Abstract
Mesoporous titanium dioxide particles (TiO2) were prepared by reactive precipitation in the supercritical anti-solvent (SAS) technique using carbon dioxide in an ambient saturated with water in the presence of the ionic liquid (IL) 1-methyl-3-octylimidazolium bis[trifluoromethylsulfonyl] imide, [C8mim][NTf2]. All experiments of reactive precipitation with SAS were conducted at 40 °C, with a pressure of 8.0 MPa, a CO2 liquid flow rate of 20 mL min–1, and a solution flow rate of 2 mL min–1. Additionally, the influence of the molar ratio of titanium(IV) isopropoxide/isopropanol and ionic liquid/titanium(IV) isopropoxide was investigated. The particles were characterized by X-ray powder diffraction, thermogravimetric analysis, N2 adsorption-desorption analysis (BET surface area), and field-emission gun scanning electron microscopy. Results indicate the formation of anatase and brookite crystalline phases after calcination at 450 °C for 2 h. Besides, the peaks related to the brookite phase were more intense on samples synthesized using the IL. The synthesized TiO2 particles have suitable structural properties, such as high surface area, controlled porosity, narrow pore size distribution (lower than 6 nm), and high thermal stability. Further, particles with spherical morphology are produced, while those synthesized with the IL present smooth surfaces. The use of the IL decreases the particle size from 1.12 ± 0.78 μm (TiO2 without IL) to particles in the range from 0.32 ± 0.13 to 0.66 ± 0.44 μm. Furthermore, the higher the precursor/alcohol ratio, the larger the particle size, thus demonstrating that the particle size also depends on the precursor/alcohol ratio.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Titanium dioxide (TiO2) is a semiconductor of relevant use due to its excellent photocatalytic, optical, and electrical properties (Dahl et al. 2014). Many studies have been carried out proving that TiO2 is adequate for several applications, such as photodegradation of organic contaminants in water and air (Humayun et al. 2018; Katal et al. 2020; Bianchi et al. 2014), dye-sensitized solar cells (Wu et al. 2015; Ahmad et al. 2017), self-cleaning windows and walls (Shen et al. 2015), hydrogen products, and solar cells (Chen et al. 2017). Among several applications, TiO2 has been widely investigated for photocatalysis and has been accepted as a promising technology for the complete degradation and mineralization of organic contaminants in the environment (Gunti et al. 2018; Shayegan et al. 2018).
A relevant aspect of the preparation of TiO2 catalysts is the development of particles of very small size, with high specific surface area, controlled porosity, and pore size distribution, seeking to enhance their catalytic activity and, therefore, the efficiency of the process (Yoo et al. 2005). Titanium dioxide particles have been synthesized through several methods, including sol-gel (Yoo et al. 2005; Agartan et al. 2015), hydrothermal (Mamaghani et al. 2019), emulsion (Dong et al. 2014), ultrasound (Ghows et al. 2010), and microwave heating (Cui et al. 2012).
Among the proposed synthesis methods, supercritical fluid (SCF) media are among the most recent. The use of supercritical CO2 (scCO2) as an alternative route for preparing new materials can considerably reduce environmental risks, as it is environmentally friendly, non-toxic, non-inflammable, cheap, and a recyclable technology. In the precipitation process using CO2, the sol-gel reaction takes place in a supercritical environment, favoring rapid hydrolysis of the precursor and a fast condensation process. Hence, the dissolution of CO2 in the precursor solution leads to a rapid expansion of the phase liquid, causing rapid precipitation of solutes (Silva et al. 2019). Likewise, precipitation of catalytic inorganic materials with particular characteristics such as particle size, size distribution, crystallinity, polymorphism, and specifically high surface area can be obtained by employing carbon dioxide as the reaction medium or an antisolvent because of its tunable thermodynamic properties (Aaymonier et al. 2006; Cansell and Aaymonier 2009; Adschiri and Yoko 2018). In this context, several studies have reported the preparation of TiO2 as micro and nanoparticles with specific catalytic properties employing supercritical carbon dioxide as reaction medium (Tadros et al. 1996; Stallings and Lamb 2003; Alonso et al. 2007; Larder et al. 2021) or as an antisolvent (Tang et al. 2006; Silva et al. 2014; Marin et al. 2015).
Tang et al. (2006) precipitated a TiO2 precursor, namely, titanium(IV) oxyacetylacetonate, using scCO2 as antisolvent by the SAS technique from a methanol solution at fixed temperature and pressure values. The aim was to prepare titanium oxide as a support for gold catalysts by calcination of precipitated particles from the precursor. After calcination, gold catalysts were supported into TiO2 particles. According to the authors, these catalysts have a high activity toward the oxidation of carbon monoxide compared with the same catalyst prepared using other methods.
