Abstract
The relationship between reproductive and physiological stress hormones in vertebrates is poorly understood. In many species of mammals and especially in humans, the most widespread idea is that there is a negative relationship between them, i.e. higher stress levels are associated with lower testosterone levels. Likewise, the subordination stress paradigm supports that subordinates suffer greater stress in a competitive situation, while the dominant ones have higher levels of testosterone. However, this predominant idea of a negative relationship between testosterone and cortisol concentrations may be influenced by unnatural circumstances, such as chronic stress in humans or forced interactions between subordinates and dominants in laboratory or captivity. Some studies have reported that dominant males under natural conditions may show higher physiological stress and also higher testosterone levels than subordinates. But for this positive relationship, the question is whether there is a causal link or whether both hormones only coincide due to other factors. We hypothesized that testosterone should be related to physiological stress only as a result of individual males being subjected to stressful situations, such as intrasexual competition. We studied this topic in Iberian red deer (Cervus elaphus hispanicus) males in two types of populations with high and low levels of intrasexual competition. We found a positive relationship between faecal testosterone and cortisol metabolite levels, but also a significant interaction showing that this relationship occurs more intensely in populations with high competition level for mating. These results reinforce the positive relationship between both hormones under natural conditions and support the hypothesis that it is mediated by male-male competition for mates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The relationships between reproductive and 'stress-related hormones', including their interactions with dominance rank and sexual behaviour of individuals, are complex (Wingfield et al. 1997). Negative relationships between testosterone and stress have been reported for some species (e.g. ratsDoerr and Pirke 1976; Cumming et al. 1983), showing that testosterone concentrations were negatively related to 'stress-related glucocorticoid concentrations, likely because stressful situations and their consequent increase of glucocorticoid concentrations led to a reduction of reproductive hormone levels and sexual behaviour (Cumming et al. 1983).
Androgens are decisive in the regulation of agonistic encounters and their secretion is correlated with the defence of territories and mates (Wingfield et al. 1997; Arteaga et al. 2008), and with the expression of sexual behaviour and sexual traits (McGraw and Ardia 2003; Cornwallis and Birkhead 2008; Karubian et al. 2011). Hence, low-androgen-level males are expected to have poor development of sexual traits and low probability of winning fights (‘Challenge Hypothesis’; Wingfield et al. 1990).
According to the subordination stress paradigm (reviewed in Blanchard et al. 2001), many studies acknowledge that in social animals, individuals with lower levels of testosterone usually lose in agonistic interactions and maintain higher levels of circulating glucocorticoids compared to dominant individuals that win agonistic encounters (Agkistrodon contortrix: Schuett et al. 1996; Mus musculus: Palanza et al. 2001), although the cause and effect are not clear in this relation, as winning itself may cause the high testosterone levels. Nevertheless, recent studies on a number of species have shown that dominant individuals have higher glucocorticoid levels compared to subordinates, including wild chimpanzees (Pan troglodytes schweinfurthii: Muller and Wrangham 2004), ring-necked pheasants (Phasianus colchicus: Mateos 2005), Iberian wolves (Canis lupus signatus: Barja et al. 2008a, b) and Arctic charrs (Salvelinus alpinus: Backström et al. 2015) (see also review in Creel 2001).
Thus, the conventional view of a negative link between both groups of hormones could be due to non-natural conditions, such as chronic individual stress (Sapolsky 2005) or laboratory/captivity studies where dominant and subordinate individuals are forced to interact (Creel 2001).
Creel et al. (2013) suggested that stress differences between individuals of different social status could be due to different allostatic situations. Coping with unpredictable situations, such as agonistic encounters, changes the glucocorticoid levels towards a new optimum. Thus, the acquisition and maintenance of a certain social status have physiological and physical effects rather than dominance rank itself. The idea of allostatic load (McEwen and Stellar 1993), as those costs associated with chronic stressful environmental challenges that any individual faces along its lifetime, can help to explain the different patterns in glucocorticoid levels according to the social rank, as well as the intra-specific glucocorticoid variations resulting from exposure to intrasexual competition.
