22, Technical Report.
Jorissen FJ (1988) Benthic foraminifera from the Adriatic Sea: principles of phenotypic variations. Utrecht Micropalaeontol Bull 37:1–174
Kannan K, Maruya KA, Tanabe S (1997) Distribution and characterization of polychlorinated biphenyl congeners in soil and sediments from a superfund site contaminated with Aroclor 1268. Environ Sci Technol 31:1483–1488
Li YH, Schoonmaker JE (2005) Chemical composition and mineralogy of marine sediments. In: Mackenzie FT (Ed.) Sediments, diagenesis, and sedimentary rocks, vol. 7, First edition: Treatise on geochemistry, Holland HD, Turekian KK, Executive Directors, pp 1–35
Lobegeier MK, Sen Gupta BK (2008) Foraminifer of hydrocarbon seeps, Gulf of Mexico. Journal of Foraminiferal Research 38(2):93–116
Loeblich R, Tappan H (1987) Foraminiferal genera and their classification. Van Nostrand Reinhold, New York
Martins V, Yamashita C, Sousa SHM, Martins P, Laut LLM, Figueira RCL, Mahiques MM, Ferreira da Silva E, Alveirinho Dias JM, Rocha F (2011) The response of benthic foraminifera to pollution and environmental stress in Ria de Aveiro (N Portugal). Journal of Iberian Geology 37(2):231–246
Martins VA, Frontalini F, Tramonte KM, Figueira RCL, Miranda P, Sequeira C, Fernández-Fernández S, Dias JA, Yamashita C, Renó R, Laut LLM, da Silva FS, Rodrigues MA, Bernardes C, Nagai R, Sousa SHM, Mahiques M, Rubio B, Bernabeu A, Rey D, Rocha F (2013) Assessment of the health quality of Ria de Aveiro (Portugal): heavy metals and benthic foraminifera. Mar Pollut Bull 70:18–33
Miller H, Croudace IW, Bull JM, Cotterill CJ, Taylor RN (2014) Modern pollution signals in sediments from Windermere, NW England, determined by micro-XRF and lead isotope analysis, In: Croudace IW, Rothwell RG (Eds), Micro-XRF studies of sediment cores. Springer
Morvan J, Le Cadre V, Jorissen F, Debenay JP (2004) Foraminifera as potential bio-indicators of the “Erika” oil spill in the Bay of Bourgneuf: field and experimental studies. Aquatic Living Resources 17:317–322
Murray JW (2003) An illustrated guide to benthic foraminifera of the Hebridean shelf, west of Scotland, with notes on their mode of life. Palaeontologia Electronica 5(1) : 31pp. http://palaeo-electronica.org/paleo/2002_2/guide/issue2_02.htm
Murray JW (2006) Ecology and applications of benthic foraminifera. Cambridge University Press, Cambridge
Parker WC, Arnold AJ (1999) Quantitative methods of data analysis in foraminifera ecology. In: Sen Gupta BK (ed) Modern foraminifera. Kluver Academic, Dordrecht, pp 71–89
Piani R, Covelli S, Biester H (2005) Mercury contamination in Marano Lagoon (Northern Adriatic Sea, Italy): source identification by analyses of Hg phases. Appl Geochem 20:546–1559
Raldúa D, Díez S, Bayona JM, Barceló D (2007) Mercury levels and liver pathology in feral fish living in the vicinity of a mercury cell chlor-alkali factory. Chemosphere 66:1217–1225
Ritchie JC, McHenry JR (1990) Application of radioactive fallout cesium-137 for measuring soil erosion and sediment accumulation rates and patterns: a review. J Environ Qual 19:215–233
Romano E, Bergamin L, Finoia MG, Carboni MG, Ausili A, Gabellini M (2008) Industrial pollution at Bagnoli (Naples, Italy): Benthic foraminifera as a tool in integrated programs of environmental characterization. Mar Pollut Bull 56:439–457
Romano E, Bergamin L, Finoia MG, Celia Magno M, Ausili A, Gabellini M (2009) The effects of human impact on benthic foraminifera in the Augusta Harbour (Sicily, Italy). In: Dahl E, Moksness E, Støttrup J (eds), Proceedings of the International Symposium on Integrated Coastal Zone Management, Wiley-Blackwell, Chichester, p 97-115
Romano E, Bergamin L, Celia Magno M, Ausili A (2013) Sediment characterization of the highly impacted Augusta Harbour (Sicily, Italy): modern benthic foraminifera in relation to grain-size and sediment geochemistry. Environ Sci: Processes Impacts 15:930–946
Samir AM, El Din AB (2001) Benthic foraminiferal assemblages and morphological abnormalities as pollution proxies in two Egyptian bays. Mar Micropaleontol 41:193–227
Samir AM, Abdou HF, Zazou SM, El-Menhawey WH (2003) Cluster analysis of recent benthic foraminifera from the northwestern Mediterranean coast of Egypt. Rev Micropaléontol 46:111–130
Sciacca S, Fallico R (1978) Presenza e concentrazione di sostanze inquinanti di origine industriale nei fanghi della rada di Augusta (Siracusa). Inquinamento 6:33–36
Scott DB, Tobin R, Williamson M, Medioli FS, Latimer JS, Boothman WA, Asioli A, Haury V (2005) Pollution monitoring in two North American estuaries: historical reconstructions using benthic foraminifera. J Foraminifer Res 35:65–82