Silva et al. (2014) synthesized TiO2 using an anti-solvent expansion with scCO2 and used the titanium (IV) isopropoxide - (TTIP) precursor with 250 bar pressure and 60 °C. They obtained an average particle size of 316.7 ± 2.7 nm and a surface area of 418.5 m2 g–1. According to the authors, using scCO2 expansion technology as the reaction medium produced a nanomaterial with a large surface area, which is attractive for adsorption processes. The same authors (Silva et al. 2019) later used the SAS technique to produce TiO2 nanoparticles with an anatase-rutile phase from the same precursor (TTIP). The precipitated material was tested as a catalyst for dye photodegradation and compared with a commercial mixed-phase TiO2. They observed that TiO2 precipitated from SAS displayed higher activity than the commercial photocatalyst. Marin et al. (2015) reported similar results in the precipitation of TiO2 particles from different titanium alkoxides as precursors employing the SAS technique. After calcination, they obtained a precipitated powder that presented a mixed anatase/rutile phase. The authors stated that the catalytic activity of SAS-TiO2 obtained in their work is equivalent to a commercial one.
On the other hand, ionic liquids (ILs) have received significant attention and have been used in different synthesis reactions. They are widely used as organic solvents or reagents in chemical reactions. ILs consist of cations and anions linked through ionic bonds, showing a liquid state around room temperature. ILs are ideal media that combine two advantages: the liquid state of molecular liquids and the ionic character of ionic compounds (Blanchard et al. 1999; Chen and Mu 2021).
They can act as stabilizers due to their low surface tension, resulting from their composition (positive and negative ions) and low vapor pressure. Consequently, ILs reduce air pollution risk, making them ideal substitutes for conventional organic solvents. Besides, theregeneration of ILs is easy since the bond between the cation and the anion in ILs is strong; thus, they can be recovered and reused repeatedly (Chen and Mu 2021). ILs have also been studied to obtain TiO2 with a high surface area through the sol-gel synthesis method (Yoo et al. 2005; Shahi et al. 2015) studied the use of different ILs to synthesize TiO2 particles via the sol-gel technique. They showed that among the ILs examined, the 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim][PF6]) was the most suitable to prepare mesoporous TiO2 particles with a BET area of approximately 478 m2 g–1.
The ILs are excellent solvents for various substances; inorganic, organic compounds, biomolecules, organometallics, and metallic ions. Considering their potential as solvents, ILs can naturally replace conventional organic solvents used in large quantities in chemical processing industries (Keskin et al. 2007). Still, they are water-soluble, and if they enter the aquatic environment through accidental spills or effluents, they can cause environmental problems (Freire et al. 2008). Furthermore, systematic studies on the toxicity and biodegradability of IL must be conducted for its safe use in industry (Chen and Mu 2021).
The low volatility of the ILs also causes product separation and recovery complications. Thus, if the product is volatile, retrodistillation can be used to remove the product from the IL; if a hydrophilic product is obtained in a hydrophobic IL, water can be used to remove the product from the IL. But if the product is not volatile or thermosensitive, it becomes impracticable to recover (Huddleston et al. 1998; Zhao et al. 2005). Therefore, scCO2 is used because it recovers different solutes from ILs without cross-contamination (Keskin et al. 2007).
The scCO2 is volatile and non-polar, forming attractive two-phase systems with non-volatile and polar ILs. The product recovery procedure with these systems is based on the premise that scCO2 is soluble in ILs, but ILs are insoluble in scCO2. As most organic compounds are soluble in scCO2 and scCO2 is soluble in ILs, these products are transferred from the IL to the supercritical phase, causing a complete separation within the systems (Blanchard et al. 2001; Zhao et al. 2005; Keskin et al. 2007).
Thus, here we report a route to synthesize mesoporous TiO2 particles using supercritical carbon dioxide as antisolvent in an ambient saturated with water in the presence of the ionic liquid [C8mim][NTf2]. Further, the synthesized particles were characterized by X-ray diffraction (XRD), thermogravimetric analysis (TG/DTA), Brunauer-Emmet-Teller (BET) using the Barrett-Joyner-Halenda (BJH) method, and field emission gun scanning electron microscopy (FEG-SEM) measurements to understand the influence of the use of the IL and operational conditions on the size and other structural characteristics of the particles. The amount of IL and the proportion between the Ti precursor and the solvent used affected the size and properties of the developed particles.
Experimental
Materials
Isopropanol (iPrOH) (99.5%) and TTIP (97.0%) were purchased from Sigma-Aldrich, the IL 1-methyl-3-octylimidazolium bis[trifluoromethylsulfonyl] imide, [C8mim][NTf2] was purchased from Ionic Liquids Technologies (Iolitec) with a purity of 99.0%, and carbon dioxide was obtained from White Martins (99.0%).
