Abstract
In this study, the formation solid solutions of titanium dioxide- zirconium dioxide (TiO2-ZrO2) system with the supercritical fluid method is described. The particles of solid solutions in the TiO2-ZrO2 system are spherical and form agglomerates, they are amorphous and have a size from 90 to 850 nm. The X-ray patterns of samples calcined above the temperatures of crystallization (450 °C) and phase transition (750 °C) demonstrate the decomposition of the solid solutions above the crystallization temperature and formation of phases in accordance with phase ratios in the TiO2-ZrO2 system at these temperatures. The formation solid solutions of the starting materials are observed in all region of concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, a large number of studies have been devoted to the synthesis of nanosized zirconium dioxide (ZrO2)[1–3] as well as nanosized titanium dioxide (TiO2).[4–6] There are examples of studies where nanosized ZrO2 was investigated as a sorbent[7] and as a catalyst support[8]. Nanostructured TiO2 used as a photocatalyst for the decomposition of a wide range of organic compounds,[9] as electrochemical energy storage,[10] and as agent for water purification.[11] Also, both of these nanosized oxides used as antimicrobial agents for oral hygiene.[12] Mixing of the oxides can produce new crystallographic phases with quite different properties than the original oxides. In particular, mixed oxides have been widely used in catalysts, because the surface characteristics of individual oxides can be changed due to the formation of new sites in the interface between the components, or by the incorporation of one oxide into the lattice of the other. It was reported that ZrO2 doped titanium solid solution showed enhanced photocatalytic activity from UV light.[13] A mixture of ZrO2 and TiO2 was used as an electrode for the dye-sensitized solar cell.[14]
The well-known method of synthesis of nanosized zirconia is via the sol-gel chemistry[15] and by the precipitation from different solutions.[16] Our earlier experiments targeting synthesis of oxides nanoparticles by crystallization on the boundary of two liquids initially led to the formation of hydroxyl carbonates,[17] and the nanoparticles of crystalline oxides were obtained only after calcination.
There are several publications where the supercritical fluid (SCF) process using different liquid media was reported.[18,19]. Particular attention was paid to solutions based on CO2 and H2O. Special attention was paid to solutions based on CO2 fluid[20] as the most promising and suitable for temperatures up to 50 °C.
The synthesis of TiO2 nanoparticles by the SCF method was investigated by several research groups.[21–24] Usually, the synthesis was performed at the pressure in the range 10–20 MPa and temperature 200–300 °C. TiO2 was obtained during thermal hydrolysis or thermal decomposition in liquids containing methanol, ethanol, isopropanol or water. At temperatures above 250 °C, well-crystallized anatase nanoparticles are obtained. The formation of 200 nm anatase particles at a temperature of 300 °C and pressure of 20 MPa was reported.[21] Synthesis of TiO2 nanoparticles with sizes 5–20 nm and degree of crystallinity of 10–100% was reported.[24] It was found that the crystallite size is determined by the ratio of isopropanol and water, and that the degree of crystallinity depends on the temperature of the reaction mixture: as the temperature increases, the degree of crystallinity increases.
Earlier our group reported on the synthesis of nanoscale TiO2 by precipitation with CO2 supercritical antisolvent.[25] The nanoparticles of controlled size obtained using supercritical technologies had X-ray amorphous structure, high specific surface area, and high porosity.
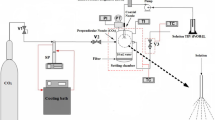
The key steps of the method are as follows: (1) preparation of the precursor solution in a polar organic solvent; (2) transfer of liquefied CO2 to the fluid state (TCT = 303.9 K, Pcr = 7.38 MPa); (3) contact of the CO2 fluid with the prepared solution, where the organic solvent dissolves in the CO2 fluid, and the precursor precipitates or decomposes into more stable substances under the experimental conditions. The advantages of the method are that the process is very fast, the product is highly pure, the dispersion of the product can be fine-tuned.
The objective of this study was to obtain nanosized ZrO2,as well as its solid solutions with TiO2, using the supercritical antisolvent precipitation (SAS) method in the CO2 subcritical fluid. It is known[26] that there is a rather wide region of solid solution in the TiO2-ZrO2 system with a component ratio of 1:1 at a temperature above 1100 °C. It was of interest to establish the possibility of forming an analogous solid solution under the conditions of the CO2 subcritical fluid.
