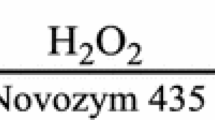Abstract
Enzymatic epoxidation of vegetable oils using a long chain fatty acid as an active oxygen carrier could produce a desirable epoxy oxygen group content (EOC); however, the acid value (AV) of final epoxidized oil is too high. The present study was to investigate the effect of different fatty acids with varying length of carbon chain on EOC and AV of the final epoxidized soybean oil (ESO); finding butyric acid was the choice of active oxygen carrier when hydrogen peroxide was used as an oxygen donor in the presence of lipase Novozyme 435. And in situ IR was used to monitor the epoxidation process, which revealed that the formation of perbutyric acid was the key step in the whole reaction. The epoxidation process was optimized as follows: molar ratio of butyric acid/C=C bonds of 0.19:1, 8% of immobilized lipase Novozyme 435 load (relative to the weight of soybean oil) and molar ratio of H2O2/C=C bonds of 3.5:1, reaction time of 4 h and reaction temperature of 45 °C. Under these conditions, ESO with a high EOC (7.62 ± 0.20%) and a lower AV value (8.53 ± 0.18 mgKOH/g) was obtained. The oxriane conversion degree was up to 97.94%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
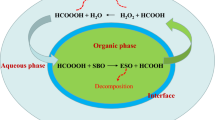
Epoxidized vegetable oil (EVO) is considered as environmental-friendly, renewable, biodegradable and less-polluting oleo-chemicals, which have been widely used in production of composites, bio-lubricants, functional fluids, plasticizers and PVC (Crivello et al. 1997; Fantoni and Simoneau 2004; Hwang and Erhan 2001; Salimon et al. 2014). In the traditional epoxidation method, using strong mineral acids such as H2SO4 and H3PO4 as catalysts will cause equipment corrosion and undesirable ring-opening reactions of oxirane in the final EVO products (Gerbase et al. 2002). Recently, enzymatic epoxidation of vegetable oil is more outstrip than the traditional chemical techniques due to the mild reaction temperature (40–50 °C), no strong mineral acids involving and much higher epoxy oxygen content (Sun et al. 2011a, b, 2014; Lu et al. 2010; Vlcek and Petrovic 2006; Daniel et al. 2014). As shown in Scheme 1, the carbon–carbon double bonds of fatty acid in vegetable oils are epoxidized by peroxy acid (RCOOOH), which is produced by the reaction of a fatty acid (RCOOH) with hydrogen peroxide (H2O2) in the presence of enzyme catalyst (Sun et al. 2011a, b).
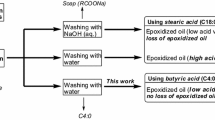
According to the previous reports, higher epoxy oxygen content (EOC) can be obtained using long chain fatty acids (e.g., stearic acid and lauric acid) as an active oxygen carrier in enzymatic epoxidation (Sun et al. 2011a, b; Vlcek and Petrovic 2006; Hosseini 2014). However, the acid value (AV) of the ultimate epoxidized oil is much higher after treatment of the final products washing with water because it is difficult to remove the long-chain free fatty acids from the final products due to their poor water solubility. In addition, introducing alkali refining (NaOH) to the final products could also probably form a large amount of soap (RCOONa), which is hard to separate from epoxidized oils. The long-chain free fatty acid (inducing high acid value) and soaps will seriously reduce the quality of the epoxidized oil products and make the post-processing unsustainable. Therefore, finding a suitable fatty acid as an active oxygen carrier, which can be also removed easily from the epoxidized oil, is highly desirable in the oleo chemical industry.
In the previous reports, short-chain fatty acids such as formic acid and acetic acid were mainly used in the presence of strong mineral acid-catalyzed epoxidation of vegetable oil. Soybean oil contains abundant unsaturated fatty acids ranging from 75 to 93%, especially linoleic acid and oleic acid, which are the excellent epoxidation materials (Sun et al. 2011a, b; Petrovic et al. 2002). As our continuing interest in enzymatic epoxidation of vegetable oils (Liu et al. 2016), the present study was, therefore, (i) to select the suitable fatty acid as an active oxygen carrier on the epoxidation of soybean oil using immobilized lipase Novozyme 435 as a catalyst; (ii) to confirm the rapid formation of perbutyric acids in the presence of enzyme catalyst; (iii) to investigate the effects of reaction time, H2O2, enzyme load, reaction temperature and fatty acids on the epoxy oxygen content (EOC) and acid value (AV) of the epoxidized soybean oil (ESO).