Reactive TiO2 precipitation by SAS technique
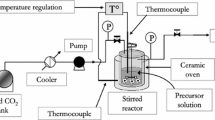
The precipitation of TiO2 particles was carried out using an experimental apparatus based on a technique that uses supercritical carbon dioxide as an anti-solvent (SAS), according to the scheme displayed in Fig. 1. More details of the experimental apparatus, with some modifications, and its operation can be found elsewhere (Franceschi et al. 2008).

Adapted from Franceschi et al. (2008)
Experimental apparatus used in the precipitation of TiO2 particles by the SAS technique employing carbon dioxide as antisolvent. V1—ball valve; V2 and V3—needle valve; SP—Syringe Pump; PT—pressure transducer; PI—pressure indicator; TI—temperature indicator; TC—temperature control.
For precipitation experiments, initially, a solution was prepared with TTIP on isopropanol (iPrOH) under an inert atmosphere with TTIP:iPrOH molar ratios of 0.01, 0.03, and 0.05. The IL [C8mim][NTF2] was then added to this solution in the molar percentage of 1, 3, and 5% IL related to TTIP and stirred for 10 min, given a homogeneous solution. Hence, the nomenclature used in this study for the synthesized particles is TiO2 x_y% IL, where x represents the TTIP:iPrOH molar ratio and y is the IL molar percentage.
Briefly, the experimental apparatus was mainly composed of a syringe pump (ISCO, model 500D) and a positive displacement HPLC pump (Acuflow Series III) that are used to flow the liquid CO2 and the solutions, respectively, into the precipitation chamber that consists of a cylindrical stainless steel vessel (homemade) with an internal volume of 500 mL. The precipitation chamber was built to support pressures and temperatures up to 30 MPa and 100 °C.
Precipitation of TiO2 was conducted at a constant pressure and temperature of 8.0 MPa, and 40°C. Carbon dioxide was pumped into the precipitation chamber through a stainless-steel tube of 1/16” external diameter at a flow rate of 20 mL min–1 and a pressure of 10 MPa. The flow inlet and outlet of the precipitation chamber were controlled by two micrometering valves (V2–V3). The solution was pumped into the top of the precipitation chamber through a silica capillary tube with an internal diameter of 150 μm at a flow rate of 2 mL min–1 and pressure of 14 MPa controlled by a backpressure regulator (GO-Regulator, Series BP-66, Model 1A11QEQ151). The pressure inside the chamber was monitored using a pressure transducer (PT) (HUBA CONTROL, model 691). The internal temperature of the precipitation chamber and the needle valve (V3) was kept through heating tapes (FISATOM, Model 5, at 200 W) coupled with temperature controllers (TC) (NOVUS, Model N1200).
The experimental procedure starts with the addition of 10 mL of ultrapure water (purified by a Milli-Q system from Millipore®) to the precipitation chamber. The chamber is then pressurized with CO2 at a constant flow of 20 mL min–1 while the temperature is raised to experimental values. The water added to the precipitation chamber promotes a CO2 water-saturated environment, thereby accelerating the hydrolysis reaction and TiO2 precipitation. When pressure and temperature are constant at the desired value, the solution is pumped into the precipitation chamber at 2 mL min–1 simultaneously with the liquid flow of CO2. After that, scCO2 continued to flow through the chamber at 40 °C under 20 mL min–1 and 8.0 MPa for 30 min more to remove non-reacted materials. Then, the precipitation chamber was slowly depressurized, and the suspension of TiO2 particles in the water was collected, filtered, and then dried at 60 °C. Further, the solid particles were calcined at 450 °C for 2 h with a heating rate of 3 °C/min in airflow. The precipitated TiO2 yield in each experiment was around 85% regarding the theoretical yield (100% of the reacted TTIP). This difference is related to unreacted TTIP and TiO2 losses during collection at the end of each experimental condition.
Characterization
X-ray diffraction analyses were conducted in a PANalytical diffractometer Empyrean, operating with CuKα radiation (λ = 0.15406 nm), with a 0.02° step and a scanning speed of 0.5° per min, in the range 20° < 2θ < 80°. The crystallite size of the particles was estimated using the Debye Scherrer equation (Eq. 1):
where in (D) is the crystallite size in nanometers, λ is the wavelength of the X-ray radiation (0.1548 nm for CuKα radiation), and β is the full width at half-peak height (FWHM). The full width values at half-maximum were obtained using the X’Pert HighScore software.
The thermal stability of the materials was tested using thermogravimetric analysis on a Shimadzu Simultaneous DTA-TG apparatus. Nearly 10 mg of each sample was analyzed from room temperature up to 1000 °C at a heating rate of 10 °C min–1, with a flow of 50 mL min–1 N2 using tin recipients. The physisorption of N2 at 77 K was measured and used for the textural characterization of the catalysts. The specific surface area and pore size distribution were obtained from the adsorption-desorption isotherms by BET and BJH methods. The physical adsorption of pure nitrogen was carried out in a Quantachrome Nova 1200 Multistation Instrument, model Autosorb 3B. Samples were pretreated under a vacuum at 100 °C for 2 h. SEM-FEG micrographs were obtained in a scanning electron microscope (JSM-6510LV) equipped with a microanalysis by energy-dispersive X-ray spectroscopy (EDS) system. No pretreatment or gold recovery was necessary. The average particle size was estimated from SEM-FEG images (counting about 400 particles in each image) by using the image analysis software Solutions Size Meter 1.1.