Intrasexual competition appears in individuals of the same sex as a result of the limited access to potential mates. The intensity of mate competition is context-dependent and can vary due to both demographic and social factors, such as mate availability and dominance stability (Baniel et al. 2018). Population structure, i. e. age and sex ratio, affects the chances for individuals to gain access to mates and hence the male intra-sexual competition situation (Andersson 1994; Rosvall 2011).
In polygynous mating systems, males often monopolize a whole group of females and the intra-sexual competition is intense. Male-male competition is usually sorted out by agonistic interactions, establishing a hierarchy between members of a group or subpopulation, which is a source of allostatic load and stress (Creel 2001, 2005). High exposure to agonistic encounters activates the hypothalamus–pituitary–adrenal axis, as an endocrine response to the stress, promoting glucocorticoid secretion (Stewart 2003). In addition, previous studies have shown the interactive role that androgens and glucocorticoids play in the changes that occur between alternative reproductive tactics affected by external environmental conditions, in terms of female availability (see Rasmussen et al. 2008).
The red deer (Cervus elaphus) is a highly polygynous species with males competing among themselves for the access to females during the rutting season (Clutton-Brock et al. 1982). It is unclear how glucocorticoid and androgen levels in red deer interact, but both hormones are involved in reproductive and aggressive behaviours (Lincoln et al. 1972; Fletcher 1978), antler development as a main secondary sexual character (Malo et al. 2009) and reproductive effort in terms of cumulative harem size in red deer (Pavvitt et al. 2015).
Studies in captive red deer found that individuals reached the lowest cortisol levels during the mating season, while the highest levels occurred in the non-breeding season, during the period of antler-growth (Ingram et al. 1999; Gaspar-López et al. 2010). However, in wild male red deer, Pavvitt et al. (2015) found a peak in cortisol metabolite levels coinciding with the rutting season, as did other studies for other polygynous species (Strier et al. 1999; Lynch et al. 2002). In the case of androgens, they peak during the mating season in both wild and captive individuals (Goss 1968; Suttie et al. 1984; Malo et al. 2009; Gaspar-López et al. 2010). Therefore, both cortisol and testosterone in wild red deer appear to peak during the rutting season, suggesting a positive, rather than negative, relationship between them.
Here, we focus on the relationship between reproductive and stress hormones in male Iberian red deer (Cervus elaphus hispanicus) in free-ranging conditions. During the rut, males compete for territories or harems (Carranza et al. 1990) as a way to get access to females, which results in strong agonistic encounters between rivals (Clutton-Brock et al. 1982; Lincoln et al. 1972; Appleby 1980; Carranza and Valencia 1999), a likely source of stress.
In Southern Spain, red deer populations occur under two different male-male competition scenarios defined by their management systems. We designated a low mate competition scenario when population structure was highly biased towards females and males were scarce and young, in contrast to a high intrasexual competition situation where most males were mature and the proportion of females was lower (Pérez-González and Carranza 2009; Pérez-González et al. 2012; Torres-Porras et al. 2014; see “Materials and methods”).
Thus, our study aimed to investigate the relationship between faecal testosterone and cortisol metabolite levels in male Iberian red deer, under two different conditions of intrasexual competition. We predicted higher faecal testosterone and cortisol metabolite levels and a strong association between both in the high competition situation, where agonistic encounters are frequent, and males invest more in sexual traits.
Materials and methods
Study area and populations
The study was conducted in Mediterranean ecosystems in South-western Spain. The study areas typically include a part of a mountain range covered by Mediterranean shrub (Cistus spp., Erica spp., Arbutus unedo, Phyllirea spp., Genista hirsuta, Lavandula spp.) and tree species (Quercus spp., Olea europaea), along with lower, flatter land, covered by open oak woodland known as ‘dehesa’.
