Abstract
This paper describes the preparation of a biobased catalyst derived from residual Jatropha curcas cake using a combination of low-temperature conversion and chemical treatment to be applied for epoxidation of vegetable oil. The catalyst was characterized and its performance for epoxidation of cottonseed oil was evaluated and compared with the cationic resin VPOC 1800. After 60 min of epoxidation reaction, the results showed that VPOC resin catalyst presented yields (conversion of double bonds to oxirane rings) of about 34 % and biochar catalyst had yield of 14 %. Both catalysts presented high selectivity to bis-allylic hydrogen attack and elimination of 95 % of conjugated double bonds. The biochar catalyst presented higher activity than VPOC 1800 resin catalyst. On the other hand, the VPOC 1800 has better reusability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epoxidation is a reaction widely used to form oxirane rings from ethylenic unsaturations (C=C) [1]. The cyclical structure of oxirane rings has a bond angle of 60 °C, making them highly strained and highly reactive [2]. Typically, the method to promote epoxidation of double bonds uses hydrogen peroxide and acetic acid as oxygen carriers in acid media (by mineral acid). The reaction causes the formation of peroxy carboxylic acid, a compound that promotes oxirane formation. Due to high reactivity, the oxirane group can be converted into other groups with interesting properties, making it one of the best organic groups for organic synthesis. Another advantage of conversion of a double bond to oxirane group is enhancement of oxidation stability of materials that have high unsaturation content. Materials like vegetable oils, biodiesel and some rubber compounds belong in this class.
Classic methods employed for the oxidation of vegetable oils use homogeneous catalytic processes that generate a lot of waste, corrode equipment and require large amounts of reagents. Heterogeneous catalysts have the advantage of easy separation and recycling of the catalyst. For this reason, ion exchange resin has been studied to promote epoxidation of vegetal oils [2, 3].
Biobased metal cutting fluid (MCF) is an important material that has wide use in industry as a substitute for cutting fluids based on fossil-derived hydrocarbons [4]. MCFs can be made from vegetable oils, but some oils have low stability, mainly at high temperature. To solve this problem, epoxidation reactions are applied to convert unsaturations to oxirane rings followed by opening of the rings with water, to form the vicinal diol [5]. In some cases it is only necessary to convert a double bond with bis-allylic hydrogen (polyunsaturated), which is more susceptible to the oxidation process, maintaining intact the double bond with allylic hydrogen only. This procedure should improve the oxidation stability but tends to maintain viscosity and the liquid state.
Several authors have investigated heterogeneous catalysts based on the sulfonation of incomplete pyrolyzed biomass, such as sucrose [7], glucose [8] and biochar [9, 10]. Some seeds have been suggested in recent years to produce biochar, but to the best of our knowledge, no studies have been carried out of biochar produced by low-temperature pyrolysis combined with sulfonation.
Catalysts based on biomass have advantages such as low cost and surface chemical properties that can be tailored appropriately. An additional advantage of this work was the use of cake from Jatropha curcas. This cake, left over after oil extraction for biodiesel production, has high toxicity and cannot be used as animal feed.
Among the desirable characteristics of the catalyst support are stability, inertness, reusability, high surface area, porosity and appropriate chemical structure [6]. The process usually produces sulfonated carbon with low surface area and low acid site content [11].
Thus, the goals of this study were to produce a catalyst based on residual biomass from vegetable oil extraction using low-temperature pyrolysis and chemical modification. This biobased catalyst was compared with the commercial ion-exchange resin Lewatit VPOC 1800 for promotion of epoxidation reactions of cottonseed oil. Efficiency and selectivity to attack bis-allylic hydrogens were evaluated under typical synthesis conditions.
Experimental Section
VPOC 1800 Resin
VPOC 1800 resin from Bayer was activated with a solution of 2 M hydrochloric acid for 24 h, washed with water and acetone and dried in an oven for 24 h at 50 °C [12]. This treatment was carried out to ensure that all sites on the resin were activated.
Biochar
The residual cake of J. curcas was pyrolyzed in a Heraeus R/O 100 fast pyrolysis reactor at 450 °C according to the method described by Romeiro and colleagues [13].
10 g ± 1 g of biochar from pyrolysis was weighed and added to 100 ml of sulfuric acid in a two-necked flask under reflux at 80 °C under vigorous stirring for 13 h. Afterward, the material was washed with water until pH 5. Then the biochar was placed in an oven for 5 h at a temperature of 130 °C to remove moisture.
Characterization of Catalysts
The catalysts were examined with a scanning electron microscope (Quanta 200). The samples were placed in aluminum brackets with carbon tape. The images were obtained using the SEM in low vacuum operating condition (0.45 Torr), with acceleration of 20 kV and magnifications of 100×, 200× and 300×.