Results and discussion
X-ray diffraction
Figure 2a and b show the XRD patterns of the samples precipitated by SAS before and after calcination at 450 °C. The XRD peaks can be associated with the crystalline phase of TiO2 anatase, with the most intense peak at 2θ = 25.4 °C (JCPDS 73-1764), even though the samples were not calcined (Fig. 2a). Yoo et al. (2005) also observed that as-prepared TiO2 particles show an anatase crystalline phase before thermal treatment. They used ionic liquids as templates in the sol-gel method to prepare mesoporous TiO2 particles. This fact prevents the fast hydrolysis of the titanium precursor when in excessive amounts of water. Hence, crystalline TiO2 particles were directly formed. However, the crystallinity of the synthesized particles was enhanced after calcination. Additionally, peaks associated with the brookite crystalline phase at 2θ = 30.80° were also observed (Fig. 2b). The XRD patterns obtained in our study are similar to those obtained by Paszkiewicz et al. (2016), Shahi et al. (2016), and Liu et al. (2009), who synthesized TiO2 using IL using hydrothermal methods. The simultaneous addition of water and (TTIP) to the supercritical carbon dioxide (scCO2) environment allows controlled precipitation of the TiO2 anatase phase due to the rapid hydrolysis of (TTIP) (Silva et al. 2019).
Reducing the hydrolysis rate provides a longer aging time for forming the sol-gel network. It makes possible an ordered array, thus resulting in the formation of highly porous TiO2 particles. The capping effect exerted by the ionic liquid resulted in an anhydrous area located between the ionic liquid and the precursor TTIP. These conditions can generally suppress the formation of hydroxide and oxyhydrate, generating amorphous species. In this case, IL also favored the formation of TiO2 in the anatase phase, as seen previously.
Thermogravimetric analysis
The weight loss of the TiO2 samples obtained from the TG thermographs is shown in Fig. 3a. The TiO2 sample precipitated without IL presented a total weight loss of approximately 17%, with no significant loss above 500 °C. Similar behaviors were observed for TiO2 0.01_1% IL and TiO2 0.01_5% IL, with total weight losses of 15% and 16%, respectively. The other three samples (i.e., TiO2 0.03_3% IL, TiO2 0.05_1% IL, and TiO2 0.05_5% IL) showed higher total weight losses of 28%, 29%, and 32%, respectively. This effect was induced by the increase in the TTIP/iPrOH ratio.
Between 25 and 120 °C, nearly 10% of weight loss occurred in the TiO2 0.03 without IL, TiO2 0.01_1% IL, and TiO2 0.01_5% IL samples, related to the evaporation of water, the physically adsorbed, and organic solvent residues that remained in the TiO2. The weight loss was 21% for both TiO2 0.03_3% IL and TiO2 0.05_1% IL and 12% for the TiO2 0.05_5% IL sample. The DTA curves of these samples (Fig. 3b) showed endothermic peaks around 78 °C that refer to both the dehydration of the samples and the vaporization of isopropanol.
The next stage of weight loss, between 121 and 300 °C, accounted for a mass loss of around 5% for samples TiO2 0.03 without IL, TiO2 0.01_1% IL, TiO2 0.01_5% IL, and TiO2 0.05_5% IL. Besides, DTA patterns showed exothermic peaks in this temperature range associated with the evaporation of water chemically adsorbed, resulting in TiOH condensation and thermal decomposition of non-hydrolyzed TTIP residues (Choi et al. 2006; Silva et al. 2014).
In the last stage of the sample transformation, all samples have a minor weight loss. Between 301 and 500 °C, a mass loss of 2–3% is associated with the thermal decomposition of organic residues strongly bound to the TiO2 structure. Above this temperature range, the sample weights remained unchanged up to 1000 °C, and a residual of 80–70% of the mass is associated with pure TiO2 (Verma et al. 2012; Silva et al. 2014). The transition phase from anatase to rutile was not observed for any sample, which agrees with a previous report that showed high thermal stability of TiO2 synthesized using an IL (Choi et al. 2008).
The DTA curve for the sample TiO2 0.03 without IL exhibits an exothermal peak at 412 °C, corresponding to the transition from the amorphous to the crystalline anatase phase of TiO2. The samples made using the IL did not present this phase transition because the anatase phase is fully structured at the end of the synthesis process, even before thermal treatment, as seen in Fig. 2a. For samples with higher proportions of IL, TiO2 0.01_5% IL, and TiO2 0.05_5% IL, there is an exothermal peak at 314 °C, resulting in an amorphous state transformation from the anatase/brookite phases. Furthermore, it can also be attributed to energy losses, which occur in a combustion reaction, as seen in the combustion of organic matter (Bu et al. 2012; Leyva-Porras et al. 2015).