Experimental section
Synthesis
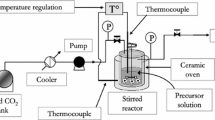
The synthesis was carried out in the experimental setup “SuperParticle SAS 50 system” (“Waters Corp.”), described earlier.[25] The reference solutions were prepared by adding isopropyl alcohol (“esp. pure”, “HimMed”) to zirconium isopropoxide or titanium isoporopoxide (98%, “Acros Organic”)in predetermined proportions, and then were fed to the reactor. After feeding the reference solution to the reactor, the fluid was fed for 15 min more to remove the solvent from the surface and from the volume of the obtained dioxide TiO2-ZrO2 system. The experimental parameters in the synthesis of ZrO2 nanoparticles varied in the following range: pressure from 7 to 25 MPa, temperature 40–70 °C, feed rate of CO2 from 35 to 50 g/min, feed rate of the reference solution from 0.25 to 1.0mL/min. The samples with Ti/Zr ratios 3:1, 3:2, 1:1, 2:3, 1:3 were synthesized.
Research methods
The obtained samples of ZrO2 and oxides with different ratios of titanium oxide to zirconium oxide were characterized by the X-ray powder diffraction (XRD) method. The XRD analysis was carried out using “Shimadzu XRD-600” diffractometer (Cu-Kα radiation, graphite monochromator). The diffraction peak positions were determined using the peak profile analysis software package PROFIT.[27] Phase analysis of the prepared samples was done using powder diffraction database PCPDFWIN. The powder 2 software package was used to index the observed diffraction peaks, calculate the spacings between the atomic planes, and relative intensities ofthe diffraction peaks. The unit sell parameters were refined using the least-squares method.
The particle size was determined by dynamic light scattering using “DelsaNano” instrument (Beckman Coulter, Inc.). The sample was prepared as suspensions in water.
The thermal properties of the samples were analyzed using a universal differential scanning calorimeter “DSC 204 F1 Phoenix”. To remove the traces of the organic solvent, the samples were dried in a vacuum oven LT-VO/20 at 0.7 bar. The sorption capacity for nitrogen at −196 °C was measured by a static volumetric method in the range of equilibrium relative nitrogen pressures from 0.01 to 0.99, using gas analyzer ASAP 2020 (Micromeritics). Before performing the measurements, the samples were degassed in a vacuum (residual pressure less than 10-3 mm Hg) directly in the measuring tube at 90 °C. The specific surface area was determined by the Brunauer-Emmett-Teller method (BET) and by the comparative method from the adsorption branch of the isotherm in the region of equilibrium relative nitrogen pressures of 0.05–0.35 and 0.4-0.8, respectively. Raman spectra were excited with 514.5 nm line of argon laser with a power density on the sample of ~100 W*cm−2. The study of the scattered radiation was carried out in the geometry of reflection from the sample surface. The spectra were analyzed using triple monochromatic multichannel spectrometer with a resolution of 3 cm−1 and CCD radiation detector cooled by liquid nitrogen. IR absorption spectra of the samples suspended in paraffinic oil between high-pressure polyethylene plates were recorded using EQUINOX55 Fourier spectrometer (Bruker).
Transmission electron microscope (TEM) FEI Osiris (FEI, USA), with the field emission gun, was used to collect TEM images. TEM micrographs were obtained at accelerating voltage 200 kV. Resolution—0.12 nm at bright field mode (TEM), the resolution—0.18 nm at the transmission scanning electron microscopy. Energy-dispersive X-ray spectroscopy was performed by highly sensitive 4-detector Super-X.
Results and discussion
The prepared samples of ZrO2 nanoparticles were examined by X-ray diffraction and it was concluded that they are amorphous (Fig. 1). The images of TiO2 particles obtained by electron microscopy were described in our earlier paper.[26] The ZrO2 particles form aggregates of strongly interconnected spherical particles with sizes up to 100 nm. The aggregate sizes reach over 1 μm. Therefore, the results of particle size measurements by dynamic light scattering do not provide a full description of particle morphology. While in the case of titanium oxide, we were able to determine the dependence of the size of the resulting nanoparticles on the parameters of the synthesis process: the concentration and the ratio of components, the feed rate of components or fluid, temperature and pressure of the process, the attempts to understand such dependencies for zirconium oxide were not successful.