Materials and methods
Materials
Soybean oil (acid value = 0.14 mgKOH/g, iodine value = 134 g I 2/100 g) was purchased from a local supermarket (Zhengzhou, Henan, China). The fatty acid composition of the soybean oil was 51.79% of linoleic acid, 25.75% of oleic acid, 5.16% of linolenic acid, 12.03% of palmitic acid and 5.27% of stearic acid. Hydrogen peroxide (30% w/w solution) was purchased from Luoyang Haohua Chemical Co., Ltd (Luoyang, Henan, China). Acetic acid (purity >99%), butyric acid (purity >99%), hexanoic acid (purity >98%), lauric acid (purity >99%) were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Stearic acid (purity >99%) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Immobilized lipase Novozyme 435 was purchased from Novozymes A/S (Bagsvaerd, Denmark). All other reagents were of analytical grade.
Preparation of epoxidized soybean oil
Soybean oil (5.0 g) was weighted into a three-neck round-bottom 250-mL flask followed by the addition of calculated amount of free fatty acid, lipase catalyst, and 30 mL of benzene in the listed order. The mixture was then heated to 50 °C by the water bath stirring at a speed of 120 rpm. Afterwards, 10 mL of hydrogen peroxide was added into the mixture dropwise through a 50-mL funnel in 10 min. The reaction was then continued to the designed time.
After the reaction was completed, Novozyme 435 was removed by filtration. The filtrate was washed by saturated salt solution and distilled hot water (70 ± 1 °C) three times, respectively. Trace water was dried by anhydrous sodium sulfate (Na2SO4) at the room temperature. Finally, the solvent and remaining trace water were distilled using a rotary evaporator and the epoxidized soybean oil (ESO) was obtained.
Analytical techniques
Epoxy oxygen group content (EOC) of the products was determined by titration method with hydrobromic acid solution in acetic acid (Paquot and Hautfenne 1987; Paquot 1979). From the oxirane content, the relative conversion percentage to oxirane was counted by the following formula:
where OOex is the content of oxirane oxygen experimentally determined, and OOth is the theoretical maximum oxirane oxygen content determined by the following equation:
where A i (126.9) and A 0 (16.0) are the atomic weights of iodine and oxygen, respectively, and IV0 is the initial iodine value of the soybean oil. The theoretical maximum oxirane oxygen content in 100 g of soybean oil (OOth) is 7.78%.
Statistical analysis
Experimental results were obtained as the mean value ± standard deviation (SD) (n = 3).
Results and discussion
Choice of fatty acids as an active oxygen carrier
Free fatty acids (mainly long chain fatty acid) in enzymatic epoxidation of vegetable oil were to act as an active oxygen carrier and to inhibit the hydrolysis of triglyceride. However, the free fatty acid residue in the final epoxidized oil products would influence theirs quality.
The first step of this study was to focus on the selection of a fatty acid, which could induce the high EOC and low AV of the epoxidation oil. The effect of five fatty acids with different length of carbon chain (acetic, butyric, hexanoic, lauric and steric acid) on the enzymatic epoxidation is shown in Fig. 1. There was no significant difference in the EOC values of epoxidized soybean oil (ESO) when butyric acid, hexanoic acid, lauric acid and stearic acid were as active oxygen carrier (5.19 ± 0.10, 5.24 ± 0.18, 5.29 ± 0.12 and 5.32 ± 0.10), while acetic acid could afford the lowest EOC (0.83 ± 0.03). This was probably because acetic acid was a relative strong organic acid which would inactivate the enzyme. Notably, there was no significant difference in AV values between acetic acid and butyric acid (1.61 ± 0.15 vs 3.01 ± 0.12). But with the increasing length of the carbon chain (from butyric acid to stearic acid), the AV of epoxidation oil was increasing rapidly (from 3.01 ± 0.12 to 31.31 ± 0.23), which might be related to the poor solubility of long-chain fatty acids in water as it could not be easily removed from the products by washing with water. The higher content of a fatty acid in the final epoxidized soybean oil was undesirable. Therefore, an extra step is needed to separate fatty acid from the final products. In this regard, butyric acid was selected as a suitable oxygen carrier in the epoxidation of soybean oil while it could not only improve EOC efficiently but also induce the lower AV. The following experiments were to optimize the epoxidation processing of soybean oil using butyric acid as oxygen carrier.