Red deer populations in the study areas occurred in hunting estates that range in size between 750 and 3000 Ha. Under one management regime, estates are fenced by 2 m high stock-proof wire mesh, while in the other regime, areas are unfenced allowing deer free movement between estates (Pérez-González and Carranza 2009; Pérez-González et al. 2012; Torres-Porras et al. 2014). Fenced hunting estates reduce hunting pressure on young males, allowing them to reach maturity. By contrast, in unfenced hunting estates, because it is not a common practice between neighbouring estates to spare young males, few stags reach old age, as no estate wants to preserve adult stags because they might be shot when crossing to adjacent estates (Torres-Porras et al. 2014). Thus, on unfenced estates, hunting pressure is not on deer with the best trophy antlers, but on almost every male above 2 years of age, excluding yearlings as it is illegal to shoot them (Torres-Porras et al. 2014). As a consequence of such contrasting management, the sex-ratio and age structure in red deer populations within unfenced areas are strongly biased towards females and young males, compared to the situation in fenced areas (Pérez-González and Carranza, 2009; Torres-Porras et al. 2014). Therefore, on fenced estates, males experience a high level of intra-sexual competition compared to that on unfenced estates, where virtually all males can mate even if they are sub-adult (Pérez-González and Carranza 2009, 2011; Pérez-González et al. 2012). Although stags reach sexual maturity at 2–3 years, males may not breed normally until they are 5–7 years old in natural populations (Clutton-Brock and Albon 1989).
We considered fenced estates as independent populations, while for open estates we defined populations with one or several estates according to natural or artificial barriers that can limit deer movements. From now on we will refer to these fenced and unfenced areas, respectively, as populations with high (HC) and low (LC) levels of intrasexual competition.
Data collection
Data were collected from October to February during the two hunting periods of 2015–2016 and 2016–2017, in estates from Sierra Morena (Province of Córdoba, UTM 37º58′ N, 5º05′ W) and Sierra San Pedro (Provinces of Badajoz and Cáceres, UTM 39º19′ N 6º42′ W). Each hunting estate constitutes a different population (average surface = 2347 ha) because of the existing natural (i.e. mountains, geographical distance) or artificial barriers between them. Iberian red deer density in these geographical regions is around 0.3 individuals/ha (0.1–1.0 indiv./ha) (Torres-Porras et al. 2014).
We sampled 236 individuals harvested during hunting activities in red deer natural occurring in 16 populations. 162 males were from LC populations and 74 males from HC populations. Hunting activities increase the physiological stress of individuals (Vilela et al. 2020). We were interested in hormone levels before the hunting action took place, hence serum level quantification was not appropriate here. Instead, we conducted hormone quantification from faeces samples by detecting hormone metabolites, a highly used and validated procedure in Iberian red deer (de la Peña et al. 2020a, b; Carranza et al. 2020) and other mammal species (Barja et al. 2007; Escribano-Ávila et al. 2013; Iglesias-Merchán et al. 2018) useful to get information on the existing hormone levels 12–24 h before sampling (Barja et al. 2012). We collected faecal samples from the rectum of each individual to avoid bacterial degradation and froze them at –20 °C until laboratory analyses. Age was estimated by counting cementum growth marks at the interradicular pad under the first molar (Carranza et al. 2004).
Quantification of steroid hormones
Faecal samples collected were used to quantify faecal testosterone and cortisol metabolite levels. Samples were extracted using a procedure established for Iberian red deer (de la Peña et al. 2020a, b; Carranza et al. 2020) and other mammal species (Escribano-Ávila et al. 2013).
Frozen faecal samples were dried and pulverized. We took 0.5 g of faeces and added 2 ml of phosphate buffer and 2 ml of 100% methanol. The mixture was vortexed. After that, samples were on the shaker for 16 h. The solvent was decanted, and the supernatant was centrifuged at 4000 rpm for 30 min.