The Fourier-transform infrared spectra of the catalysts were obtained with a Thermo Nicolet Nexus 470 spectrophotometer from a piece of about 0.5 % sample in KBr. The small piece was compressed using a pressure of 105 N/m2 under dry condition in an oven at 110 °C for 24 h. The spectra were recorded in the range 4000–600 cm−1 with resolution of 4 cm−1 and 32 scans.
Surface Area
The specific surface area (S) of the catalysts was measured by nitrogen physisorption using a Micromeritics ASAP 2020 automatic physical adsorption analyzer. The specific surface area was calculated according to the Brunnet–Emmet–Teller (BET) equation by adsorption of nitrogen (N2).
The treatment of the samples was performed at 150 °C for about 10 h and the analyses were carried out for about 3 h.
Determination of the –SO3H Groups
The content of –SO3H groups on the surface of the solid acids was determined by potentiometric titration with sodium hydroxide. An aqueous sodium chloride solution (0.01 M, 20 mL) was added to the biochar (0.020 g). The mixture was stirred for 60 min at room temperature under ultrasonic vibration. The supernatant was titrated with sodium hydroxide (0.01 M) until there was no color change of phenolphtalein [14].
Epoxidation Reactions
Epoxidation reactions were performed in the same way for both catalysts. In a two-necked flask, 100 g of cottonseed oil, 16 g of acetic acid and 22 g of catalyst were mixed. Then the mixture was heated to 75 °C under magnetic stirring. After 30 min of stirring, 89 g of H2O2 was slowly added during 15 min [2]. After this time, the reaction was carried out for 300 min. A sample of 10 mL of the reaction mixture was collected every hour and transferred to a separating funnel. Then the sample was washed with deionized water until the pH became neutral. The reaction was followed by analysis of iodine value and 1H nuclear magnetic resonance (NMR).
Determination of Iodine Value
This parameter was analyzed according to EN 14111, using a Metrohm 857 Titrando potentiometric titrator.
Nuclear Magnetic Resonance (NMR)
Bruker Avance 200 and Avance 300 spectrometers operating at frequencies of 300 MHz for hydrogen were used. Samples were prepared in tubes with diameter of 5 mm, using 0.6 ml of deuterated chloroform (CDCl3) as solvent.
Reusability Test
The reusability was tested under the same conditions as the initial reaction. Each catalyst was tested with five cycles. After each reaction, the catalyst was removed by filtration and used without treatment. The reaction product was analyzed by 1H NMR after each cycle.
Results and Discussion
Characterization of Catalysts
SEM micrographs of the catalysts show different surface features between them (Fig. 1). Figure 1a shows VPOC 1800 resin, with spherical shape and small size distributions (400–500 μm). The resin beads present smooth and homogeneous surfaces without the presence of cracks, fissures or pores. As can be seen in Fig. 1b, the resin surfaces are not rough, indicating low porosity. In addition, there is no detectable surface area by ASAP. These results are in agreement with gel-type morphology of VPOC resin. Resin beads with gel-type morphology are compact and are not porous in the dry state [15].
Figure 1c shows that biochar particles had wide size distribution, including particles with diameters of several micrometers, but they had a large number of large cavities with diameters around of 10 µm (Fig. 1d). The biochar also presented no detectable surface area by ASAP, probably due to the macroporous morphology, as can be seen in Fig. 1d. Sulfuric acid was the chemical treatment employed in this work. Thus, the large reduction of surface area could be due to high destruction from the surplus water vapor released via H2SO4 dehydration. This produces an over-gasification of parent biochar by converting micropores into meso- and macropores, and therefore causes a detrimental effect on the BET and micropore surface areas [16].
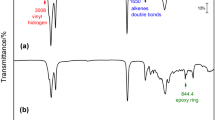
Infrared spectroscopy of the catalysts showed the presence of SO2 in both catalysts. Regions of characteristic bending absorption of SO2 groups (1215 cm−1) and strong axial stretching of S=O at 1034 cm−1 are present. The large absorption at 3000–3500 cm−1 has been widely associated OH bonds, as hydroxyl from phenol, cellulose or from –SO3H. Theses adsorptions bands match with the presence of sulfonic acid groups (–SO3H) in both catalyst. The data from acid base titration showed that biochar (1 × 10−3 mol/g) have lower number of acid sites than resin (4 × 10−3 mol/g) (Fig. 2).
Epoxidation with H2O2
Figure 3 shows the results of monitoring the epoxidation reaction promoted with VPOC 1800 resin or biochar. The blank curve represents the reaction performed without any catalyst where no meaningfully yield was obtained.
