Abstract
Epoxidized soybean oil was produced in this study by in situ generated peroxycitric with citric acid and hydrogen peroxide. Different production techniques were applied, namely conventional heating epoxidation and microwave-assisted epoxidation, using the Prileschajew method. Three different processes were studied in the conventional heating process: homogeneous catalysis with sulfuric acid, heterogeneous catalysis with Amberlite IR-120, and a process with no catalyst. Compared to acetic acid, citric acid is less toxic, safer for the epoxidation process, and does not require a strong acid catalyst for the reaction to occur, although oxirane oxygen content is higher when acetic acid is used as the oxygen carrier. Thermal runaway risks can be reduced by replacing acetic acid with citric acid since the latter is less volatile and more susceptible to steric hindrance. Citric acid is more acid than acetic acid, evidenced by a lower pKa, and then tends to favor ring-opening reactions once the epoxy group is produced. In the microwave heating process, epoxidation using both acetic acid and citric acid was studied. Microwave-assisted epoxidation allowed a decrease in reaction time from 2 h to 15 min when citric acid epoxidation was performed with similar resulting oxirane oxygen contents. In epoxidation with acetic acid, time was reduced from 2.3 h to 10 min in homogeneous catalysis with sulfuric acid and from 5 h to 25 min in heterogeneous catalysis with Amberlite IR-120.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epoxidized vegetable oils have been largely produced and studied for being originated from a renewable feedstock, presenting lower cost than petroleum raw-materials (Gupta et al. 2011; Jin and Park 2008; Saremi et al. 2012) and multiple applications such as lubricant (Adhvaryu and Erhan 2002), biodegradable polymers (Al-Mulla et al. 2011), and plasticizer (Yang et al. 2014). Epoxides are highly reactive precursors of various substances such as alcohols and polyesters, being derived from natural or synthetic substances (Chua et al. 2012). Epoxidized vegetable oils can be used as intermediates in the production of polyurethanes (Dworakowska et al. 2012; Pérez-Sena et al. 2018) and the production of composite materials as a replacement for polymeric matrices (Bhalerao et al. 2016; Espinoza-Perez et al. 2011; Sahoo et al. 2018; Vu et al. 2018).
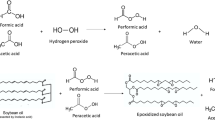
An epoxide is a functional group in which an oxygen atom is bonded to two carbon atoms in a cyclic structure. Epoxidation occurs when unsaturated carbon compounds are treated with hydrogen peroxide in the presence of oxygen donating substances. In the first step, hydrogen peroxide reacts with a carboxylic acid in an acid medium to form a peroxyacid (Bennett 1956) in a perhydrolysis reaction, as shown in Fig. 1:
The second step is the epoxidation reaction, in which the epoxy group is produced from unsaturations in the vegetable oil: Fig. 2.
In Figs. 1 and 2 it was described epoxidation with the Prileschajew method. Different oxygen carriers can be used in the production of epoxidized vegetable oils, such as formic acid (Campanella et al. 2008; Mushtaq et al. 2011), acetic acid (Boyacá and Beltrán 2010; Kim and Sharma 2012; Lage et al. 2014; Perez et al. 2009; Petrovic et al. 2002) and propionic acid (Aguilera et al. 2018; Leveneur 2017). In the case of acetic acid and especially formic acid, thermal runaway situations can occur in the reacting system (Leveneur et al. 2015; Pérez-Sena et al. 2020), leading to high risks in operation. Epoxidation can also occur directly with hydrogen peroxide without an oxygen carrier; that approach can reduce thermal runaway risks, as reported elsewhere (Pérez-Sena et al. 2020). In this study, citric acid is proposed as an oxygen carrier that can minimize thermal runaway issues, as it is less volatile than both acetic acid and formic acid and more susceptible to steric hindrance due to a more ramified structure.
Citric acid is a relatively weak carboxylic acid, vastly common in nature, used by factories for many purposes. In the food industry, citric acid is regulated as an acidulant, antioxidant, acidity regulator, and sequestrant (ANVISA 1999). It can also be used as a cleaning agent (Bermudez-Aguirre and Barbosa-Canovas 2013), a catalyst in reticulated fiber production (Widsten et al. 2014), and an anticoagulant (Li et al. 2012). Although it is mainly known to be used for food applications, it has been studied as a crosslinking agent for epoxy resins (Altuna et al. 2013; Gogoi et al. 2015a, b), producing bio-based polymer networks with reasonable properties, with a major advantage of reducing toxicity when compared with some of the commercial curing agents (Ding and Matharu 2014). The use of citric acid as an epoxidation agent has also been demonstrated (Suzuki et al. 2018), and the self-catalyst property of CA allows the process to be completely eco-friendly, with no need for other catalysts and no toxic residue production.
Epoxidized vegetable oils production can be assisted by both heterogeneous and homogeneous catalysis. In homogeneous catalysis, a peroxyacid is produced in the presence of a strong mineral acid such as sulfuric acid or phosphoric acid, allowing a faster epoxidation compared to heterogeneous catalysis, although selectivity may be reduced (Santacesaria et al. 2011; Zheng et al. 2016). Goud et al. (2006) studied epoxidation of Karanja oil (Pongamia glabra) with acetic acid, hydrogen peroxide, and sulfuric acid as a catalyst. Authors studied the molar ratio between reagents, and they found that beyond 1500 rpm in mechanical stirring, epoxidation reaction is free from mass transfer resistance, and the kinetic regime controls the process.
Heterogeneous catalysis, frequently chosen due to the simplicity of separation at the end of the reaction, involves utilizing solid catalysts with acid groups on their surface. One example is ion-exchange resins such as Amberlite IR-120, which are hydrophilic in general, and allow only small molecules to enter their pores but not the epoxidized vegetable oil, avoiding oxirane ring-opening (Cooney 2009). The pores’ size will dictate the ability to catalyze the epoxidation reaction and the selectivity of the catalyst (Sinadinovic-Fiser et al. 2001).
Somidi et al. (2014) tested synthesized sulfated SnO2 as a catalyst for canola oil epoxidation. The optimized conditions resulted in 100% conversion of double bonds and excellent selectivity to oxirane of 97% in the conventional process using acetic acid and hydrogen peroxide and a reaction time of 6 h. A catalyst based on a molybdenum complex on montmorillonite K-10 was produced by Farias et al. (2011) in the epoxidation of soybean and castor oils. Tert-butyl hydroperoxide was used as the oxygen carrier, and reaction times were varied from 2 to 24 h. With 4 h of reaction in castor oil epoxidation, researchers obtained 82% conversion of double bonds and 78% selectivity to oxirane. Jatropha curcas press cake, residual biomass, was used to produce biochar applied in the epoxidation of cottonseed oil with acetic acid and hydrogen peroxide in the study of Silva et al. (2017). In their study, the maximum conversion of double bonds was 73%, and the yield of epoxidation was 82% with biochar, although catalytic activity decreased after the second cycle, which may limit the application of the produced catalyst. Di Serio et al. (2012) studied soybean epoxidation using hydrogen peroxide and a heterogeneous catalyst composed of a mixture of Nb2O5 and SiO2. However, researchers obtained at most 30% of conversion of double bonds when 2.5% Nb2O5 catalyst was applied. More recently, Pérez-Sena et al. (2021) studied methyl oleate’s epoxidation using direct oxidation with hydrogen peroxide in a process assisted by AlO2 catalyst. The authors compared a batch and a semi-batch reactor, and it was found that a continuous addition of H2O2 in the process can improve conversion to oxirane in 30%.