Based on the enzymatic epoxidation mechanism proposed in Scheme 1, it is concluded that the most important step in enzymatic epoxidation of vegetable oils is the first step: fatty acid reacting with H2O2 to generate perfatty acid in situ in the presence of enzyme. Therefore, in situ IR which can directly detect unstable reaction intermediates in real time is used to confirm the mechanism of the reaction (Leadbeater 2010; Fukui et al. 2012; De Souza and Cajaiba da Silva 2013). The mixture of butyric acid, H2O2 and Novozyme 435 was monitored by in situ IR to observe the formation of perfatty acid (Fig. 2). Butyric acid (peak at 1710 cm−1) was consumed rapidly as soon as the addition of H2O2 and perbutyric acid (peak at 1636 cm−1) was produced, suggesting that perbutyric acid indeed could generate rapidly in situ and would participate in the next step reaction immediately. So the formation of perbutyric acid was the key step in the whole reaction which decides the reaction rate.
Optimization epoxidation of soybean oil
Effect of reaction time on the enzymatic epoxidation of soybean oil
The effect of reaction time on the enzymatic epoxidation of soybean oil was examined (Fig. 3). The results showed that the EOC value reached the highest when the reaction time was lasting to 4 h (5.19 ± 0.10). However, the EOC of epoxidized soybean oil began to decrease with the increasing reaction time (6–12 h), which was ascribed to the formation of more by-products water inducing the hydrolysis of epoxy group (Sun et al. 2011a, b; Hosseini 2014). Meanwhile, the AV had an unremarkable increase before 6 h, increased rapidly thereafter and reached the highest at 12 h, probably because the fatty acid content from the hydrolysis of triglyceride was increased with the increasing reaction time. Furthermore, the side reactions between water and epoxidized soybean oil would generate dihydric alcohol which could induce the emulsification of epoxidized soybean oil (ESO). Therefore, 4 h was selected in the following experiments.
Effect of hydrogen peroxide (H2O2) on epoxidation
The effect of H2O2 was also investigated and the results are presented in Fig. 4. The highest EOC (5.19 ± 0.10) was obtained at molar ratio of H2O2/C=C of 3.5:1, because perbutyric acid was produced. But the further increase of H2O2 (>3.5:1) would decrease the EOC of epoxidized soybean oil. This was probably because hydrogen peroxide was a kind of strong oxidant, which would inactivate the enzyme. Besides, extra H2O2 would bring much water into the reaction mixture and dilute the substrates. This phenomenon was consistent with the previous study reported by Sun et al. (2011a, b) and Rafiee-mongaddam et al. (Hosseini 2014). However, the hydrogen peroxide had little effect on AV (Fig. 4b). So molar ratio of H2O2/C=C of 3.5:1 was the optimal amount of active oxygen donor in this study.
Effect of enzyme load on the enzymatic epoxidation of soybean oil
The effect of enzyme load (immobilized lipase Novozyme 435) was also studied in the range of 0–12% relative to the weight of soybean oil (Fig. 5). The maximum EOC (6.39 ± 0.16) could be achieved at 8% of enzyme load. Further increase in the enzyme (8–12%) caused a decline of EOC (from 6.39 ± 0.16 to 4.47 ± 0.20) because excess enzyme load could accelerate the hydrolysis of the epoxy oil. The AV of epoxidation soybean oil was increasing rapidly (from 3.01 ± 0.12 to 11.54 ± 0.35) with the increasing immobilized lipase Novozyme 435 (2–12%). This was because the enzyme could catalyze the hydrolysis of triglycerides. Therefore, 8% of enzyme load was considered optimum.
