Abstract
The mesoporous anatase form of TiO2 was prepared by modified sol–gel method using ionic liquid as a template agent. The prepared nanosize TiO2 particle was characterized by N2-physisorption, XRD, TEM, and SEM. The physical properties of prepared TiO2 particles were compared with that prepared by conventional sol–gel method without template. It has been proved that the anatase phase prepared by modified sol–gel process using ionic liquid was preserved well even if the TiO2 samples were treated at high temperatures up to 800 °C while those prepared by conventional sol–gel method were transformed from anatase to rutile phase gradually during calcinations at 600 °C. Moreover, there was no phase transition in the sample obtained by sol–gel method with ionic liquid in spite of prolonged calcination for 60 h at 600 °C. However, in case of those samples prepared by conventional sol–gel method, the portion of rutile form was continuously increased with the increase in the calcination period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Titanium dioxide (TiO2, titania) has received widespread attention due to its numerous catalytic applications such as photocatalyst and catalyst support [1]. For catalysis applications, the crystalline structure and the degree of crystallinity of the TiO2 play an important role in determining its performance. It is well known that anatase crystalline phase of TiO2 is metastable and transform into rutile at higher temperatures [2]. Especially, the porous anatase form, as compared to the rutile phase, is of greater importance and interest due to its better catalytic properties [3]. So, an important aspect in the preparation of TiO2 as a catalyst and/or support is the development of high surface area TiO2 with controlled porosity and higher anatase phase thermal stability. Recently, various techniques of surfactant templating have been employed on preparing nano-structured TiO2 materials with enhanced physical properties [4].
Crystalline nano-structured TiO2 particle with high surface area and narrow pore size distribution was synthesized [5] at low temperature using ionic liquid by sol–gel method. However, the method has crucial drawbacks such as high consumption of expensive ionic liquid and additional process for removal of ionic liquid with organic solvents. In this work, thermally stable TiO2 particles with high surface area and anatase phase were synthesized using modified sol–gel method with small amount of ionic liquid (IL). The prepared samples were characterized to identify the physical properties by N2-physisorption, XRD, TEM, and SEM.
2 Experimental
2.1 Reagents
Titanium (IV) isopropoxide (TTIP) (97%, Aldrich Chemical Co.), acetic acid (99.99% glacial, biochemical grade, Acros Organics), 2-propanol (99.5%, Samchun Chemical, South Korea) and ionic liquid (C-Try Co., South Korea) were used to prepare TiO2 in the present study. Deionized water was used throughout in this work.
2.2 Preparation of TiO2 Particle
A small amount of IL was applied to the conventional sol–gel method using acetic acid as a chelating agent [6]. First, TTIP was reacted with acetic acid at room temperature with CH3COOH/TTIP molar ratio of 1 under Ar atmosphere. Ionic liquid (1-butyl-3-methylimidazolium hexafluorophosphate, [bmim][PF6]) was then added into the mixture at an IL/TTIP molar ratio of 0.03 (this content is one-hundredth of earlier experiment [5]) with stirring for 10 min. The resulting mixture was diluted with 2-propanol and stirred for additional 5 min. The titanium alkoxide solution was hydrolyzed by adding 1 M hydrochloric acid solution dissolved in 2-propanol. After several minutes, the solution turns into a white emulsion. This emulsion was then aged for 7 days and was dried thoroughly at 80 °C for 3 days. The entrapped IL and organics were removed by calcination without additional extraction treatment.
2.3 Characterization of TiO2 Particle
From adsorption–desorption isotherm, BET surface area and total pore volume of TiO2 prepared were measured by N2-physisorption analyzer (Quantachrome Co., Autosorb-1C). All samples were degassed at 150 °C for 4 h before N2-physisorption study. The powder XRD patterns of samples were recorded at an ambient temperature in air using XRD (Shimazdu Co., XRD-6000) with a Cu-Kα radiation (λ = 1.54056 Å), operated at 40 kV and 30 mA. It was used to determine the phase and crystallinity of the TiO2 sample and also to identify the change of the major phase as a function of calcination temperature. The identification of peaks was accomplished by the comparison of the recorded spectra of the samples with a reference TiO2 samples from JCPDS powder diffraction file data. The morphology and crystal size were measured by using SEM (Hitachi Co., S-4200) and TEM (Philips Co., CM30).
3 Results and Discussion
Two samples designated as TiO2–SG–0.03 IL and TiO2–SG–without IL were prepared in the present study by modified sol–gel method using a small amount of ionic liquid (0.03 IL) and conventional sol–gel method without ionic liquid, respectively.