Specific surface area
The N2 adsorption isotherms (BET method) and pore size distribution (BJH method) obtained for the samples synthesized with and without IL are shown in Fig. 4. All samples presented isotherms that can be identified as type IV, according to IUPAC classifications, characteristic of mesoporous materials (Sing et al. 1985). The pore size distribution of the samples is similar and relatively narrow, in the range of 2.80–3.21 nm (Table 1), which indicates a high homogeneity of the pores, associated with the high mechanical stability of the materials, which is an appreciated prerequisite for its use as a photocatalyst (Yoo et al. 2005; Miao et al. 2006). However, note that the addition of IL increases both the pore volume and pore size of the powders; therefore, the TiO2 0.05_5% IL showed a pore size of 5.8 nm due to the higher IL content (Zhang et al. 2015b).
Adsorption-desorption isotherms of N2 and pore size distribution graphs (BJH) (inset) of particles calcined at 450 °C for samples synthesized in different proportions of TTIP/iPrOH and IL concentration through scCO2: a TiO2_0.03 without IL, b TiO2_0.01_1% IL, c TiO2_0.01_5% IL, d TiO2_0.03_3% IL, e TiO2_0.05_1% IL, and f TiO2_0.05_5% IL
The BET surface area of the TiO2 particles was reduced by the thermal treatment conducted at 450 °C, whereas the final area was highly similar (around 100 m2 g–1), independent of the IL content. The particles prepared without IL or low IL content showed the greatest reduction in surface area after calcination, around 50%. Alternatively, TiO2 powder prepared with 3% IL presented higher thermal stability, with less than 40% surface area reduction. The sample with the highest IL content (TiO2 0.05_5% IL) lost only 26% of its surface area, maintaining a highly porous structure after calcination. These findings agree with previous studies by Yoo et al. (2005) and Hu et al. (Hu et al. 2008), who prepared TiO2 in the presence of ILs by the sol-gel method under ambient temperature and pressure conditions.
The total volume of pores of TiO2 particles prepared without ionic liquid was 0.225 cm3/g before calcination and 0.224 cm3/g after calcination at 450 °C; hence, there was an insignificant decrease in the total volume of pores, and these results agree with those observed by Yoo et al. (2005). Conversely, the total pore volume was 0.208 cm3/g and 0.240 cm3/g before and after heat treatment at 450 °C for the samples prepared with 3% ionic liquid (TiO2_0.03_3% IL). This increase in total pore volume may be related to the presence of ionic liquid under supercritical CO2 conditions. This behavior is contrary to those found in the reports of Yoo et al. (2005), who prepared TiO2 in the presence of IL using the sol-gel method, and Sui et al. (2011), who prepared TiO2 particles via supercritical CO2. Notably, the total pore volume increased significantly (twice) in samples with the maximum proportion of IL (TiO2_0.05_5% IL), which changed from 0.173 cm3/g before calcination to 0.346 cm3/g after calcination, indicating a positive effect of the use of the ionic liquid in the synthesis of TiO2 particles under supercritical conditions.
Scanning electron microscopy
FEG-SEM images of all samples are shown in Fig. 5. The developed TiO2 particles have a spherical morphology and a wide particle size distribution (Fig. 6). The mean particle size of the particles is summarized in Table 1.
The particles made without IL (Fig. 5a) have an average diameter of 1.12 ± 0.8 μm, while the samples made with IL have an average diameter size of 0.536 ± 0.350 μm. Additionally, TiO2 particles made with IL showed uniform morphology and smooth surfaces, indicating the strong capacity of IL as a structuring agent (Sui et al. 2011; Paszkiewicz et al. 2016).
Although the use of IL decreases the particle size, the higher the IL concentration, the higher the particle size. The particles made using 1% IL have an average diameter of 0.519 ± 0.370 μm, while the powder made with 5% IL has an average diameter of 0.657 ± 0.440 μm. Similar behavior occurs for the smaller ratio of TTIP/iPrOH but with lower intensity. The results found here are in accordance with previous studies by Paszkiewicz et al. (2016), who synthesized TiO2 particles using the solvothermal method in the presence of different ILs ([BMIM][Cl] and [DMIM][Cl]) and with different concentrations of IL. These authors concluded that an increase in the IL concentration increases the particle size, independent of the IL used.