The iodine value is related to the number of double bonds present in the sample. The decreasing iodine value indicates conversion of double bonds to oxirane rings. Reaction without catalyst did not produce a meaningful yield. The initial iodine value of cottonseed oil was 111.62 g of I2/100 g of sample. After 60 min with resin VPOC 1800 as catalyst, the iodine value decreased to 73.80 g of I2/100 g. The iodine value decreased further with time, finally reaching 28.90 g of I2/100 g of sample (yield of 73.9 %).
The reaction for 300 min with the modified biochar as catalyst was effective, reaching an iodine value of 30.97 g of I2/100 g of sample (yield of 72.4 %). The epoxidation reactions using both VPOC 1800 resin and biochar as catalysts were very effective, eliminating almost 75 % of the unsaturations present in cottonseed oil. Although the two catalysts produced similar yields after 300 min, the behavior of the decay curves was very different.
1H NMR
The iodine value indicates the total presence of double bonds from either mono or polyunsaturations. NMR allowed determining whether hydrogen abstraction occurred on allylic or bis-allylic hydrogens during the epoxidation reaction, indicating if there is selectivity of bis-allylic attack by the catalyst.
Figure 4 presents 1H NMR spectra of parent cottonseed oil and products of reactions over time. Use of biochar catalyst led to very similar chemical shifts in NMR spectra. The intensities of signals were used to determine the yield and selectivity. The signals with chemical shift about 2.0 ppm are related to allylic hydrogens (neighboring the double bond). The integral value of 8.25 indicates the presence of unsaturated compounds. The signal with chemical shift at 2.70 ppm (triplet) is related to bis-allylic hydrogens (hydrogen atoms on the carbon between two double bonds), with integral value of 3.09. Both signals depend on the levels of mono and polyunsaturated fatty acids in the oil. Therefore, monitoring the signals of allylic and bis-allylic hydrogens allows monitoring the double bonds. The presence of an epoxide ring was confirmed in the epoxidized fluid by the chemical shift around 1.4–1.5 ppm (hydrogen atoms of carbon neighboring the epoxy rings) and chemical shifts in 2.8–3.1 ppm (hydrogens of the epoxide group). Table with total epoxy rings overtime can be found at supplementary material. Hydrogen integration signals were determined using the terminal methyl group as an internal standard (0.6 ppm).
The kinetic curve for the VPOC 1800 resin (Fig. 5a) shows pseudo-first-order behavior of both hydrogen types (allylic and bisallylic), but with different yields: while bis-allylic hydrogens produced a yield of 93.5 %, allylic hydrogens caused a yield of 66.5 %. The general yield (sum of all double bond consumption) was 73.89 %, close to the 74.1 % obtained by iodine value.
Epoxidation of vegetable oil with biochar as catalyst reaches yield for allylic hydrogens at 61.2 % while bis-allylic hydrogens yielded 93 %. Overall yield was about 72 %, close to that obtained by the iodine value.
Both catalytic systems preferentially attack bis-allylic hydrogens (present only on polyunsaturated fatty esters), allowing a reduction of polyunsaturation without completely removing monounsaturated (oleic) esters. This reduction of the polyunsaturated chain (bis-allylic hydrogens) tends to increase the oxidation resistance of the product. In addition, avoiding the complete reaction of monounsaturated ester prevents excessive increase of the product’s melting point.
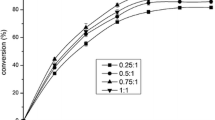
Reusability is an important advantage of solid acid catalysts over homogeneous acid catalysts. Reuse tests were performed under the same conditions as the initial reaction (Fig. 6). The results show that conversion of double bonds to oxirane rings of biochar is higher (up to 72–82 %) than resin until a second cycle, when undergoing substantial deactivation. The resin maintained activity of over 60 % for five cycles. Since the catalysts were removed by filtration in each cycle, without any treatment, the decrease of the conversion in both catalysts can be attributed to the pore blockage of recovered catalyst during the reaction by reactant residues. Dehkhoda and Ellis [17] previously reported this behavior for biochar catalysts.
Both catalysts can react with a double bond of the polyunsaturated ester while preserving some double bonds of the monounsaturated ester. This fact is important because it allows a liquid metal cutting fluid to be used at room temperature with high oxidation resistance. The results show that both catalysts are adequate for MCF production, with low generation of wastes. The recovery capacity of resin is better than biochar, but the latter’s recovery could be improved, allowing a greater the number of recycles.
The use of biomass to prepare catalyst is an advance, allowing a new application of residual biomass reducing the disposal of wastes from industrial processes.