The scanning calorimetric analysis shows (Fig. 2) that a sample obtained in a SCF medium with heating to ~400 °C demonstrates a large mass loss, with this process happening in two stages. The IR spectrum of the sample shows the large content of organic solvent (isopropyl alcohol and di-isopropyl ether which is one of the reaction by-products) these solvents are removed at temperatures up to 240 °C while water is removed at the second stage 370 °C.
The specific surface area of the ZrO2 samples measured by nitrogen adsorption method is in the range 8-10 m2/g. The volume of mesopores is 59.55% of the total pore volume. At a temperature of 456 °C, the effect associated with the transition of matter from pseudo-amorphous to the crystalline state is observed. At 633 °C, a phase transition to m-ZrO2 is observed. This process is accompanied by the removal of the residual amount of liquid phase (~4%).
Amorphous powders of complex oxides prepared via the sol-gel method have been investigated previously[28] and the results have many commonalities with the results of the present study. The tetragonal phase ZrO2 (P42/nmc; a = 3.60(7), c = 5.1(4)A)was identified in the samples with the stoichiometry 3ZrO2-TiO2, 3ZrO2-2TiO2,ZrO2-TiO2 (75ZrO2-25TiO2,60ZrO2-40TiO2, 50ZrO2-50TiO2%mol) after annealing at 520 °C. At 950 °C the tetragonal phase dissolves in ZrTi2O6, which has an orthorhombic crystal structure (space group Pbcn; PCPDFWIN file 46–1265). Compound ZrTi2O6 is observed after annealing at 950 °C in compositions 3ZrO2-2TiO2, 2ZrO2-3TiO2, ZrO2-3TiO2 (60ZrO2-40TiO2, 40ZrO2-60TiO2, 25ZrO2-75TiO2. Formation of Zr5Ti7O24, was reported previously[29,30] this phase, however, was not observed in our study. The monoclinic and orthotetragonal phases of ZrO2 co-existed after annealing at 520 °C. The orthotetragonal phase converted into monoclinic after annealing at 950 °C. Formation of ZrTi2O6 was not observed. The transition towards lower crystallographic symmetry most likely is an indication of the system approaching an equilibrium state. The transition of anatase to rutile without formation of the monoclinic phase was reported after annealing at 1000 °C.[29] In case of ZrO2-3TiO2 (25ZrO2-75TiO2 mol%) composition, we detected the presence of TiO2 anatase after annealing at 950 °C. Anatase usually stable up to temperatures around 700 °C and it transitions to rutile at higher temperatures. In our case, such transition was not observed probably because of stabilization of anatase phase with a very small amount of zirconium. The increase of the anatase unit cell parameter by approximately 1% could be a manifestation of this effect. Crystallographic data for the identified phases are summarized in Table 1. Data obtained in the present study is in a good agreement with the phase diagram reported in-[30] and contradict with the phase diagram reported in.[26]
It was of interest to consider the possibility of formation of complex phases of this binary system under conditions of CO2 SCF. The ratiosoftitaniumandzirconium oxides, forwhichnano-forms of the samples were obtained, are indicated above. The quality of the X-ray patterns did not allow us to clarify their nature either as a mixture of solid solutions or individual compounds.
The FTIR absorption spectra of the samples obtained under SCF conditions and exposed to vacuum drying (in the spectral region above 100 cm−1) contain a large number of absorption bands in a wide spectral range up to 3600 cm−1. This is consistent with the presence of a large amount of a liquid phase in the samples: water and an organic solvent. However, the spectra analysis makes it possible to identify bands that are shifting with the change in the cation ratio. The most significant frequency shifts are observed for the bands at 646–656, 580–587, 503–515, 430–444, 243–253 and 194–200 cm−1 for the phases with the ratios TiO2/3ZrO2 and 3TiO2/ZrO2, respectively. Such single-mode behavior of some vibrational frequencies is observed throughout the concentration range studied, indicating the formation of a continuous series of solid solutions in the pseudo-amorphous state of TiO2-ZrO2 system. The formation of single-phase agglomerates is confirmed by the analysis of electron micrographs (Fig. 3).
The X-ray patterns of samples calcined above the temperatures of crystallization (450 °C) and phase transition (750 °C) demonstrate the decomposition of the solid solutions above the crystallization temperature and formation of phases in accordance with the known[26,30] phase ratios in the TiO2-ZrO2 system at these temperatures.
Thus, it is established that under the conditions of the CO2 SCF experiment, the formation of nanosized solid solutions is possible in the entire range of ratios of titanium oxides and zirconium oxides having a pseudo-amorphous structure.