Effect of reaction temperature on the enzymatic epoxidation of soybean oil
Figure 6 shows the effect of reaction temperature on the EOC and AV of epoxidation soybean oil. The maximum EOC was obtained at 50 °C (6.39 ± 0.16). When the temperature was over 50 °C, the EOC of soybean oil was declined, probably because the higher temperature would induce the enzyme inactivation and ring opening reaction of the epoxides (Campanella and Baltanas 2005; Tornvall et al. 2007). There was no significant difference in AV value of epoxidation products at temperatures of 40–50 °C (8.77 ± 0.23, 7.98 ± 0.15, 8.46 ± 0.06). However, AV value was lower at 30 °C (6.00 ± 0.21) primarily because the hydrolysis of triglyceride was low at lower temperature. It was interesting that AV value of the products began to decrease when the temperature was over 50 °C, which might be due to the partial inactivation of enzyme and poor triglyceride hydrolysis. Considering that AV would affect the quality of epoxidized soybean oil, 45 °C was considered an optimal reaction temperature.
Effect of butyric acid concentration on the enzymatic epoxidation of soybean oil
In the last step, different molar ratios of butyric acid/C=C bonds from 0.04:1 to 0.31:1 on the EOC and AV values of enzymatic epoxidation were examined (Fig. 7). EOC of epoxidized soybean oil was increased with the increasing molar ratio of butyric acid/C=C bonds (≤0.19:1) (from 5.89 ± 0.38 to 7.62 ± 0.20). However, EOC value of epoxidized soybean oil was declined slightly when the molar ratio of butyric acid/C=C bonds increased further (0.23:1, 7.48 ± 0.30; 0.31:1, 6.57 ± 0.30). This could be explained that H2O was also produced with the formation of perbutyric (Scheme 1), thus inducing the ring opening of the oxirane and the decrease of EOC of the epoxidized soybean oil. The AV was increased slightly with the increasing molar ratio of butyric acid/C=C (0.04:1, 7.16 ± 0.04; 0.31:1, 9.50 ± 0.12). So 0.19:1 was considered as the optimal molar ratio of butyric acid/C=C bonds. Notably, under the optimal conditions, the epoxidized soybean oil with higher EOC (7.62 ± 0.28) and lower acid value (8.53 ± 0.18 mgKOH/g) was achieved. And the oxriane conversion degree was up to 97.94%.
Conclusions
The epoxidized soybean oil with excellent epoxy oxygen content (EOC) and much lower AV was achieved when butyric acid was chosen as an oxygen carrier and immobilized lipase Novozyme 435 was the catalyst. And in situ IR was used to monitor the epoxidation process, which revealed that the formation of perbutyric acid was the key step in the whole reaction. The optimal epoxidation conditions were offset at 45 °C, molar ratio of butyric acid: C=C bonds of 0.19:1, molar ratio of H2O2:C=C bonds of 3.5:1, 8% of immobilized lipase Novozyme 435 load and 4 h of reaction time. Under these conditions, EOC was up to 7.62 ± 0.20 while AV was 8.53 ± 0.18 mgKOH/g. Moreover, the oxriane conversion degree was up to 97.94%.
Abbreviations
- AV:
-
Acid value
- EOC:
-
Epoxy oxygen group content
- ESO:
-
Epoxidized soybean oil
- EVO:
-
Epoxidized vegetable oil
- PV:
-
Peroxide value
- RCO:
-
Relative conversion oxriane
References
Campanella A, Baltanas MA (2005) Degration of the oxirane ring of exopoxidized vegetable oils in liquid–liquid systems: II. Reactivity with solvated acetic and peracetic. Lat Am Appl Res 35:211–216. doi:10.5702/massspec.54.235
Crivello JV, Narayan R, Stemstein SS (1997) Fabrication and mechanical characterization of glass fiber reinforced UV-cured composites from epoxidized vegetable oils. J Appl Polym Sci 64:2073–2087
Daniel MS, Nicolas RL, Vicente G, Vicente GF (2014) Chemoenzymatic epoxidation of alkenes based on peracid formation by a Rhizomucor miehei lipase-catalyzed perhydrolysis reaction. Chemistry 70:1144–1148. doi:10.1016/j.tet.2013.12.084