Microwave technology has already been proven as an efficient method to decrease production times, applied in many different processes, including transesterification to produce biodiesel (Azcan and Yilmaz 2013; Rabelo et al. 2015), production of activated carbon (Barbosa et al. 2015), and epoxy curing (Maenz et al. 2015). Microwaves can fasten chemical reactions due to both thermal and non-thermal effects. One possible mechanism is the selective absorption of microwave energy by polar molecules, while non-polar molecules are transparent to microwaves (El Sherbiny et al. 2010). Microwaves can also increase the accessibility of the susceptible bonds, promoting an efficient chemical reaction and reduced by-products (Azcan et al. 2008). An essential drawback in the epoxidation of vegetable oils using Prileschajew oxidation is the reaction time; it may take up to 10 h for achieving conversion levels of 80 to 90%, which allows epoxidation intensification and a consequent reduction in reaction times (Chavan et al. 2012). The use of microwaves in epoxidation leads to a reduction in reaction times due to a higher interfacial mass transfer than the conventional heating process (Aguilera et al. 2016). The microwave-assisted epoxidation should be well controlled since ring-opening is accelerated as well (Aguilera et al. 2016), and non-isothermal heating could lead to a thermal runaway (Leveneur et al. 2014). Several studies were published in the microwave-assisted epoxidation of vegetable oils, but none of them study epoxidation with citric acid as an oxygen carrier.
Leveneur et al. (2014) published a study in the epoxidation of oleic acid with peroxypropionic acid and peroxyacetic acid formed in situ, and the microwave irradiation provided a faster epoxide conversion. However, a 70% conversion of oleic acid was attained in approximately 3 h, for an average absorbed power of 10 W. Saifuddin et al. (2011) performed a heat-modeling of epoxidation of palm oil using formic acid and an enzyme catalyst. Results showed that the highest conversion to oxirane oxygen was obtained in approximately 33 min, and the optimum temperature was 75 °C. Other published papers in microwave epoxidation technology involve oxirane ring-opening and production of polyols (Dworakowska et al. 2012; Nikje et al. 2011; Saifuddin et al. 2010). Piccolo et al. (2019) studied the effects of temperature and agitation in microwave-assisted epoxidation, results showed a reduction in epoxidation time from 3 h in conventional heating to 1.5 h in microwave heating, with a similar yield for both methods. More recently, Aguilera et al. (2016) developed a new microwave irradiated epoxidation system of oleic acid. Shortly, the system is comprised of a batch microwave reactor with a loop, allowing recycling of the products, which are then heated and mechanically stirred before being fed once again to the microwave reactor. In that system, acetic acid and hydrogen peroxide were used to produce in situ peroxyacetic acid and then epoxidized oleic acid. Best results under microwave irradiation are obtained with an oleic acid: hydrogen peroxide: acetic acid ratio of 1–4.5–2.4 in approximately 9 h. 98% of double bonds were consumed by the peroxyacid.
In this study, citric acid is evaluated as an oxygen carrier in both conventional and microwave-assisted epoxidation, in which different catalysts were explored: sulfuric acid in homogeneous catalysis and Amberlite IR-120 in heterogeneous catalysis, apart from citric acid itself. No studies have been published in literature with citric acid as an oxygen carrier in microwave epoxidation, and, as far as we know, no study performed a comparison between citric acid and acetic acid as oxygen carriers for epoxidation. This study investigates citric acid epoxidation in the conventional heating process, using a comparative evaluation of different catalysts, investigates citric acid and acetic acid epoxidation under microwave heating, and performs a preliminary evaluation of energy consumption and reduction of reaction times by using the microwaves.
Materials and methods
Materials
Soybean oil (LIZA, PR, Brazil) was purchased in local grocery stores. Reagents glacial acetic acid (99,7%), anhydrous citric acid, sulfuric acid 98%, iodine-monochloride Wijs solution, potassium iodate, potassium iodide, hydrochloric acid, sodium thiosulfate, soluble starch, chloroform, hydrobromic acid 48%, diethyl ether (99%) and gentian violet were acquired from Synth (Sao Paulo, Brazil). Catalyst Amberlite IR-120 (hydrogen form) was purchased from Sigma-Aldrich (St. Louis, United States).
Methods
In this section, methodologies for conventional and micro-wave irradiated routes are described briefly.
Conventional routes
Conventional route epoxidation was performed adapted from a previous study in our research group (Suzuki et al. 2018), with slight modifications. In the heterogeneous catalyst approach, fifty grams of soybean oil and catalyst Amberlite IR-120 H (22.1 g) were added to a semi-batch jacketed reactor coupled with a thermostatic bath (Lucadema—SP, Brazil) equipped with a mechanical stirrer and a reflux condenser. A mixture of hydrogen peroxide 50% (wt/wt) and anhydrous citric acid was added to the reactor when the temperature reached a specified value. Fifteen different tests were performed according to Table 1, varying the unsaturation to citric acid to hydrogen peroxide molar ratio (UNS:CA:HP), reaction times, and the temperatures. Time zero was set when the specified temperature was reached, which was about 60 min.
Three different kinetic epoxidation studies were performed, using Amberlite IR-120 as the heterogeneous catalyst, sulfuric acid as the homogeneous catalyst, and a citric acid autocatalyzed process. Two hundred fifty grams of soybean oil were used in each test. An unsaturation to citric acid to hydrogen peroxide (UNS:CA:HP) molar ratio of 1.0:0.5:2.2 was applied in all tests, the temperature was set to 80 °C, and the reaction mixtures were mechanically stirred (500 rpm) in a semi-batch jacketed reactor coupled to a thermostatic bath, equipped with a reflux condenser. In the heterogeneous catalyst approach, 110.35 g of Amberlite IR-120 was used, and the reaction times ranged from 30 min to 5 h; in the homogeneous catalyst approach, 2700 µL of sulfuric acid 98% wt/wt was used, and the reaction times were varied from 30 min to 2 h. Finally, in the process with no external catalyst, reaction times ranged from 30 min to 4.5 h. Aliquots of 10 mL were taken from the reactor at the specified times; the samples were washed with distilled water, extracted with diethyl ether, roto-evaporated to recover the solvent, and dried overnight at 50 °C.