We used commercial enzyme immunoassay kits (DEMEDITEC Diagnostics GmbH, Kiel, Germany; testosterone: DEMEDITEC DE1559; cortisol: DEMEDITEC DEH3388) to determine the faecal metabolite hormone levels by the same procedure published in previous studies (de la Peña et al. 2020a, b; Carranza et al. 2020). DEMEDITEC-kits have been successfully analytically validated by carrying out the corresponding parallelism, accuracy, and precision tests. Parallel displacement curves were obtained by comparing serial dilutions (1:32, 1:16, 1:8, 1:4, 1:2, 1:1) of pooled faecal extracts with the standard curves. The results corroborated that both curves were parallel for all hormones considered in the present study (Cortisol: R2 = 0.972; P = 0.001; Testosterone: R2 = 0.994; P = 0.001). Accuracy (recovery) was 93.6% for cortisol and 104.4% for testosterone, with mean recovery percentages of red deer faecal extracts being similar for both hormones. Precision was tested through intra- and inter-assay coefficients of variation for faecal samples, being the testosterone intra-assay coefficient of variation 10.8% and inter-assay 10.6%. The cortisol intra-assay coefficient of variation was 9.2% and inter-assay 10.2%. The assay sensitivity for testosterone and cortisol were 0.083 and 2.5 ng/g, respectively. Faecal extracts were analysed in duplicates. These results clearly supported that the used kits were correctly measuring cortisol and testosterone concentrations in the collected samples without specifically requiring an ACTH test.
We did not perform ACTH challenge test or specific validation in this study because as biological validation, we compared the faecal metabolite cortisol and testosterone levels within the male age classes—juveniles and adults, and health status, specifically if they resulted positive or negative in a tuberculosis seroprevalence test. We found significant differences in faecal testosterone metabolite levels between juvenile and adult males (ANOVA: (\({F}_{1, 234}\) \({\mathrm{F}}_{1, 234}\)=7.342; p-value = 0.007) and between those resulting positive or negative in the tuberculosis test (ANOVA: \({F}_{5, 151}\)=2.990; p value = 0.013). We did not find such significant differences in faecal cortisol metabolite levels between both age classes (ANOVA: \({F}_{1, 234}\)=0.373; p value = 0.542) nor between tuberculosis positive and negative animals (ANOVA: \({F}_{5, 151}\)=0.270; p value = 0.929).
Statistical analyses
We built Generalized Linear Mixed Models (GLMMs) to investigate factors associated with variations in faecal cortisol metabolite levels fitted to a gamma distribution. We performed models with different predictor variables and combinations between explanatory variables, all of them with a biological sense. As explanatory variables, we included age, faecal testosterone metabolite concentrations and the average of the antler length as covariables. We considered mate competition as a factor staged in two levels (low and high levels of competition).
Even though there was no collinearity between the variables included in our models (see below), previous studies support that the level of intra-sexual competition affects antler length in Iberian red deer populations (Pérez-González and Carranza 2009; Torres-Porras et al. 2009; Carranza et al. 2020), establishing a biological relationship between these variables. Hence, we conducted a second GLMM after removing antler length to see potential relationships of other variables hidden by the effects of antler size.
To facilitate model convergence, all quantitative variables were z-transformed, being the mean of zero and a standard deviation of one (using the scale function, Eager 2017). We also considered two-way interactions and, to avoid risks of over-parameterization, we removed non-significant interactions sequentially (p value > 0.05) following a backwards-stepwise selection procedure. The sample size was 236 observation values from 16 populations, some of them sampled in 2 years. Average age (± SE) of individuals was 3.32 ± 0.12 years ( = 2–11 years) and average antler size was 60.11 ± 1.12 ( = 10.35–101.85). Specifically, 162 observations were from LC populations, where the average age (± SE) was 2.60 ± 0.09 (= 2–11) and average antler size was 52.32 ± 0.93 ( = 10.35–79.90). Seventy-four observations correspond to males from HC populations where the average age (± SE) was 4.89 ± 0.25 ( = 2–11) and average antler size was 77.16 ± 1.68 ( = 45.55–101.85).