The particle size distribution for all experimental conditions can be observed in Fig. 6. Note that samples with smaller ratios of TTIP/iPrOH (Fig. 5c; Table 1) have the smallest average particle sizes (i.e., 0.326 ± 130 μm and 0.328 ± 0.120 μm) and narrow granulometric distribution. It indicates that the initial concentration of the Ti precursor plays a vital role in crystallization and, consequently, the size of the particles. Furthermore, the average crystallite sizes estimated using the Scherrer equation for all samples range from 6.1 to 8.0 nm. These values of crystallite sizes demonstrate that the TiO2 particles are formed of nanocrystalline grains.
The dissolution of titanium alkoxide and the stabilization of aqueous dispersions in supercritical CO2 are necessary to form spherical particles. The injector nozzle produces very small liquid droplets of precursor solutions that, when in contact with scCO2, rapidly expand the liquid phase. The low amount of water can deplete the hydrolysis of the titania precursor and favor the formation of spherical particles (Silva et al. 2019; Tadros et al. 1996).
Conclusions
Here, we proposed a novel method for TiO2 particles by reactive precipitation using scCO2 as an antisolvent saturated with water and an IL as an adjuvant. The influence of the TTIP/IPrOH molar ratio (0.01, 0.03, and 0.05) and the molar percentage of IL regarding TTIP (1, 3, and 5%) on the characteristics of TiO2 particles were investigated. The synthesized TiO2 nanoparticles are crystalline, in the anatase and brookite phases, after thermal treatment at 450 °C for 2 h. Notably, the samples synthesized using the IL displayed the crystalline phase of anatase without thermal treatment, indicating that the IL suppresses hydrolysis and results in crystalline particles.
Thermogravimetric data indicate that the transition from amorphous to crystalline anatase phase of TiO2 does not occur for samples made using the IL, which agrees with the XRD data. The synthesized particles have high values of the BET surface area. Nevertheless, the higher the amount of IL used, the smaller the surface area after the calcination step. The calcined samples showed high surface area values, ranging from 100 to 118 m2 g–1. The pore size increases as the IL concentration increases, shifting from 2.80 for the sample synthesized without IL to 5.8 nm for a sample made using the IL.
Spherical particles were produced using the developed method. The average particle size also increases as the TTIP/iPrOH ratio increases. Nevertheless, the use of ionic liquid to synthesize TiO2 particles decreases the mean particle size compared to those synthesized without ionic liquid. However, the higher the ionic liquid concentration, the larger the mean particle size, showing that the IL concentration is an essential parameter in the control of precipitated TiO2 particles. Therefore, the developed method opens up the opportunity to synthesize spherical mesoporous TiO2 nanoparticles with high surface area and controlled size.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. Additionally, raw data are available on request from the corresponding author.
References
Aaymonier C et al (2006) Review of supercritical fluids in inorganic materials science. J Supercrit Fluids 38:242–251. https://doi.org/10.1016/j.supflu.2006.03.019
Adschiri T, Yoko A (2018) Supercritical fluids for nanotechnology. J Supercrit Fluids 134:167–175. https://doi.org/10.1016/j.supflu.2017.12.033
Agartan L et al (2015) Effect of initial water content and calcination temperature on photocatalytic properties of TiO2 nanopowders synthesized by the sol–gel process. Ceram Int 41:12788–12797. https://doi.org/10.1016/j.ceramint.2015.06.114
Ahmad MS, Pandey AK, Rahim NA (2017) Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renew Sustain Energy Rev 77:89–108. https://doi.org/10.1016/j.rser.2017.03.129
Alonso E et al (2007) Synthesis of titanium oxide particles in supercritical CO2: Effect of operational variables in the characteristics of the final product. J Supercrit Fluids 39:453–461. https://doi.org/10.1016/j.supflu.2006.03.006
Bianchi CL et al (2014) Photocatalytic degradation of acetone, acetaldehyde and toluene in gas-phase: comparison between nano and micro-sized TiO2. Appl Catal B-Environ 146:123–130. https://doi.org/10.1016/j.apcatb.2013.02.047
Blanchard LA et al (1999) Green processing using ionic liquids and CO2. Nature 399:28–29. https://doi.org/10.1038/19887
Blanchard LA et al (2001) High-pressure phase behavior of ionic liquid/CO2 systems. J Phys Chem B 105:2437–2444. https://doi.org/10.1021/jp003309d