Microwave-assisted epoxidation
Epoxidized soybean oil with citric acid was produced in this study under different processing conditions, namely time, temperature, and nominal power. Proportions between reagents were the same as the conventional route. Citric acid epoxidation was performed in a multimode microwave-assisted reactor StartSYNTH (Milestone Inc., United States) equipped with a quartz magnetic stirrer, a reflux condenser, and temperature control. Stirring was defined in 69% of equipment maximum capacity or about 600 rpm. Twenty-five grams of soybean oil was added to the reactor, and after 5 min, the time needed to reach the target temperature, a catalytic mixture comprised of citric acid (12.31 g), hydrogen peroxide (16.0 mL), and concentrated sulfuric acid (270 μL, 2% wt/wt) was added slowly to the reactor, which is equivalent to a UNS:CA:HP molar ratio of 1:0.5:2.2. The temperature was adjusted to 80 °C, reaction times were varied from 5 to 17.5 min, and nominal powers of 200 W, 300 W, and 400 W were tested. The system was then cooled with air for 10 min before washing with water. At the end of the process, 200 mL of distilled water was added to the system the reaction to halt any reaction that might be still occurring. To study the effect of forced cooling in epoxidation, several tests were done with the addition of distilled water immediately after reaction time was completed.
Acetic acid (AA) epoxidation was adapted from literature studies (Boyacá and Beltrán 2010; Perez et al. 2009). 4.5 mL of glacial acetic acid and 16 mL of hydrogen peroxide 50% wt/wt were used in the processes with sulfuric acid used for homogeneous catalysis (equivalent to an UNS:AA:HP molar ratio of 1:0.67:2.2) and Amberlite IR-120 (hydrogen form) ion-exchange resin was used for heterogeneous catalysis. Amounts of sulfuric acid (98% wt/wt) and Amberlite IR-120 were 270 μL and 5.28 g, respectively. Nominal power was 200 W in all experiments. Times ranged from 5 to 15 min for homogenous catalysis and from 5 to 25 min for heterogeneous catalysis, respectively.
Purification steps were performed exactly as in the conventional route, i.e., by washing the organic layer with distilled water until the pH of residual water was neutral, extracting with diethyl ether, roto-evaporating the solvent, and drying overnight at 50 °C in an oven.
Characterization of produced epoxidized vegetable oils
Samples were characterized employing iodine value determination according to ASTM Standard D5554-15 (ASTM 2015). Soybean oil iodine value is used to calculate theoretical maximum oxirane oxygen content, according to Eq. (1):
In Eq. (1), IVO is the vegetable oil iodine value, AI is the iodine atomic number, and AO is the atomic number of oxygen.
Equation (2) was used to calculate the percentage conversion of double bonds, in which IVo represents soybean oil iodine value and IVexp represents epoxidized soybean oil iodine value.
Oxirane oxygen content was determined according to AOCS Standard Cd9-57 (AOCS 2009). Oxirane oxygen content analysis indicates the experimental procedure’s efficiency by comparing the results with the theoretical maximum value. Equation (3) was used to calculate relative conversion to oxirane oxygen, in which Oexp is the experimental value and OTM is the maximum theoretical value.
All the characterization analyses were performed in triplicate, and mean values and standard deviations were calculated.
Results and discussion
Conventional epoxidation: heterogeneous catalyst
The experimentally determined iodine value of soybean oil was 131.8 ± 0.4, like the one obtained by Perez et al. (2009), 133.6 ± 0.3. Theoretical maximum oxirane oxygen content, determined by Eq. (1), was 7.67% wt.
Iodine value, double-bond conversion, oxirane oxygen content, and relative conversion to oxirane results are summarized in Table 2. Maximum oxirane oxygen content, 5.26%, was obtained in sample 3, with unsaturation to citric acid to hydrogen peroxide molar ratio (UNS:CA:HP) of 1:0.5:2.2, a temperature of 80 °C, and reaction time of 4 h. It was observed that higher reaction times increased double-bond conversion but did not increase oxirane oxygen content (samples 12 and 15), which is a sign of oxirane ring-opening. When the time reached more than 6 h, the reaction mixture started to increase viscosity and solidify, which might be due to epoxy polymerization with citric acid, as studied by some researchers (Altuna et al. 2013; Gogoi et al. 2015a, b).
One can also compare samples 2 and 3, in which the same reaction time (4 h) and the same amounts of reagents were added to the reaction flask, but temperature changed from 70 to 80 °C. Conversion of double bonds increased from 43.6% to 82.6%, and the average conversion to oxirane oxygen increased from 23 to 68%. Specifically, 80 °C tends to favor epoxidation reaction at the expense of other reactions, such as oxirane ring-opening reaction, for example. An increase in temperature to 85 °C (sample 4) did not favor either the epoxy group production or the conversion of double bonds.
It is possible to compare samples 6, 7, and 8, in which reaction time was 5 h, the temperature was 70 °C, and the unsaturation to citric acid to hydrogen peroxide molar ratios were 1:0.17:2.0, 1:0.33:2.0, and 1:0.5:2.0). Higher amounts of citric acid favored epoxidation reaction and the conversion of double bonds. This result agrees with pKa values for citric acid: 3.15, 4.76, and 6.40 (Haynes and Lide 2012). Although citric acid displays three carboxylic groups in its structure, just one is reactive enough to contribute significantly to Prileschajew oxidation.
Finally, one can compare samples 9, 10, and 11, in which the only difference was the amount of hydrogen peroxide (H2O2). In sample 9, 2.2 mol of H2O2 per mole of unsaturation was enough to quickly dissolve the desired amount of citric acid. When the volume of H2O2 was increased further (samples 10 and 11) neither the conversion of double bonds nor the relative conversion to oxirane increased.
The kinetic profile for conventional epoxidation assisted by Amberlite IR-120 is plotted in Fig. 3. The maximum relative conversion to oxirane was 68% in a reaction time of 4 h. If the reaction can continue far from this point, the conversion of double bonds increases but the conversion to oxirane decreases, which is evidence of the epoxy ring-opening process.
Conventional epoxidation: no catalyst
According to a previous study, conventional epoxidation with citric acid can be performed without a catalyst (Suzuki et al. 2018). A preliminary kinetic study was performed to better understand how time influences the epoxidation process without the ion-exchange resin due to citric acid’s ability to auto-catalyze the perhydrolysis reaction to produce the peroxycitric acid, which favors the epoxidation process. This ability is associated in part with its lower value of first pKa (pKa1 = 3.13) compared to acetic acid (pKa = 4.756) (Haynes and Lide 2012). Formic acid (pKa = 3.75) (Haynes and Lide 2012) is able to autocatalyze perhydrolysis reaction as well (Bhalerao et al. 2016; Campanella et al. 2008; Wu et al. 2016).