We checked the normal distribution of the model residuals, when explaining variation in the faecal cortisol metabolite levels, using a Shapiro–Wilks test and we assessed the assumptions of homogeneity of variance plotting residual versus fitted values. We also examined the presence of outliers and potential influential data points using Cook’s distance graphs. To avoid multicollinearity between variables, we calculated the variance inflation factors (VIFs; Alin 2010) of each built model, using the package usdm (Babak 2015). In all cases, we did not find any evidence of collinearity (VIF < 1.910, see "Appendices 1, 2"). Analyses were carried out with R 3.2.4 (Rstudio Team 2017), using the package “lme4” (Bates 2015). The means are given ± SE and the level of statistical significance was P < 0.05. Predictions were visualized with ‘ggeffects’ (Ludecke 2018) and ‘ggplot2′ (v3.1.1) was used for graphics (Wickham 2016).
Results
Faecal cortisol metabolite (FCM) levels were positively related to faecal testosterone metabolite (FTM) levels and to the interaction between antler length and FTM levels (Table 1), indicating that the relationship between FTM and FCM levels was more intense when antlers were bigger (Fig. 1). Age and the level of male-male intrasexual competition (HC or LC) were non-significant factors.
Predictions of the model on Table 1 of faecal cortisol metabolite (FCM) against faecal testosterone metabolite (FTM) levels and antler length of male red deer. Points are raw data for the FCM (high competition, HC: grey filled points; low competition, LC, grey open points)
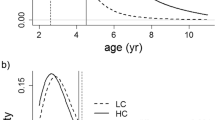
Results derived from the second model, in which we removed antler length as a covariate (see “Materials and methods”), are shown in Table 2. FCM levels appeared related to FTM levels and the interaction between FTM and the level of intrasexual competition (HC vs. LC populations), i.e. the relationship between FTM and FCM levels was stronger in populations with high levels of male intrasexual competition (Fig. 2).
Predictions of the model on Table 2 of faecal cortisol metabolite (FCM) against faecal testosterone metabolite (FTM) levels of male from populations with two levels of male-male sexual competition for mating opportunities (high competition, HC solid line, and low competition, LC dashed line). Points are raw data for the FCM (HC: grey filled points; LC, grey open points)
Discussion
We studied the relationship between concentrations of both FTM and FCM in wild male Iberian red deer under two different scenarios of male-male competition. In contrast with previous studies in this species (Gaspar-López et al. 2010), we found a positive relationship between levels of FTM and FCM.
It has been claimed that concentrations of both hormones should be inversely related (e.g. Wingfield et al. 1994) due to the important role of glucocorticoids as a suppressor of gonadal function in many species. In accordance with this prediction, several studies have shown a negative relationship between both testosterone and cortisol metabolite levels. Also, the subordination stress paradigm (reviewed in Schuett et al. 1996; Blanchard et al. 2001), predicts that subordinate males will show higher glucocorticoid levels than dominant males while at the same time they may have lower levels of sexual hormones in concordance with their lower mating chances and their lesser expression of reproductive behaviour and traits.
Our results disagree with these predictions, showing a positive correlation between glucocorticoids and reproductive hormones. This positive link, however, has also been found in the most recent studies on the topic (Muller and Wrangham 2004; Mateos 2005; Barja et al. 2008a, b; Backström et al. 2015), arguing that the stress situation of dominants and subordinates depends on the social context. Thus, in captive conditions subordinates may be subjected to the most stressful situation, for instance, if they are not able to avoid undesirable interactions with dominants. However, in the wild or under natural conditions, these individuals have a great chance to be the least stressed in the group. In red deer, the strong male-male competition to defend resources and females leads to agonistic encounters and other stressful situations mostly suffered by dominant stags (Lincoln et al. 1972; Clutton-Brock et al. 1982).