The effects of calcination temperature on the BET specific surface areas and total pore volume of two TiO2 samples prepared in this work are illustrated in Fig. 1. With increase in calcination temperature, the BET surface area was found to decrease for both samples due to pore collapse and shrinkage. TiO2–SG sample showed higher surface area than TiO2–SG–0.03 IL sample up to 550 °C. However, it was found that the sample prepared by modified sol–gel method with an IL/TTIP molar ratio of 0.03 (TiO2–SG–0.03 IL) showed significant thermal stability above 600 °C than the TiO2–SG prepared by conventional sol–gel method without IL. The BET surface area of TiO2–SG–0.03 IL sample was relatively high even after heat treatment at 800 °C. The thermal stability of the TiO2 particles prepared with IL was in good agreement with their structural characteristics.
The total pore volume and BET surface area of the TiO2 particles prepared without IL decreased rapidly from 0.159 to 0.013 cc/g and from 197 to 2 m2/g, respectively, upon heat treatment from 100 °C to 800 °C. TiO2 prepared using small amount of IL shows low total pore volume below 400 °C. However, it shows higher pore volume than TiO2–SG without IL after calcinations at 400 °C and up to 800 °C. It seems that TiO2 particles prepared by modified sol–gel method using 0.03 IL possesses IL template and organics at low temperature below 400 °C. Then through calcination, the entrapped template and organics in pore structure were removed thoroughly.
The N2 adsorption–desorption isotherms of TiO2 samples prepared by modified and conventional sol–gel methods heated at different temperatures are presented in Fig. 2. The TiO2–SG–0.03 IL sample heated at 100 °C has shown adsorption of N2 nearly to constant value. But samples heated at 400, 600 and 800 °C has exhibited a hysteresis loop of type II with almost vertical and nearly parallel adsorption and desorption branches which is similar to the commercial TiO2–P25 (Degussa, not shown) [7]. The limiting adsorption at P/P 0 = 1 in all the samples calcined at 400–800 °C attains an extremely large value of almost 100 cc/g. In samples heated at 400–800 °C, the relative pressure P/P 0 for the sudden rise in the adsorption increases with increase in heating temperature. TiO2–SG sample heated at different temperature showed isotherms of type IV with hysteresis loop, which indicates the mesoporous nature of TiO2, but the hysteresis loop at high relative pressures confirms that the mesopores in the samples were not regular. Because the mesopores and nanoparticles coexist in the obtained samples, it would be reasonable to say that the hysteresis loops can be attributed to the total contribution of both intraparticle pores and an interparticle pores. The inset figures show the pore size distribution curves for their samples. The TiO2–SG without IL shows pore size around 5 nm at 400 °C, which was found to be almost collapsed around 600 °C. However, inset TiO2–SG–0.03 IL sample shows pore around 10 nm which were increased to ∼18 nm, at 600 °C, may be due to the removal of organic part from the pores.
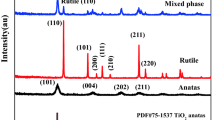
Figure 3 shows XRD patterns of TiO2–SG–0.03 IL and TiO2–SG–without IL samples calcined at different temperatures. The 2θ values of the most intense peak of anatase is 25.3 and that of rutile is 27.5, respectively [8]. It was observed that the crystallinity of TiO2 particles increased with increasing calcination temperature. The TiO2 particles prepared without IL (TiO2–SG) were amorphous after drying at 100 °C (not shown in figure), but they were transformed to anatase phase after the calcination at 400 °C. The crystalline phase of anatase was partially transformed to rutile at around 600 °C and then completely at 800 °C. On the other hand, TiO2–SG–0.03 IL was found thermally very stable. It was found that the sample retained anatase phase crystallinity even after calcination at 800 °C.
Although the surface area of TiO2–SG–0.03 IL sample was less than other sample (Fig. 1), the IL played an important role in retaining their anatase phase at high temperature treatment as shown in the Fig. 3. Especially, TiO2 prepared by conventional sol–gel method without IL showed a rapid collapse of pore structure and crystalline phase transition. It can be concluded that TiO2 prepared by modified sol–gel method using a small amount of IL showed higher thermal stability than the TiO2 sample prepared by conventional method.
The phase transition behavior was investigated with many circumstances at 600 °C. In order to examine the effect of calcination time, TiO2 particles prepared by conventional sol–gel and ionic liquid assisted sol–gel methods were calcined at 600 °C for 2–60 h. It was found that TiO2 particles prepared by conventional sol–gel method start transforming from anatase to rutile phase gradually during calcination at 600 °C for 2 h. The portion of rutile form was continuously increasing with increase in the calcination period at constant temperature as shown in Fig. 4. However, there was no phase transition in the TiO2 particles calcined at 600 °C for 60 h prepared by modified sol–gel method with [Bmim][PF6]/TTIP molar ratios of 0.03. The results suggest that the modified sol–gel method is an attractive preparation method for the support having ability for reaction at high temperature [9].