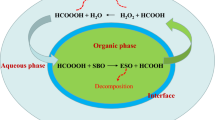
Peroxycitric acid (PCA) synthesis studies in the literature are not frequently found. Ferdousi et al. (2006) synthesized PCA using citric acid and hydrogen peroxide with sulfuric acid as catalyst and without a catalyst. The final concentration of PCA was significantly lower when no catalyst was used. This is evidence that autocatalysis of PCA production by citric can also be explained by chemical equilibrium considerations and Le Chatelier principle. In our process, PCA is produced in the perhydrolysis reaction (Fig. 1), catalyzed by citric acid itself. Once PCA is produced, it is consumed quickly in epoxidation reaction, which shifts the equilibrium on the perhydrolysis reaction to the right according to Le Chatelier’s Principle, contributing to the process’s continuity.
Results from iodine value and oxirane oxygen content determination are shown graphically in Fig. 4.
Results exhibited in Fig. 4 demonstrate that the highest relative conversion to oxirane was obtained in 4.5 h, 57%, which represents a significant reduction compared to previous tests, in which ion-exchange resin Amberlite IR-120 (hydrogen form) was used (sample 3 in Table 2). Although epoxide content decreased by eliminating catalyst, the production cost was also decreased, as the residue production. For instance, there is a reduction in water consumption during the washing steps compared to acid-catalyzed processes. If the reaction continues after 4.5 h, a reduction in oxirane oxygen content and selectivity, probably due to epoxide ring-opening with the formation of polyols, and also by crosslink reactions, since citric acid has been studied as a curing agent (Altuna et al. 2013; Gogoi et al. 2015a, b). This kinetic profile agrees with a trend obtained by Wu and coworkers in the study of conventional heating epoxidation using formic acid and no catalyst (Wu et al. 2016).
Conventional epoxidation: homogeneous catalyst
Conventional heating epoxidation with citric acid and sulfuric acid as catalyst results are plotted in Fig. 5.
Results in Fig. 5 show that with sulfuric acid as the catalyst, reaction times can be decreased significantly compared to heterogeneous catalysis and the process without the catalyst discussed previously. A relatively high oxirane oxygen content was obtained in 2 h, 5.15% wt/wt, which is 4% lower than the maximum value obtained with heterogeneous catalyst Amberlite IR-120 H, which is more expensive than sulfuric acid in the international market. However, reaction time was reduced by 56%. The maximum value in homogeneous catalysis is 15% greater than the maximum value without the catalyst, which was 4.38% wt/wt. The reaction is faster when sulfuric acid catalysis is used, compared to the other approaches. A similar trend was shown in the study of Kurańska et al. (2019). That can be associated with the strength of the acid catalyst (Dinda et al. 2008) and also with the concentration of the catalyst in the system (Abolins et al. 2020). In our study, due to stirring speed, we can infer that process is controlled by mass transfer (Goud et al. 2006), in which a homogeneous catalyst is more effective in reducing mass transfer restrictions. When reaction time surpasses 2 h, the viscosity started to increase quickly, leading to solidification of the mixture.
Boyacá and Beltrán (2010) studied epoxidation with sulfuric acid and acetic acid, obtaining a 6.4% wt/wt oxirane oxygen content in 2.3 h. The oxirane oxygen content for citric acid epoxidation was also lower than the values obtained in other studies when acetic (Kim and Sharma 2012; Lage et al. 2014; Perez et al. 2009) and formic (Galli et al. 2014; Meadows et al. 2018; Musik et al. 2018) acids are employed as oxygen carriers, probably due to steric hindrance restrictions.
One can compare kinetic profiles shown in Figs. 3, 4, 5. Amberlite IR-120 was the most selective catalyst and also the one which led to a higher relative conversion to oxirane. That may be in part due to acid-catalyzed reactions occurring just in the pores of ion exchange resin, and it is more difficult for epoxidized soybean oil to enter these pores. Then, once the epoxy group is formed, its ring-opening will be more difficult to occur in heterogeneous catalysis. When no catalyst was used, relative conversion to oxirane was at most 57%, which is lower than relative conversions obtained with acid-catalyzed processes, in which conversions near 70% were obtained.
The epoxidation with acetic acid and formic acid results in higher oxirane oxygen contents and higher conversions to oxirane than the process with citric acid. When acetic acid is used as an oxygen carrier for the production of epoxidized soybean oil, relative conversions to oxirane in the range of 80–93% were reported (Boyacá and Beltrán 2010; Kim and Sharma 2012; Lage et al. 2014; Perez et al. 2009; Sinadinovic-Fiser et al. 2001); when formic acid is applied, relative conversions to oxirane are beyond 80% (Campanella et al. 2008; Mushtaq et al. 2011). However, citric acid can auto catalyze epoxidation reaction in the same way as formic acid and differently from acetic acid and propionic acid. The ability of autocatalysis in epoxidation reaction for an oxygen carrier depends on dissociation constant (pKa), as citric acid first pKa is 3.13 and pKa for formic acid is 3.75. Both demonstrated to be able to auto catalyze epoxidation reaction. In contrast, neither acetic acid nor propionic acid acts in the same way, as pKa values for these carboxylic acids are 4.756 and 4.87, respectively (Haynes and Lide 2012).
Microwave-assisted epoxidation
Differently from the conventional route, epoxidation with citric acid without a catalyst was not successful in our microwave system, probably due to mass transfer limitations. Aguilera et al. (2016) claimed that speed stirring is essential to epoxidation as a complete emulsion must be formed to reduce mass transfer restrictions. Piccolo et al. (2019) showed that the threshold stirring speed for epoxidation is about 250–300 rpm; below 250 rpm the organic and aqueous phases are entirely segregated, and epoxidation yields are very low. However, the actual threshold stirring speed depends on some factors such as impeller diameter and the volume of the reacting mixture. Indeed, citric acid is highly soluble in water, and then it tends to remain in the aqueous phase, not in the oil phase. Probably, peroxycitric acid formed in situ in the homogeneous catalysis is more soluble in the oil; thus, epoxidation is facilitated. Microwave equipment used in our study includes a magnetic stirring system, which is not as efficient as the mechanical stirrer used in the conventional heating process, especially when the viscosity increases as the epoxidation reaction continues.
In the first tests in microwave epoxidation, samples were forced to cool down immediately with the addition of distilled water to the system. Any reactions that might still be in progress in the reactor were halted. Different nominal powers were tested, and results are summarized in Table 3.
The best result obtained in terms of oxirane oxygen and double bonds conversion was in 24 min for the power of 200 W. In this case, energy consumption is at most 288 kJ. Clearly, the longer the reaction time, the greater the double bond conversion and the greater the relative conversion to oxirane. However, there is no clear pattern comparing the same times and different powers, particularly when power is increased to 400 W. This behavior is due to this microwave equipment works with a temperature curve. When the temperature reaches a specified value, in this case, 80 °C, actual power is gradually decreased to keep the temperature around the set point. Nominal power of 200 W was enough to heat the reaction mixture to 80 °C, and because of that, 200 W was selected as default to the following tests.