Conclusions
The successful preparation of biochar catalyst from biomass by applying low-temperature conversion and chemical modification is reported. At one hour of epoxidation reaction of cottonseed oil, the VPOC 1800 resin catalyst presented higher conversion of double bonds to oxirane rings than biochar catalyst. The biochar catalyst was as selective and reactive to bis-allylic hydrogens (polyunsaturated esters) as VPOC 1800 catalyst. However, the biochar reusability needs to be improved because catalyst deactivation started at the third cycle. Both catalysts can be used as green chemistry approaches to produce epoxidized metal cutting fluid from vegetable oils.
References
Patil, H., Waghmare, J.: Catalyst for epoxidation of oils: a review. Discovery 3, 10–14 (2013)
Mungroo, R., Pradhan, N.C., Goud, V.V., Dalai, A.K.: Epoxidation of canola oil with hydrogen peroxide catalyzed by acidic ion exchange resin. J. Am. Oil Chem. Soc. 85, 887–896 (2008)
Turco, R., Vitiello, R., Russo, V., Tesser, R., Santacesaria, E., Di Serio, M.: Selective epoxidation of soybean oil with performic acid catalyzed by acidic ionic exchange resins. Green process Synth. 2, 427–434 (2013)
Kuram, E., Babur, O., Demirbas, E.: Environmentally friendly machining: vegetable based cutting fluids. Green Manuf. Process. Syst. 1, 23–47 (2013)
Alam, M., Akram, D., Sharmin, E., Zafar, F., Ahmad, S.: Vegetable oil based eco-friendly coating materials: a review article. Arab. J. Chem. 7, 469–479 (2014)
Mavrogiorgou, A., Papastergiou, M., Deligiannakis, Y., Loulodi, M.: Activated carbon functionalized with Mn(II) Schiff base complexes as efficient alkene oxidation catalysts: solid support matters. J. Mol. Catal. A: Chem. 393, 8–17 (2014)
Peng, L., Philippaerts, A., Ke, X.X., Van Noyen, J., De Clippel, F., Van Tendeloo, G., Jacobs, P.A., Sels, B.F.: Preparation of sulfonated ordered mesoporous carbon and its use for the esterification of fatty acids. Catal. Today 150, 140–146 (2010)
Zong, M.H., Duan, Z.Q., Lou, W.Y., Smith, T.J., Wu, H.: Preparation of a sugar catalyst and its use for highly efficient production of biodiesel. Green Chem. 9, 434–437 (2007)
Dehkhoda, A.M., West, A.H., Ellis, N.: Biochar based solid acid catalyst for biodiesel production. Appl. Catal. A-Gen. 382, 197–204 (2010)
Deng, J., Xiong, T., Xu, F., Li, M., Han, C., Gong, Y., Wang, H., Wang, Y.: Inspired by bread leavening: one-pot synthesis of hierarchically porous carbon for supercapacitors. Green Chem. 7, 453–460 (2015)
Geng, L., Wang, Y., Yu, G., Zhu, Y.: Efficient carbon-based solid acid catalysts for the esterification of oleic acid. Catal. Commun. 13, 26–30 (2011)
Maria, L.C.S., Oliveira, R.O., Merçon, F., Borges, M.E.R.S.P., Barud, H.S., Ribeiro, S.J.L., Messaddeq, Y., Wang, S.H.: Preparation and bactericidal effect of composites based on crosslinked copolymers containing silver nanoparticles. Polímeros 20, 227–230 (2010)
Romeiro, G.A., Salgado, E.C., Silva, R.V.S., Figueiredo, M.K.-K., Pinto, P.A., Damasceno, R.N.: A study of pyrolysis oil from soluble coffee ground using low temperature conversion (LTC) process. J. Anal. Appl. Pyrolysis. 93, 47–51 (2012)
Aldana-Pérez, A., Lartundo-Rojas, L., Gómez, R., Niño-Gómez, M.E.: Sulfonic groups anchored on mesoporous carbon Starbons-300 and its use for the esterification of oleic acid. Fuel 100, 128–138 (2012)
Corain, B., Zecca, M., Jerabek, K.: Catalysis and polymer networks—The role of morphology and molecular acessibility. J. Mol. Catal. A Chem. 177, 3–20 (2001)
Yakout, S.M., Daifullah, A.E.H.M., El-Reefy, S.A.: Pore structure characterization of chemically modified biochar derived from rice straw. Environ. Eng. Manage. J. 14, 473–480 (2015)
Dehkhoda, A.M., Ellis, N.: Biochar-based catalyst for simultaneous reactions of esterification and transesterification. Catal. Today 207, 86–92 (2013)
Acknowledgments
We gratefully acknowledge CNPq for a scholarship to Viviane F. Silva.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Silva, V.F., Batista, L.N., Cunha, V.S. et al. Production of Catalyst to Vegetable Oil Epoxidation from Toxic Biomass Residue. Waste Biomass Valor 8, 1265–1271 (2017). https://doi.org/10.1007/s12649-016-9616-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9616-z