The particles of titanium oxide TiO2 are completely amorphous, which is confirmed, in particular, by the ring diffraction pattern [Fig. 4(a)], they have a spherical shape, and form agglomerates measuring from 180 to 800 nm [Fig. 5(a)]. Furthermore, the small particles with sizes up to 30 nm were also observed [Fig. 5(b)].
Amorphous state of ZrO2 particles is also confirmed by ring diffraction [Fig. 4(b)]. The size of spherical particles lies in the limit from 100 to 450 nm; the agglomerates of particles of different sizes are shown in Figs. 5(c) and 5(d).
The particles of solid solutions in the TiO2-ZrO2 system are spherical and form agglomerates [Fig. 3(a)], they have a size from 90 to 850 nm, and they are amorphous, as evidenced by the ring diffraction pattern [Fig. 4(c)]. There is a uniform distribution of titanium and zirconium in the particles [Fig. 3(b)].
Conclusions
The principal conclusion of this study is that the SCF method of particle synthesis describes the formation solid solutions of the starting materials in all region of concentrations. The individual crystalline phases are known in this system, can be obtained from solid solutions with the corresponding ratio of the components by heating above 450 °C.
References
H. Cui, Q. Li, S. Gao, and J.K. Shang: Strong adsorption of arsenic species by amorphous zirconium oxide nanoparticles. J. Ind. Eng. Chem. 18, 1418–1427 (2012).
R. Chakravarty, R. Shukla, R. Ram, A.K. Tyagi, A. Dash, and M. Venkatesh: Development of a nano-zirconia based 68Ge/68Ga generator for biomedical applications. Nucl. Med. Biol. 38, 575–583 (2011).
F.V. Hajiyeva: Luminescent properties of nanocomposites on the basis of isotactic polypropylene and zirconium dioxide nanoparticles. J. Nanomed. Nanotechnol. 7, (2015).
T. Peng, D. Zhao, K. Dai, W. Shi, and K. Hirao: Synthesis of titanium dioxide nanoparticles with mesoporous anatase wall and high photocatalytic activity. J. Phys. Chem. B. 109, 4947–4952 (2005).
X. Chen and S.S. Mao: Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 107, 2891–2959 (2007).
M. Wu, G. Lin, D. Chen, G. Wang, D. He, S. Feng, and R. Xu: Sol-hydrothermal synthesis and hydrothermally structural evolution of nanocrystal titanium dioxide. Chem. Mater. 14, 1974–1980 (2002).
Y. Su, H. Cui, Q. Li, S. Gao, and J.K. Shang: Strong adsorption of phosphate by amorphous zirconium oxide nanoparticles. Water Res. 47, 5018–5026 (2013).
S.J. Roberts, J.J. Dodson, P.L. Carpinone, and H.E. Hagelin-Weaver: Evaluation of nanoparticle zirconia supports in the thermochemical water splitting cycle over iron oxides. Int. J. Hydrog. Energy. 40, 15972–15984 (2015).
R. Daghrir, P. Drogui, and D. Robert: Modified TiO2 for environmental photocatalytic applications: a review. Ind. Eng. Chem. Res. 52, 3581–3599 (2012).
A. Al-Ahmed, B. Mukhtar, S. Hossain, S.M. Javaid Zaidi, and S. U. Rahman: Application of titanium dioxide (TiO2) based photocatalytic nanomaterials in solar and hydrogen energy: a short review in materials science forum. Trans Tech Publications. 712, 25–47 (2012).
Y.S. Kim, M.Y. Song, E.S. Park, S. Chin, G.N. Bae, and J. Jurng: Visible-light-induced bactericidal activity of vanadium-pentoxide (V2O5)-loaded TiO2 nanoparticles. Appl. Biochem. Biotechnol. 168, 1143–1152 (2012).
S.T. Khan, A.A. Al-Khedhairy, and J. Musarrat: ZnO and TiO2 nanoparticles as novel antimicrobial agents for oral hygiene: a review. J. Nanopart. Res. 17, 276 (2015).
X. Fu, L.A. Clark, Q. Yang, and M.A. Anderson: Enhanced photocatalytic performance of titania-based binary metal oxides. Environ. Sci. Technol. 30, 647–653 (1996).