Table 4 presents microwave-assisted citric acid epoxidation results with homogeneous catalysis and cooling time of 10 min with air inside the equipment. During this time, magnetic stirring continued. Epoxidation reaction clearly continues during these 10 min.
In the experiment group presented in Table 4, the best result was obtained in 15 min, with an oxirane oxygen content equal to 4848%. Energy consumption is estimated to be at most 180 kJ, representing a significant reduction compared to the conventional route. For comparison purposes, our thermostatic bath heats 13 L of water from 25 to 80 °C. Considering water heat capacity of 4.186 kJ/kg K and water density 1000 kg/m3 by simplicity, energy consumption only to heat the referred amount of water is estimated at almost 3000 kJ, or 833.3 W h. These calculations do not consider the energy required to keep microwave equipment switched on; however, as no external heating is required, saving the amount of energy employed to heat thermostatic baths and other instruments is possible. Therefore, apart from significantly reducing reaction time, energy consumption using microwave-assisted epoxidation is about 94%. Oxirane ring polymerization, a side reaction, possibly occurs with citric acid in an aqueous solution, as it was demonstrated (Altuna et al. 2013; Gogoi et al. 2015a, b). That explains in part why the latter produced resin did not dissolve in any of the common organic solvents, i.e., chloroform, diethyl ether, acetone, cyclohexane, etc.
Comparing Tables 3 and 4, it is possible to observe that cooling with air inside the microwave reactor plays a vital role in epoxidation, as oxirane oxygen content was decreased from 485% to 424% wt/wt, corresponding to a 12.5% reduction. Forced cooling inside the equipment increases conversion to oxirane, increases the conversion of double bonds and allows a 37% reduction in microwave energy consumption. More studies are needed to better understand this effect, with different air-cooling times. In part, that behavior can be explained by additional time in the target reaction temperature.
The results from a preliminary study with soybean oil epoxidation with acetic acid and sulfuric acid as the catalyst are presented in Table 5.
Analyzing results from Table 5, 10 min is enough to promote a high epoxide formation, as the maximum oxirane oxygen content is greater than that obtained in Boyacá and Beltrán study (2010). The theoretical oxirane oxygen content of 7.67% is not attainable by this homogeneous catalysis approach, which may be in part because of reduced control and selectivity of homogeneous catalysis compared to heterogeneous catalysis. If the reaction can continue, other secondary compounds are probably formed by side reactions, as oxirane oxygen content is reduced to 5.5% after 15 min. Reaction time compared to the conventional heating process (Boyacá and Beltrán 2010) was reduced from 2.3 h to just 10 min. Energy consumption in this microwave process is estimated to be 120 kJ. In Leveneur et al. study (2014), which involves oleic acid epoxidation with acetic acid, an 80% conversion of oleic acid was obtained after 5 h under microwave irradiation with an average 10 W of absorbed power. Thus, we can estimate total microwave energy consumption in 180 kJ.
Results from a preliminary study with soybean oil epoxidation with acetic acid and Amberlite IR-120 H as a catalyst are presented in Table 6.
Results from Table 6 show that epoxidation with acetic acid and with heterogeneous catalyst occurs slower than with homogeneous catalyst, as expected. This result is coherent with the literature, as Pérez et al. (2009) obtained the highest conversion with heterogeneous catalysis in 5 h and Boyacá and Beltrán (2010) obtained the highest conversion in 2.3 h when sulfuric acid was employed as catalyst.
In heterogeneous catalysis, after 25 min of microwave irradiation, 6.59% wt/wt oxirane oxygen content is obtained, which represents a high conversion to oxirane. If more time is given to the system, probably oxirane oxygen content will be increased, but it is important to consider the cost and energy consumption of this additional time to the process. An important advantage of heterogeneous catalysis compared to homogeneous catalysis is the reduction of cost in separation steps and greater selectivity (Cooney 2009). However, a mineral acid is often cheaper than an ion-exchange resin, being in general preferred in an industrial scale (Boyacá and Beltrán 2010; Danov et al. 2017).
Figure 6 shows a comparison between citric acid and acetic acid epoxidation under microwave irradiation. It can be observed that oxirane oxygen content is greater when acetic acid is used, which is desirable for applications such as structural composite materials that require more rigidity after the curing process (Espinoza-Perez et al. 2011; Suzuki et al. 2016). However, for applications in the food industry, for example, oxirane oxygen and rigidity may not be the most critical parameters, but it is crucial to decrease toxicity final product’s toxicity. Bueno-Ferrer et al. (2010) published a study in which epoxidized soybean oil is used to stabilize polyvinyl chloride (PVC) in the production of films for the food industry. Another application for epoxy resins is as a plasticizer. Yang et al. (2014) demonstrated the feasibility of applying epoxidized soybean oil to produce films with ethyl cellulose. Waste cooking epoxidized soybean oil was applied as a plasticizer in Suzuki and coworkers’ study (Suzuki et al. 2018), to produce PVC films.
A comparison with other studies involving microwave-assisted epoxidation is shown in Table 7.
It can be depicted from Table 7 that the results are compatible with other studies published in the literature, especially in the case of acetic acid epoxidation, and the reaction time is significantly reduced when microwave irradiation is used. Leveneur et al. (2014) reached an 85% conversion of oxirane using sulfuric acid as a catalyst and acetic acid as an oxygen carrier. In the latter study, reaction time was 300 min for an average absorbed power of 10 W; thus, we can estimate energy consumption of 180 kJ. Although oxirane oxygen content is lower than other epoxidation methods when citric acid is used, it is essential to consider time reduction and energy consumption with a microwave and a possible reduction of cost by eliminating a catalyst in purification steps for conventional epoxidation. Besides, citric acid is less toxic than other common oxygen carriers such as formic acid and acetic acid. Oxirane oxygen content can be increased with the addition of Amberlite IR-120 H catalyst, even though the catalyst cost should be considered according to the final application. The major drawback of epoxidation with citric acid without a catalyst is mass transfer restriction due to the presence of an aqueous and an oil phase, which can be addressed with an effective catalyst or a robust agitation system.
In terms of the catalyst, sulfuric acid is cheaper than Amberlite IR 120 H and is easier to purchase with local reagents vendors, and industrial epoxidized soybean oil is in general produced with sulfuric acid (Danov et al. 2017). However, sulfuric acid is corrosive to iron-based equipment. Hence, it requires more expensive reactor constructive materials (Boyacá and Beltrán 2010; Danov et al. 2017).
When cross-linked with a hardener, epoxidized vegetable oils produced with citric acid tend to be more flexible than the ones produced with formic acid and acetic acid due to a lower crosslink density (Sue et al. 2000) which leads to more linear and flexible chains. However, the most common application for epoxidized vegetable oils is in surface coating, especially in the production of polyurethanes and polyols (Suzuki et al. 2016), in which rigidity is not the most important parameter.