The morphology of TiO2 samples prepared by modified sol–gel method using [Bmim][PF6] was compared with the sample prepared by conventional method without IL. The two images (a) and (b) in Fig. 5 are the TEM micrographs of TiO2–SG–0.03 IL samples calcined at 600 °C for 2 and 60 h, respectively. They show particle sizes in between 15 and 30 nm when calcined at 600 °C for 2 and 60 h, respectively. The images (c) and (d) are those of TiO2–SG sample calcined at 600 °C for 2 and 60 h, respectively. Sample calcined at 2 h has large agglomerates in various shapes and size and are found to increase with increase in calcination period to 60 h. TEM data reveals that the length of calcination period up to 60 h does not have much effect on the size of the TiO2 particles prepared using ionic liquids. The size of particles was found in nanometer scale.
The morphology of TiO2 sample prepared using ionic liquid was also studied by SEM and was compared with TiO2 sample prepared by conventional sol–gel technique. The images (a) and (b) in Fig. 6 are SEM micrographs of TiO2–SG–0.03 IL sample calcined at 600 °C for 2 and 60 h, respectively. The particles are uniform in size and in shape. The TiO2 prepared by conventional sol–gel technique and calcined at 600 °C for 2 and 60 h are shown in (c) and (d), respectively. Sample calcined at 2 h consists of irregular shaped particles of various size (30–40 nm) which on calcining for 60 h, agglomerates into big particles varying in size (50–200 nm). It can be concluded that the TiO2 samples prepared with ionic liquids are not peered to agglomeration and are stable to its morphology at prolonged heat treatment.
Resistance to sintering effect of the nano-sized TiO2 particles for a prolonged period may play an important role in suppression of its phase transition at higher temperature [10]. XRD data support their result.
4 Conclusions
The mesostructured TiO2 with controlled porosity and regular nanoparticle size was prepared using a small amount of ionic liquid as a pore forming agent via a modified sol–gel process. Though TiO2 was not crystallized as an anatase phase at low temperature, its anatase phase crystallinity was found to increase with increase in calcination temperature and was found to be thermally very stable in its phase and morphology, resistant to pore collapse and not decline to anatase to rutile phase transformation during calcination at higher temperature unlike the TiO2 prepared by a conventional sol–gel method. Also its physical and thermal stability was well retained even during prolong heat treatment at 600 °C up to 60 h. It may be inferred that the property of nanosize TiO2 samples resistant to heat treatment at high temperature, helped them to preserve their anatase crystalline phase.
The residual template in the TiO2 samples prepared by the modified sol–gel method was removed by calcination at 400 °C without any extraction process. Though the surface areas of prepared TiO2 samples were found to decrease with calcination, the degree of reduction in surface area was not so high compared to TiO2 sample prepared by the conventional sol–gel method.
It can be concluded further that controlling materials at a nanosize level can accelerate the development of new types of products with improved properties and functionality for several other applications.
References
Fujishima A, Hashimoto K, Watanabe T (1999) TiO2 photocatalysis, fundamentals and applications. BKC Inc., Tokyo
Vittadini A, Casarin M, Selloni A (2007) Theor Chem Acc 117:663
Phonthammachai N, Chairassameewong T, Bulari E, Jamieson AM, Wongkasemjit S (2003) Microporous Mesoporous Mater 66:261
Antonelli DM, Ying JY (1995) Angew Chem Int Ed Engl 34:2014
Yoo KS, Choi H, Dionysiou DD (2005) Catal Commun 6:259
SaKKa S (ed) (2003) Sol–gel science and technology. Kluwer Academic Publishers, Boston
Wang JA, Cuan A, Salmones J, Nava N, Castillo S, Moran-Pineda M, Rojas F (2004) Appl Surf Sci 230:94
Liquiang J, Xiaojun S, Bifu X, Baiqi W, Weimin C, Honggan F (2004) J Solid State Chem 177:3375
Moon DJ, Choi EH, Rye JW, Yoo KS, Lee SD, Ahn BS (2005) Korea Patent 10–753055
Choi EH, Kim DH, Shim JG, Lee H, Lee SD, Moon DJ (2006) 11th APCChE
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, E.H., Hong, SI. & Moon, D.J. Preparation of Thermally Stable Mesostructured Nano-sized TiO2 Particles by Modified Sol–Gel Method Using Ionic Liquid. Catal Lett 123, 84–89 (2008). https://doi.org/10.1007/s10562-008-9398-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9398-4