Sgarrella F, Moncharmont-Zei M (1993) Benthic foraminifera of the gulf of Naples (Italy): systematic and autoecology. Boll Soc Paleontol Ital 32(2):145–264
Shannon CE (1948) A mathematical theory of communication. Bell Syst Technical Journal 27(3):379–423
Spearman C (1904) The proof and measurement of association between two things. Am J Psychol 15:72–101
Spezzaferri S, Basso D, Koral H (2000) Holocene palaeoceanographic evolution of the Iskenderun Bay, south-eastern Turkey, as a response to river mouth diversions and human impact. Mediterr Mar Sci 1:19–43
Sprovieri M, Oliveri E, Di Leonardo R, Romano E, Ausili A, Gabellini M, Barra M, Tranchida G, Bellanca A, Neri R, Budillon F, Saggiomo R, Mazzola S, Saggiomo V (2011) The key role played by the Augusta basin (southern Italy) in the mercury contamination of the Mediterranean Sea. J Environ Monit 13:1753–1760
Stouff V, Geslin E, Debenay JP, Lesourd M (1999) Origin of morphological abnormalities in Ammonia (Foraminifera): studies in laboratory and natural environments. J Foraminifer Res 29:152–170
Ullrich SM, Ilyushchenko MA, Tanton TW, Uskov GA (2007) Mercury contamination in the vicinity of a derelict chlor-alkali plant part II: contamination of the aquatic and terrestrial food chain and potential risks to the local population. Sci Total Environ 381:290–306
Vilela CG, Batista DF, Baptista Neto JA, Crapez M, McAllister JJ (2004) Benthic foraminifera distribution in high polluted sediments from Niterói Harbor (Guanabara Bay), Rio de Janeiro, Brazil. An Acad Bras Cienc 76(1):161–171
Yanko V, Bresler V, Hallock P (1994a) Defense and transport system against xenobiotics in some benthic foraminifera. Isr J Zool 40:114
Yanko V, Kronfeld J, Flexer A (1994b) Response of benthic foraminifera to various pollution sources: implications for pollution monitoring. J Foraminifer Res 24(1):1–17
Acknowledgments
The authors are grateful to many people who gave their support for the sediment analysis: Giancarlo Pierfranceschi and Francesco Venti for grain size, Giulio Sesta and Anna Lauria for PAHs and PCBs, and Chiara Maggi and Maria Teresa Berducci for heavy metals.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Thomas Hein
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Results of quantitative analysis of benthic foraminifera. Relative abundance of commonly occurring species (i.e., >5 % in at least one sample) is reported. These data were used as a matrix for HCA (Fig. 4) and PCA (Fig. 8). In the following full species names are reported. A_park: Ammonia parkinsoniana; A_tepi: Ammonia tepida; A_mami: Asterigerinata mamilla; B_aena: Bolivina aenariensis; B_semi: Bolivina seminuda; B_marg: Bulimina marginata; C_invo: Cornuspira involvens; E_vitr: Epistominella vitrea; H_depr: Haynesina depressula; L_loba: Lobatula lobatula; M_subr: Miliolinella subrotunda; P_corr: Patellina corrugata; Q_lata: Quinqueloculina lata; Q_stel: Quinqueloculina stelligera; Ros_spp.; Rosalina spp.; S_cost: Sigmoilinita costata; S_vivi: Spirillina vivipara. (XLSX 14 kb)
ESM 2
Analytical results for grain size, Ba, Hg, PAHs and PCB used, after standardization by means of z-score method, as a matrix Principal Component Analysis (Fig. 7). (XLSX 10 kb)
ESM 3
Abiotic parameters (grain size, Ba, Hg, PAHs and PCBs), Faunal parameters (FN, FSI, a-index, H-index) and relative abundance of commonly occurring species. These data were used, standardized by means of z-score method, as a matrix for the CCA (Fig. 8). In the following full species names are reported. A_park: Ammonia parkinsoniana; A_tepi: Ammonia tepida; A_mami: Asterigerinata mamilla; B_aena: Bolivina aenariensis; B_semi: Bolivina seminuda; B_marg: Bulimina marginata; C_invo: Cornuspira involvens; E_vitr: Epistominella vitrea; H_depr: Haynesina depressula; L_loba: Lobatula lobatula; M_subr: Miliolinella subrotunda; P_corr: Patellina corrugata; Q_lata: Quinqueloculina lata; Q_stel: Quinqueloculina stelligera; Ros_spp.; Rosalina spp.; S_cost: Sigmoilinita costata; S_vivi: Spirillina vivipara. (XLSX 13 kb)
Rights and permissions
About this article
Cite this article
Romano, E., Bergamin, L., Ausili, A. et al. Evolution of the anthropogenic impact in the Augusta Harbor (Eastern Sicily, Italy) in the last decades: benthic foraminifera as indicators of environmental status. Environ Sci Pollut Res 23, 10514–10528 (2016). https://doi.org/10.1007/s11356-015-5783-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5783-x