Conclusion
Citric acid was demonstrated as an efficient oxygen carrier for epoxidation. In the conventional route, an appreciable conversion was obtained without the need to add an acid catalyst, thus allowing process cost reduction. This ability of citric acid is due to its lower pKa comparing to acetic acid, allowing, however, steric hindrance and the possibility of cross-linking limit conversion to oxirane oxygen. In this regard, epoxidation with no catalyst allows a significant reduction in water consumption during washing steps. Thermal runaway risks can also be reduced by replacing acetic acid or formic acid by citric acid, which is less volatile and less reactive due to steric hindrance. Microwave technology was applied successfully to reduce epoxidation time, and reaction times were decreased from 4 h to just 15 min in epoxidation with citric acid and from 2.3 h to 10 min for epoxidation with acetic acid using a homogeneous catalysis. Also, microwave heating allowed a significant reduction in energy consumption in the epoxidation of soybean oil, estimated at up to 94%. It was demonstrated that forced air cooling after ceasing the microwave irradiation until room temperature was a critical controlling parameter to increase conversion to epoxide.
References
Abolins A, Kirpluks M, Vanags E, Fridrihsone A, Cabulis U (2020) Tall oil fatty acid epoxidation using homogenous and heterogeneous phase catalysts. J Polym Environ 28(6):1822–1831. https://doi.org/10.1007/s10924-020-01724-9
Adhvaryu A, Erhan SZ (2002) Epoxidized soybean oil as a potential source of high-temperature lubricants. Ind Crops Prod 15(3):247–254. https://doi.org/10.1016/s0926-6690(01)00120-0
Aguilera AF, Tolvanen P, Eränen K, Leveneur S, Salmi T (2016) Epoxidation of oleic acid under conventional heating and microwave radiation. Chem Eng Process Process Intensif 102:70–87. https://doi.org/10.1016/j.cep.2016.01.011
Aguilera AF, Tolvanen P, Heredia S, Muñoz MG, Samson T, Oger A, Verove A, Eränen K, Leveneur S, Mikkola J-P, Salmi T (2018) Epoxidation of fatty acids and vegetable oils assisted by microwaves catalyzed by a cation exchange resin. Ind Eng Chem Res 57(11):3876–3886. https://doi.org/10.1021/acs.iecr.7b05293
Al-Mulla EAJ, Suhail AH, Aowda SA (2011) New biopolymer nanocomposites based on epoxidized soybean oil plasticized poly(lactic acid)/fatty nitrogen compounds modified clay: Preparation and characterization. Ind Crops Prod 33(1):23–29. https://doi.org/10.1016/j.indcrop.2010.07.022
Altuna FI, Pettarin V, Williams RJJ (2013) Self-healable polymer networks based on the cross-linking of epoxidised soybean oil by an aqueous citric acid solution. Green Chem 15(12):3360–3366. https://doi.org/10.1039/c3gc41384e
ANVISA (1999) Regulamento Técnico sobre Aditivos Utilizados Segundo as Boas Práticas de Fabricação e Suas Funções. In: Resolução no 386, de. ANVISA—Agência Nacional de Vigilância Sanitária. http://portal.anvisa.gov.br/wps/wcm/connect/0556e3004745787485bdd53fbc4c6735/RESOLUCAO_386_1999.pdf?MOD=AJPERES. Accessed 12 Dec 2019
AOCS (2009) AOCS Official Method Cd 9–57. In: Oxirane Oxygen. AOCS—The American Oil Chemists’ Society. https://aocs.personifycloud.com/PersonifyEBusiness/Default.aspx?TabID=251&productId=111548. Accessed 12 Dec 2019
ASTM (2015) Standard test method for determination of the iodine value of fats and oils. West Conshohocken, ASTM International, p 3. https://doi.org/10.1520/D5554-15
Azcan N, Yilmaz O (2013) Microwave assisted transesterification of waste frying oil and concentrate methyl ester content of biodiesel by molecular distillation. Fuel 104:614–619. https://doi.org/10.1016/j.fuel.2012.06.084
Azcan N, Danisman A, Azcan Danisman AN (2008) Microwave assisted transesterification of rapeseed oil. Fuel 87(10–11):1781–1788. https://doi.org/10.1016/j.fuel.2007.12.004
Barbosa TM, Franca AS, Oliveira LS, Valle RM (2015) Comparative evaluation of acid and basic thermo-chemical treatments in the production of adsorbents based on biodiesel production solid residu. Int J Environ Sci Dev 7(4):234–239. https://doi.org/10.7763/IJESD.2016.V7.775
Bennett GM (1956) Organic peroxides. Nature 177(4514):814. https://doi.org/10.1038/177814b0 (1st ed., John Wiley & Sons)
Bermudez-Aguirre D, Barbosa-Canovas GV (2013) Disinfection of selected vegetables under nonthermal treatments: Chlorine, acid citric, ultraviolet light and ozone. Food Control 29(1):82–90. https://doi.org/10.1016/j.foodcont.2012.05.073
Bhalerao MS, Patwardhan AV, Bhosale MA, Kulkarni VM, Bhanage BM (2016) Epoxidised soybean oil–Cu/Cu 2 O bio-nanocomposite material: synthesis and characterization with antibacterial activity. RSC Adv 6(45):38906–38912
Boyacá LA, Beltrán ÁA (2010) Producción de epóxido de soya con ácido peracético generado in situ mediante catálisis homogénea TT - Soybean epoxide production with in situ peracetic acid using homogeneous catalysis. Ingeniería e Investigación 30(1):136–140
Bueno-Ferrer C, Garrigos MC, Jimenez A (2010) Characterization and thermal stability of poly(vinyl chloride) plasticized with epoxidized soybean oil for food packaging. Polym Degrad Stab 95(11):2207–2212. https://doi.org/10.1016/j.polymdegradstab.2010.01.027
Campanella A, Fontanini C, Baltanas MA (2008) High yield epoxidation of fatty acid methyl esters with performic acid generated in situ. Chem Eng J 144(3):466–475. https://doi.org/10.1016/j.cej.2008.07.016
Chavan VP, Patwardhan AV, Gogate PR (2012) Intensification of epoxidation of soybean oil using sonochemical reactors. Chem Eng Processing Process Intensif 54:22–28. https://doi.org/10.1016/j.cep.2012.01.006
Chua S-C, Xu X, Guo Z (2012) Emerging sustainable technology for epoxidation directed toward plant oil-based plasticizers. Process Biochem 47(10):1439–1451. https://doi.org/10.1016/j.procbio.2012.05.025