Bu X, Zhang G, Zhang C (2012) Effect of nitrogen doping on anatase–rutile phase transformation of TiO2. Appl Surf Sci 258:7997–8001. https://doi.org/10.1016/j.apsusc.2012.04.154
Cansell F, Aymonier C (2009) Design of functional nanostructured materials using supercritical fluids. J Supercrit Fluids 47:508–516. https://doi.org/10.1016/j.supflu.2008.10.002
Chen Y, Mu TC (2021) Revisiting greenness of ionic liquids and deep eutectic solvents. Green Chem Eng 2:174–186. https://doi.org/10.1016/j.gce.2021.01.004
Chen PC, Chen CC, Chen SH (2017) A review on production, characterization, and Photocatalytic applications of TiO2 nanoparticles and nanotubes. Curr Nanosci 13:373–393. https://doi.org/10.2174/1573413713666170511163542
Choi H et al (2006) Thermally stable nanocrystalline TiO2 photocatalysts synthesized via sol–gel methods modified with ionic liquid and surfactant molecules. Chem Mater 18:5377–5384. https://doi.org/10.1021/cm0615626
Choi EH, Hong SI, Moon DJ (2008) Preparation of thermally stable mesostructured nano-sized TiO2 particles by modified sol–gel method using ionic liquid. Catal Lett 123:84–89. https://doi.org/10.1007/s10562-008-9398-4
Cui L et al (2012) Facile microwave-assisted hydrothermal synthesis of TiO2 nanotubes. Mater Lett 75:175–178. https://doi.org/10.1016/j.matlet.2012.02.004
Dahl M, Liu Y, Yin Y (2014) Composite Titanium Dioxide Nanomaterials. Chem Rev 114:9853–9889. https://doi.org/10.1021/cr400634p
Dong R et al (2014) TiO2 microspheres with variable morphology, size and density synthesized by a facile emulsion-mediated hydrothermal process. Mater Lett 123:135–137. https://doi.org/10.1016/j.matlet.2014.03.008
Franceschi E et al (2008) Phase behavior and process parameters effects on the characteristics of precipitated theophylline using carbon dioxide as antisolvent. J Supercrit Fluid 44:8–20. https://doi.org/10.1016/j.supflu.2007.09.031
Freire MG et al (2008) Mutual solubilities of water and the [Cnmim][Tf2N] hydrophobic ionic liquids. J Phys Chem B 112:1604–1610. https://doi.org/10.1021/jp7097203
Ghows N, Entezari MH (2010) Ultrasound with low intensity assisted the synthesis of nanocrystalline TiO2 without calcination. Ultrason Sonochem 17:878–883. https://doi.org/10.1016/j.ultsonch.2010.03.010
Gunti S, Kumar A, Ram MK (2018) Nanostructured photocatalysis in the visible spectrum for the decontamination of air and water. Int Mater Rev 63:257–282. https://doi.org/10.1080/09506608.2017.1379264
Hu S et al (2008) Synthesis of mesostructure anatase TiO2 particles in room-temperature ionic liquids. Mater Lett 62:2954–2956. https://doi.org/10.1016/j.matlet.2008.01.082
Huddleston JG et al (1998) Room temperature ionic liquids as novel media for clean liquid–liquid extraction. Chem Commun 16:1765–1766. https://doi.org/10.1039/a803999b
Humayun M et al (2018) Modification strategies of TiO2 for potential applications in photocatalysis: a critical review. Green Chem Lett Rev 11:86–102. https://doi.org/10.1080/17518253.2018.1440324
Katal R et al (2020) A review on the synthesis of the various types of anatase TiO2 facets and their applications for photocatalysis. Chem Eng J 384:123384. https://doi.org/10.1016/j.cej.2019.123384
Keskin et al (2007) A review of ionic liquids towards supercritical fluid applications. J Supercrit Fluids 43:150–180. https://doi.org/10.1016/j.supflu.2007.05.013
Larder RR et al (2021) Porous hollow TiO2 microparticles for photocatalysis: exploiting novel ABC triblock terpolymer templates synthesized in supercritical CO2. Polym Chem 12:2904–2913. https://doi.org/10.1039/D1PY00334H
Leyva-Porras C et al (2015) Low-temperature synthesis and characterization of anatase TiO2 nanoparticles by an acid assisted sol–gel method. J Alloys Compd 647:627–636. https://doi.org/10.1016/j.jallcom.2015.06.041
Liu H et al (2009) Hydrothermal synthesis of mesostructured nanocrystalline TiO2 in an ionic liquid–water mixture and its photocatalytic performance. Solid State Sci 11:1655–1660. https://doi.org/10.1016/j.solidstatesciences.2009.06.011
Mamaghani AH, Haghighat F, Lee CS (2019) Hydrothermal/solvothermal synthesis and treatment of TiO2 for photocatalytic degradation of air pollutants: Preparation, characterization, properties, and performance. Chemosphere 219:804–825. https://doi.org/10.1016/j.chemosphere.2018.12.029
Marin RP et al (2015) Supercritical antisolvent precipitation of TiO2 with tailored anatase/rutile composition for applications in redox catalysis and photocatalysis. Appl Catal A-Gen 504:62–73. https://doi.org/10.1016/j.apcata.2015.02.023