De Souza AVA, Cajaiba da Silva JF (2013) Biodiesel synthesis evaluated by using real-time ATR-FTIR. Org Process Res Dev 17:127–132. doi:10.1021/op300318k
Fantoni L, Simoneau C (2004) European survey of contamination of homogenized baby food by epoxidized soybean oil migration from plasticized PVC gaskets. Food Addit Contam 20:1087–1096. doi:10.1080/026520303/0001615186
Fukui YK, Oda S, Suzuki H, Hakogi T, Yamada D, Takagi Y, Aoyama Y, Kitamura H, Ogawa M, Kikuchi J (2012) Process optimization of aldol-type reaction by process understanding using in situ IR. Org Process Res Dev 16:1783–1786. doi:10.1021/op300186p
Gerbase AE, Gregorio JR, Martinelli M, Brasil MC, Mendes ANF (2002) Epoxidation of soybean oil by the methyltrioxorhenium-CH2Cl2/H2O2 catalytic biphasic system. J Am Oil Chem Soc 79:179–181. doi:10.1007/s11746-002-0455-0
Hosseini S (2014) Lipase epoxidation optimlzing of Jatropha curcas oil using perlauric acid. Dig J Nano Bios 9:1159–1169
Hwang HS, Erhan SZ (2001) Modification of epoxidized soybean oil for lubricant formulations with improved oxidative stability and low pour point. J Am Oil Chem Soc 12:1179–1184. doi:10.1007/s11745-001-0410-0
Leadbeater NE (2010) In situ reaction monitoring of microwave-mediated reactions using IR spectroscopy. Chem Commun 46:6693–6695. doi:10.1039/c0cc01921f
Liu W, Chen JN, Liu RL, Bi YL (2016) Revisiting the enzymatic epoxidation of vegetable oils by perfatty acid: perbutyric acid effect on the oil with low acid value. J Am Oil Chem Soc 93:1479–1486. doi:10.1007/s11746-016-2897-3
Lu H, Sun SD, Bi YL, Yang GL, Ma RL, Yang HF (2010) Enzymatic epoxidation of soybean oil methyl esters in the presence of free fatty acids. Eur J Lipid Sci Technol 112:1101–1105. doi:10.1002/ejlt.201000041
Paquot C (1979) Standard methods for the analysis of oils, fats and derivatives part, 6th edn. Pergamon, Oxford, pp 66–70
Paquot C, Hautfenne A (1987) Standard methods for the analysis of oils, fats and derivatives, 7th edn. Blackwell Scientific Publications, Oxford, pp 118–119
Petrovic ZS, Zlatanic A, Lava CC, Sinadinovic-Fiser S (2002) Epoxidation of soybean oil in toluene with peroxoacetic and peroxoformic acids Kinetics and side reactions. Eur J Lipid Sci Technol 104:293–299
Salimon J, Abdullah BM, Yusop RM, Salih N (2014) Synthesis, reactivity and application studies for different biolubricants. Chem Cent J 8:3225–3233. doi:10.1186/1752-153x-8-16
Sun SD, Ke XQ, Cui LL, Yang GL, Bi YL, Song FF, Xu XD (2011a) Enzymatic epoxidation of Sapindus mukorossi seed oil by perstearic acid optimized using response surface methodology. Ind Crops Prod 33:676–682. doi:10.1016/jindcrop.2011.01.002
Sun SD, Yang GL, Bi YL, Liang H (2011b) Enzymatic epoxidation of corn oil by perstearic acid. J Am Oil Chem Soc 88:1567–1571. doi:10.1007/s11746-0011-1820-1
Sun SD, Yang GL, Bi YL, Xia FG (2014) Enzymatic epoxidation of soybean oil using ionic liquid as reaction media. J Oleo Sci 63:383–390. doi:10.5650/ios.ess13197
Tornvall U, Orellana-Coca C, Hatti-Kaul R, Adlercreutz D (2007) Stability of immobilized Candida antarctica lipase B during chemo-enzymatic epoxidation of fatty acids. Enzyme Microb Technol 40:447–451. doi:10.1016/j.enzmictec.2006.07.019
Vlcek T, Petrovic ZS (2006) Optimization of the chemoenzymatic epoxidation of soybean oil. J Am Oil Chem Soc 83:247–252. doi:10.1007/s11746-006-1200-4
Acknowledgements
The authors gratefully acknowledge the financial support from the Technical Innovation Talent Funds in Henan University of Technology (No. 2013CXRC01), High Level Talent Funds of Henan University of Technology (No. 2013BS038) and the National Natural Science Foundation of China (No. 31601537).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Chen, J., Zhou, J., Liu, W. et al. Enzymatic epoxidation of soybean oil in the presence of perbutyric acid. Chem. Pap. 71, 2139–2144 (2017). https://doi.org/10.1007/s11696-017-0206-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-017-0206-8