Cooney T (2009) Epoxidised resins from natural renewable resources [University of Southern Queensland]. In: Faculty of Engineering and Surveying: Vol. Bachelor o. https://eprints.usq.edu.au/8468/1/Cooney_2009.pdf. Accessed 28 Dec 2019
Danov SM, Kazantsev OA, Esipovich AL, Belousov AS, Rogozhin AE, Kanakov EA (2017) Recent advances in the field of selective epoxidation of vegetable oils and their derivatives: a review and perspective. Catal Sci Technol 7(17):3659–3675
Di Serio M, Turco R, Pernice P, Aronne A, Sannino F, Santacesaria E (2012) Valuation of Nb2O5–SiO2 catalysts in soybean oil epoxidation. Catal Today 192(1):112–116. https://doi.org/10.1016/j.cattod.2012.03.069
Dinda S, Patwardhan AV, Goud VV, Pradhan NC (2008) Epoxidation of cottonseed oil by aqueous hydrogen peroxide catalysed by liquid inorganic acids. Bioresour Technol 99(9):3737–3744. https://doi.org/10.1016/j.biortech.2007.07.015
Ding C, Matharu AS (2014) Recent developments on biobased curing agents: a review of their preparation and use. ACS Sustain Chem Eng 2(10):2217–2236. https://doi.org/10.1021/sc500478f (American Chemical Society)
Dworakowska S, Bogdal D, Prociak A (2012) Microwave-assisted synthesis of polyols from rapeseed oil and properties of flexible polyurethane foams. Polymers 4(3):1462–1477. https://doi.org/10.3390/polym4031462
El Sherbiny SA, Refaat AA, El Sheltawy ST (2010) Production of biodiesel using the microwave technique. J Adv Res 1(4):309–314. https://doi.org/10.1016/j.jare.2010.07.003
Espinoza-Perez JD, Nerenz BA, Haagenson DM, Chen Z, Ulven CA, Wiesenborn DP (2011) Comparison of curing agents for epoxidized vegetable oils applied to composites. Polym Compos 32(11):1806–1816. https://doi.org/10.1002/pc.21213
Farias M, Martinelli M, Rolim GK (2011) Immobilized molybdenum acetylacetonate complex on montmorillonite K-10 as catalyst for epoxidation of vegetable oils. Appl Catal A General 403(1):119–127. https://doi.org/10.1016/j.apcata.2011.06.021
Ferdousi B, Islam M, Awad MI, Okajima T, Kitamura F, Ohsaka T (2006) Preparation and potentiometric measurement of peroxycitric acid. Electrochemistry -Tokyo- 74:606–608. https://doi.org/10.5796/electrochemistry.74.606
Galli F, Nucci S, Pirola C, Bianchi CL (2014) Epoxy methyl soyate as bio-plasticizer: two different preparation strategies. In: Iconbm: International Conference on Biomass, Pts 1 and 2, vol 37, pp 601–606. https://doi.org/10.3303/cet1437101
Gogoi P, Horo H, Khannam M, Dolui SK (2015b) In situ synthesis of green bionanocomposites based on aqueous citric acid cured epoxidized soybean oil-carboxylic acid functionalized multiwalled carbon nanotubes. Ind Crops Prod 76:346–354. https://doi.org/10.1016/j.indcrop.2015.06.057
Gogoi P, Boruah M, Sharma S, Dolui SK (2015a) Blends of epoxidized alkyd resins based on jatropha oil and the epoxidized oil cured with aqueous citric acid solution: a green technology approach. Acs Sustain Chem Eng 3(2):261–268. https://doi.org/10.1021/sc500627u
Goud VV, Pradhan NC, Patwardhan AV (2006) Epoxidation of karanja (Pongamia glabra) oil by H2O2. J Am Oil Chemi Soc 83(7):635–640. https://doi.org/10.1007/s11746-006-1250-7
Gupta AP, Ahmad S, Dev A, Habib F, Bajpai M (2011) Synthesis and characterization of acrylated epoxidized soybean oil for UV cured coatings. Polym Eng Sci 51(6):1087–1091. https://doi.org/10.1002/pen.21791
Haynes WM, Lide DR (2012) CRC handbook of chemistry and physics (93th NV-). CRC, New York
Jin FL, Park SJ (2008) Thermomechanical behavior of epoxy resins modified with epoxidized vegetable oils. Polym Int 57(4):577–583. https://doi.org/10.1002/pi.2280
Kim JR, Sharma S (2012) The development and comparison of bio-thermoset plastics from epoxidized plant oils. Ind Crops Prod 36(1):485–499. https://doi.org/10.1016/j.indcrop.2011.10.036
Kurańska M, Beneš H, Prociak A, Trhlíková O, Walterová Z, Stochlińska W (2019) Investigation of epoxidation of used cooking oils with homogeneous and heterogeneous catalysts. J Clean Prod 236:117615. https://doi.org/10.1016/j.jclepro.2019.117615
Lage FC, Franca AS, Oliveira LS, Bracarense AQ (2014) Epoxidized vegetable oil as a sustainable ingredient in welding electrode coatings. Adv Mater Res 856:87–91 (10.4028/www.scientific.net/AMR.856.87)
Leveneur S (2017) Thermal safety assessment through the concept of structure–reactivity: application to vegetable oil valorization. Org Process Res Dev 21(4):543–550. https://doi.org/10.1021/acs.oprd.6b00405
Leveneur S, Ledoux A, Estel L, Taouk B, Salmi T (2014) Epoxidation of vegetable oils under microwave irradiation. Chem Eng Res Des 92(8):1495–1502. https://doi.org/10.1016/j.cherd.2014.04.010
Leveneur S, Estel L, Crua C (2015) Thermal risk assessment of vegetable oil epoxidation. J Therm Anal Calorim 122(2):795–804. https://doi.org/10.1007/s10973-015-4793-8
Li L, Cheng C, Xiang T, Tang M, Zhao W, Sun S, Zhao C (2012) Modification of polyethersulfone hemodialysis membrane by blending citric acid grafted polyurethane and its anticoagulant activity. J Membr Sci 405:261–274. https://doi.org/10.1016/j.memsci.2012.03.015
Maenz S, Muehlstaedt M, Jandt KD, Bossert J (2015) Mechanical properties of microwave cured glass fibre epoxy composites prepared by resin transfer moulding. J Compos Mater 49(23):2839–2847. https://doi.org/10.1177/0021998314557295
Meadows S, Hosur M, Celikbag Y, Jeelani S (2018) Comparative analysis on the epoxidation of soybean oil using formic and acetic acids. Polym Polym Compos 26(4):289–298
Mushtaq M, Tan IB, Devi C, Majidaie S, Nadeem M, Lee S (2011) Epoxidation of fatty acid methyl esters derived from jatropha oil. In: IEEE (Ed.), National Postgraduate Conference, pp. 1–4
Musik M, Milchert E, Malarczyk-Matusiak K (2018) Technological parameters of epoxidation of sesame oil with performic acid. Pol J Chem Technol 20(3):53–59