Miao S et al (2006) Synthesis of mesoporous TiO2 films in ionic liquid dissolving cellulose. Micropor Mesopor Mat 95:26–30. https://doi.org/10.1016/j.micromeso.2006.04.013
Paszkiewicz M et al (2016) The ILs-assisted solvothermal synthesis of TiO2 spheres: the effect of ionic liquids on morphology and photoactivity of TiO2. Appl Catal B-Environ 184:223–237. https://doi.org/10.1016/j.apcatb.2015.11.019
Shahi SK et al (2015) Green synthesis of photoactive nanocrystalline anatase TiO2 in recyclable and recoverable acidic ionic liquid [Bmim] HSO4. J Mater Sci 50:2443–2450. https://doi.org/10.1007/s10853-014-8799-6
Shahi SK, Kaur N, Singh V (2016) Fabrication of phase and morphology controlled pure rutile and rutile/anatase TiO2 nanostructures in functional ionic liquid/water. Appl Surf Sci 360:953–960. https://doi.org/10.1016/j.apsusc.2015.11.092
Shayegan Z, Lee CS, Haghighat F (2018) TiO2 photocatalyst for removal of volatile organic compounds in gas phase – A review. Chem Eng J 334:2408–2439. https://doi.org/10.1016/j.cej.2017.09.153
Shen W et al (2015) Preparation of titanium dioxide nanoparticle modified photocatalytic self-cleaning concrete. J Clean Prod 87:762–765. https://doi.org/10.1016/j.jclepro.2014.10.014
Silva EP et al (2014) scCO2-based synthesis of semi-crystalline TiO2 nanoparticles: a rapid and direct strategy. Mater Lett 136:133–137. https://doi.org/10.1016/j.matlet.2014.07.156
Silva EP et al (2019) Effect of phase composition on the photocatalytic activity of titanium dioxide obtained from supercritical antisolvent. J Colloid Interf Sci 535:245–254. https://doi.org/10.1016/j.jcis.2018.09.098
Sing KSW et al (1985) Reporting physisorption data for gas-solid systems. Pure Appl Chem 57:603–619. https://doi.org/10.1351/pac198254112201
Stallings WE, Lamb HH (2003) Synthesis of nanostructured titania powders via hydrolysis of titanium isopropoxide in supercritical carbon dioxide. Langmuir 19:2989–2994. https://doi.org/10.1021/la020760i
Sui R, Rizkalla A, Charpentier PA (2011) Experimental study on the morphology and porosity of TiO2 aerogels synthesized in supercritical carbon dioxide. Micropor Mesopor Mat 142:688–695. https://doi.org/10.1016/j.micromeso.2011.01.016
Tadros ME et al (1996) Synthesis of titanium dioxide particles in supercritical CO2. J Supercrit Fluids 9:172–176. https://doi.org/10.1016/S0896-8446(96)90029-7
Tang ZR et al (2006) Preparation of TiO2 using supercritical CO2 antisolvent precipitation (SAS): a support for high activity gold catalysts. Stud Surf Sci Catal 162:219–226. https://doi.org/10.1016/S0167-2991(06)80910-9
Verma YL, Singh MP, Singh RK (2012) Ionic liquid assisted synthesis of nano-porous TiO2 and studies on confined ionic liquid. Mater Lett 86:73–76. https://doi.org/10.1016/j.matlet.2012.07.025
Wu D et al (2015) Tunable synthesis of single-crystalline-like TiO2 mesocrystals and their application as effective scattering layer in dye-sensitized solar cells. J Colloid Interf Sci 456:125–131. https://doi.org/10.1016/j.jcis.2015.06.023
Yoo KS, Lee TG, Kim J (2005) Preparation and characterization of mesoporous TiO2 particles by modified sol–gel method using ionic liquids. Micropor Mesopor Mat 84:211–217. https://doi.org/10.1016/j.micromeso.2005.05.029
Zhang B et al (2015) Ionic liquid-assisted synthesis of morphology controlled TiO2 particles with efficient photocatalytic activity. RSC Adv 5:81108–81114. https://doi.org/10.1039/C5RA17213F
Zhao H, Xia S, Ma P (2005) Use of ionic liquids as ‘green’solvents for extractions. J Chem Technol Biotechnol: Int Res Process Environ Clean Technol 80(10):1089–1096. https://doi.org/10.1002/jctb.1333
Acknowledgements
The authors thank the National Council of Technological and Scientific Development–CNPq (grants 304419/2015-0, 305438/2018-2, 313453/2018-7), the Coordination for the Improvement of Higher Education Personnel–CAPES (Finance Code 001) and the Sergipe State Research and Technological Innovation Foundation (FAPITEC/SE) from Brazil for financial support and scholarships supply for this work. This research used facilities of the Multiuser Centre for Nanotechnology at UFS (CMNano-UFS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prado, L.R., Figueiredo, R.T., Silva, R.S. et al. Reactive precipitation of titanium dioxide particles in supercritical CO2 by SAS technique with an ionic liquid as adjuvant. Braz. J. Chem. Eng. 41, 287–298 (2024). https://doi.org/10.1007/s43153-023-00319-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-023-00319-w