Derived from these results, we realized that some LC males were those that presented the highest levels of faecal metabolites of testosterone but not that of cortisol (Fig. 2). According to previous studies (de la Peña et al. 2019), these males have greater chance of mating, due to the higher availability of females per male. Testosterone is related to aggressiveness and territorial behaviours (Wingfield et al. 1997; Arteaga et al. 2008). However, high faecal testosterone metabolite levels may refer to their reproductive status (Martin et al. 2014) rather than to the dominance rank, and it is remarkable that these LC males with high FTM in conditions of low intrasexual competition showed relatively low levels of FCM (Fig. 2).
Therefore, this work provides clear evidence that the positive relationship between testosterone and cortisol depends on the level of intrasexual competition, it being stronger when stags are under high competition level and hence stronger sexual selection that occurs in HC populations (Pérez-González and Carranza 2011). Pavvitt et al. (2015) showed that faecal cortisol metabolite concentrations in male red deer were positively correlated with cumulative harem size, a proxy of male reproductive effort and/or mating success during the rut. Our results for different levels of male-male competition populations point to intrasexual interactions as the source of stress for those males with higher faecal testosterone metabolite levels who are those more likely involved in the intrasexual competition (Lincoln et al. 1972; Malo et al. 2009).
The positive relationship between faecal testosterone and cortisol metabolite levels might also be interpreted under the framework of the immunocompetence handicap hypothesis (Folstad and Karter 1992). Under this hypothesis, males with higher testosterone levels will incur costs in terms of immunocompetence, which may also elevate their stress levels. However, a causal link between sexual hormone levels and stress is not supported by our results, since metabolite levels of both hormones were not related in populations with low levels of intrasexual competition. Rather, our results indicate that the link between both hormones may be mediated by the stressful situation produced by the sexual competition between males.
Previous papers dealing with the relationship between testosterone and stress hormones, such as cortisol, have mainly focused on dominance differences between individuals (Schuett et al. 1996; Blanchard et al. 2001; Muller and Wrangham 2004; Mateos 2005; Barja et al. 2008a, b; Backström et al. 2015). However, dominance rank per se is not likely responsible for such relationships. Differences in population structure, such as skewed sex-ratio or age distribution, should modulate the source of stress for both dominants and subordinates (Muller and Wrangham 2004). Levels of stress hormones should relate to the ability of the individuals to cope with an unpredictable disturbance (allostasis models view in Creel et al. 2013), not just because of its rank alone. The conventional point of view is that subordinate individuals suffer elevated stress levels due to frustrated attempts at reproduction. However, if we understand stress as a result of physiological and psychological processes by which an individual tries to return to the balanced state after an unpredictable disturbance (Creel et al. 2013), our understanding on the social and individual reproductive context changes. Investment in sexual characters and reproductive effort, as well as unpredictable agonistic interactions, should result in costs in terms of stress. Red deer antlers constitute an important sexual trait, with high production costs (Goss 1983). Antler size is positively correlated with winning fights and mating success (Clutton-Brock et al. 1982; Malo et al. 2009). Our results support that dominant and high-testosterone individual have bigger antlers, and show elevated cortisol faecal levels, in agreement with their higher rate of agonistic encounters compared to subordinates (Creel et al. 1996). Also, antler length appeared in our study associated with high faecal cortisol metabolite concentrations regardless the competitive level in the population (HC or LC), but we also found a significant interaction between testosterone and antler length, indicating that those males with small antlers did not show a relationship between both hormones, while males with bigger antlers did. And also, we already know that males in HC populations produce bigger antlers at the cost of lower lifespan expectancies due to elevated tooth wear (Carranza et al. 2020), which is in agreement with higher FCM levels in males either with bigger antlers or experiencing a situation of higher intrasexual competition.
To summarize, we found a positive relationship between faecal testosterone and cortisol metabolites in male Iberian red deer in free-ranging conditions, and the strength of this relationship to be context-dependent: under conditions of high male-male competition there was a stronger positive relationship between these two hormones. These results may contribute to our understanding of the nature of the costs involved in sexual competition and investment in sexual traits.