A. Kitiyanan, S. Ngamsinlapasathian, S. Pavasupree, and S. Yoshikawa: The preparation and characterization of nanostructured TiO2-ZrO2 mixed oxide electrode for efficient dye-sensitized solar cells. J. Solid State Chem. 178, 1044–1048 (2005).
Q. Chang, J.E. Zhou, Y. Wang, and G. Meng: Formation mechanism of zirconia nano-particles containing pores prepared via sol-gel-hydrothermal method. Adv. Powder Technol. 21, 425–430 (2010).
K. Geethalakshmi, T. Prabhakaran, and J. Hemalatha: Dielectric studies on nano-zirconium dioxide synthesized through co-precipitation process. World Acad. Sci. Eng. Technol. 64, 179–182 (2012).
T. Buslaeva, E. Kopylova, V. Popenko, A. Potapova, and V. Fomichev: Cerium (III) Carbonate hydroxide nanoparticles encrusted by metallic palladium. Synthesis and investigation. Fine Chem. Technol. 11, 23–28 (2016).
A. Baiker: Supercritical fluids in heterogeneous catalysis. Chem. Rev. 99, 453–474 (1999).
H. Machida, M. Takesue, and R.L. Smith: Green chemical processes with supercritical fluids: properties, materials, separations and energy. J. Supercritical Fluids. 60, 2–15 (2011).
E. Reverchon and R. Adami: Nanomaterials and supercritical fluids. J. Supercritical Fluids. 37, 1–22 (2006).
T. Lu, S. Blackburn, C. Dickinson, M.J. Rosseinsky, G. Hutchings, S. Axon, and G.A. Leeke: Production of titania nanoparticles by a green process route. Powder Technol. 188, 264–271 (2009).
R.P. Marin, S. Ishikawa, H. Bahruji, G. Shaw, S.A. Kondrat, P.J. Miedziak, and M. Bowker: Supercritical antisolvent precipitation of TiO2 with tailored anatase/rutile composition for applications in redox catalysis and photocatalysis. Appl. Catalysis A: General. 504, 62–73 (2015).
J.L. Mi, S. Johnsen, C. Clausen, P. Hald, N. Lock, L. Sø, and B.B. Iversen: Highly controlled crystallite size and crystallinity of pure and iron-doped anatase-TiO2 nanocrystals by continuous flow supercritical synthesis. J. Mater. Res. 28, 333–339 (2013).
M. Tuncer and B. Ozdemir: Photocatalytic activity of TiO2 powders synthesized by supercritical gas antisolvent method. Growth 14, 15 (2013).
I.A. Konovalov, B.N. Mavrin, N.A. Prokudina, and V.V. Fomichev: Synthesis of nanoscale titanium dioxide by precipitation using supercritical anti-solvent. Russ. Chem. Bull. 65, 2795–2800 (2016).
U. Troitzsch and D.J. Ellis: The ZrO2-TiO2 phase diagram. J. Mater. Sci. 40, 4571–4577 (2005).
V.V. Zhurov and S.A. Ivanov: PROFIT computer program for processing powder diffraction data on an IBM PC with a graphic user interface. Crystallogr. Rep. 42, 202–206 (1997).
E.L. Sham, M.A. Aranda, E.M. Farfan-Torres, J.C. Gottifredi, M. Martinez-Lara, and S. Bruque: Zirconium titanate from sol-gel synthesis: thermal decomposition and quantitative phase analysis. J. Solid State Chem. 139, 225–232 (1998).
P. Bordet, A. McHale, A. Santoro, and R.S. Roth: Powder neutron diffraction study of ZrTiO4, Zr5Ti7O24, and FeNb2O6. J. Solid State Chem. 64, 30–46 (1986).
A. McHale and R.S. Roth: Low-temperature phase relationships in the system ZrO2-TiO2. J. Am. Ceram. Soc. 69, 827–832 (1986).
Acknowledgments
The research was carried out using the equipment of the Central Scientific-Technical Center Crystallography and Photonics of the Russian Academy of Sciences, with the support of the Ministry of Education and Science. This work was performed using the equipment of the Shared Research Center IC RAS and supported by The Ministry of Education and Science of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sokolov, I.E., Konovalov, I.A., Zakalyukin, R.M. et al. Synthesis of nanosized zirconium dioxide and its solid solutions with titanium dioxide from the CO2 supercritical fluid. MRS Communications 8, 59–64 (2018). https://doi.org/10.1557/mrc.2018.3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrc.2018.3