Nikje MA, Abedinifar F, Idris A, Mohammad M, Nikje A, Abedinifar F, Idris A (2011) Epoxidized soybean oil ring opening reaction under MW irradiation. Arch Appl Sci Res 3(3):383–388. http://scholarsresearchlibrary.com/aasr-vol3-iss3/AASR-2011-3-3-383-388.pdf
Perez JDE, Haagenson DM, Pryor SW, Ulven CA, Wiesenborn DP (2009) Production and characterization of epoxidized canola oil. Trans ASABE 52(4):1289–1297
Pérez-Sena WY, Cai X, Kebir N, Vernières-Hassimi L, Serra C, Salmi T, Leveneur S (2018) Aminolysis of cyclic-carbonate vegetable oils as a non-isocyanate route for the synthesis of polyurethane: a kinetic and thermal study. Chem Eng J 346:271–280
Pérez-Sena WY, Salmi T, Estel L, Leveneur S (2020) Thermal risk assessment for the epoxidation of linseed oil by classical Prisleschajew epoxidation and by direct epoxidation by H2O2 on alumina. J Therm Anal Calorim 140(2):673–684. https://doi.org/10.1007/s10973-019-08894-2
Perez-Sena WY, Wärnå J, Eränen K, Tolvanen P, Estel L, Leveneur S, Salmi T (2021) Use of semibatch reactor technology for the investigation of reaction mechanism and kinetics: heterogeneously catalyzed epoxidation of fatty acid esters. Chem Eng Sci 230:116206. https://doi.org/10.1016/j.ces.2020.116206
Petrovic ZS, Zlatanic A, Lava CC, Sinadinovic-Fiser S (2002) Epoxidation of soybean oil in toluene with peroxoacetic and peroxoformic acids—kinetics and side reactions. Eur J Lipid Sci Technol 104(5):293–299. https://doi.org/10.1002/1438-9312(200205)104:5%3c293::aid-ejlt293%3e3.0.co;2-w
Piccolo D, Vianello C, Lorenzetti A, Maschio G (2019) Epoxidation of soybean oil enhanced by microwave radiation. Chem Eng J 377. https://doi.org/10.1016/j.cej.2018.10.050
Rabelo SN, Ferraz VP, Oliveira LS, Franca AS (2015) FTIR analysis for quantification of fatty acid methyl esters in biodiesel produced by microwave-assisted transesterification. Int J Environ Sci Dev 6(12):964–969. https://doi.org/10.7763/ijesd.2015.v6.730
Sahoo SK, Mohanty S, Nayak SK (2018) Mechanical, dynamic mechanical, and interfacial properties of sisal fiber-reinforced composite with epoxidized soybean oil-based epoxy matrix. Polym Compos 39(6):2065–2072. https://doi.org/10.1002/pc.24168
Saifuddin N, Wen C, Zhan W, Ning X (2010) Palm oil based polyols for polyurethane foams application. In: Proceedings of international conference on advances in energy technologies
Saifuddin N, Zhan LW, Ning KX (2011) Heat-modeling of microwave assisted epoxidation of palm acid oil. Am J Appl Sci 8(3):217–229. https://doi.org/10.3844/ajassp.2011.217.229
Santacesaria E, Tesser R, Di Serio M, Turco R, Russo V, Verde D (2011) A biphasic model describing soybean oil epoxidation with H2O2 in a fed-batch reactor. Chem Eng J 173(1):198–209
Saremi K, Tabarsa T, Shakeri A, Babanalbandi A (2012) Epoxidation of soybean oil. Ann Biol Res 3(9):4254–4258
Silva VF, Batista LN, Cunha VS, Costa MAS (2017) Production of catalyst to vegetable oil epoxidation from toxic biomass residue. Waste Biomass Valoriz 8(4):1265–1271. https://doi.org/10.1007/s12649-016-9616-z
Sinadinovic-Fiser S, Jankovic M, Petrovic ZS (2001) Kinetics of in situ epoxidation of soybean oil in bulk catalyzed by ion exchange resin. J Am Oil Chem Soc 78(7):725–731. https://doi.org/10.1007/s11746-001-0333-9
Somidi AKR, Sharma RV, Dalai AK (2014) Synthesis of epoxidized canola oil using a sulfated-SnO2 catalyst. Ind Eng Chem Res 53(49):18668–18677. https://doi.org/10.1021/ie500493m
Sue H-J, Puckett PM, Bertram JL, Walker LL (2000) The network structure of epoxy systems and its relationship to toughness and toughenability. ACS Publications, Washington DC
Suzuki A, Lage F, Oliveira L, Franca A (2016) Biological materials as precursors for the production of resins. Nova Publishers, Hauppauge, pp 1–38
Suzuki AH, Botelho BG, Oliveira LS, Franca AS (2018) Sustainable synthesis of epoxidized waste cooking oil and its application as a plasticizer for polyvinyl chloride films. Eur Polymer J 99:142–149. https://doi.org/10.1016/j.eurpolymj.2017.12.014
Vianello C, Piccolo D, Lorenzetti A, Salzano E, Maschio G (2018) Study of soybean oil epoxidation: effects of sulfuric acid and the mixing program. Ind Eng Chem Res 57(34):11517–11525. https://doi.org/10.1021/acs.iecr.8b01109
Vu CM, Nguyen DD, Sinh LH, Choi HJ, Pham TD (2018) Improvement the mode I interlaminar fracture toughness of glass fiber reinforced phenolic resin by using epoxidized soybean oil. Polym Bull 75(10):4769–4782. https://doi.org/10.1007/s00289-018-2296-z
Widsten P, Dooley N, Parr R, Capricho J, Suckling I (2014) Citric acid crosslinking of paper products for improved high-humidity performance. Carbohydr Polym 101:998–1004. https://doi.org/10.1016/j.carbpol.2013.10.002
Wu Z, Nie Y, Chen W, Wu L, Chen P, Lu M, Yu F, Ji J (2016) Mass transfer and reaction kinetics of soybean oil epoxidation in a formic acid-autocatalyzed reaction system. Can J Chem Eng 94(8):1576–1582. https://doi.org/10.1002/cjce.22526
Yang D, Peng X, Zhong L, Cao X, Chen W, Zhang X, Liu S, Sun R (2014) “Green” films from renewable resources: Properties of epoxidized soybean oil plasticized ethyl cellulose films. Carbohyd Polym 103:198–206. https://doi.org/10.1016/j.carbpol.2013.12.043
Zheng JL, Wärnå J, Salmi T, Burel F, Taouk B, Leveneur S (2016) Kinetic modeling strategy for an exothermic multiphase reactor system: application to vegetable oils epoxidation using Prileschajew method. AIChE J 62(3):726–741
Acknowledgements
The authors acknowledge Brazilian government agencies CAPES and CNPq for funding this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lage, F.C., Suzuki, A.H. & Oliveira, L.S. Comparative evaluation of conventional and microwave assisted epoxidation of soybean oil with citric acid, acetic acid using homogeneous and heterogeneous catalysis. Braz. J. Chem. Eng. 38, 327–340 (2021). https://doi.org/10.1007/s43153-021-00096-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-021-00096-4