References
Alin A (2010) Multicollinearity. Wiley Interdisciplinary Rev 3:370–374. https://doi.org/10.1002/wics.84
Andersson M (1994) Sexual selection. Princeton University Press, New Jersey, p 624. doi: https://doi.org/10.1515/9780691207278
Appleby M (1980) Social rank and food access in red deer stags. Behaviour 74:294–309. https://doi.org/10.1163/156853980X00519
Arteaga L, Bautista A, Martínez-Gómez M, Nicolás L, Hudson R (2008) Scent marking, dominance and serum testosterone levels in male domestic rabbits. Physiol Behav 94:510–515. https://doi.org/10.1016/j.physbeh.2008.03.005
Babak N (2015) Uncertainty analysis for species distribution models. R package version 1.1–15
Backström T, Heynen M, Brännäs E, Nilsson J, Magnhagen C (2015) Dominance and stress signalling of carotenoid pigmentation in Arctic charr (Salvelinus alpinus): lateralization effects? Physiol Behav 138:52–57. https://doi.org/10.1016/j.physbeh.2014.10.003
Baniel A, Cowlishaw G, Huchard E (2018) Context dependence of female reproductive competition in wild chacma baboons. Anim Behav 139:37–49. https://doi.org/10.1016/j.anbehav.2018.03.001
Barja I, Silván G, Rosellini S, Piñeiro A, González-Gil A, Camacho L, Illera JC (2007) Stress physiological responses to tourist pressure in a wild population of European pine marten. J Steroid Biochem Mol Biol 104:136–142. https://doi.org/10.1016/j.jsbmb.2007.03.008
Barja I, Silván G, Illera JC (2008a) Relationships between sex and stress hormone levels in feces and marking behavior in a wild population of Iberian wolves (Canis lupus signatus). J Chem Ecol 34:697–701. https://doi.org/10.1007/s10886-008-9460-0
Barja I, Silvá F, Rosellini S, Piñeiro A, Illera MJ, Illera JC (2008b) Quantification of sexual steroid hormones in faeces of Iberian wolf (Canis lupus signatus): a non-invasive sex typing method. Reprod Domest Anim 43:701–707. https://doi.org/10.1111/j.1439-0531.2007.00974.x
Barja I, Escribano-Ávila G, Lara-Romero C, Virgós E, Benito J, Rafart E (2012) Non-invasive monitoring of adrenocortical activity in European badgers (Meles meles) and effects of sample collection and storage on faecal cortisol metabolite concentrations. Animal Biology 62(4):419–432
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Blanchard RJ, McKittrick CR, Blanchard C (2001) Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol Behav 73:261–271. https://doi.org/10.1016/S0031-9384(01)00449-8
Carranza J, Valencia J (1999) Red deer females collect on male clumps at mating areas. Behav Ecol 10:525–532. https://doi.org/10.1093/beheco/10.5.525
Carranza J, Álvarez F, Redondo T (1990) Territoriality as a mating strategy in red deer. Anim Behav 40:79–88. https://doi.org/10.1016/S0003-3472(05)80667-0
Carranza J, Alarcos S, Sánchez-Prieto C, Valencia J, Mateos C (2004) Disposable-soma senescence mediated by sexual selection in an ungulate. Nature 432:215–218. https://doi.org/10.1038/nature03004
Carranza J, Pérez-Barbería J, Mateos C, Alarcos S, Torres-Porras J, Pérez-González J, Sánchez-Prieto CB, Valencia J, Castillo L, de la Peña E, Barja I, Seoane J, Reglero M, Flores A, Membrillo A (2020) Social environment modulates investment in sex trait versus lifespan: red deer produce bigger antlers when facing more rivalry. Sci Rep 10:9234. https://doi.org/10.1038/s41598-020-65578-w
Clutton-Brock TH, Albon SD (1989) Red deer in the highlands. Blackwell Scientific, Oxford
Clutton-Brock TH, Guinness FE, Albon SD (1982) Red deer: behaviour and ecology of two sexes. University of Chicago Press, Chicago
Cornwallis CK, Birkhead TR (2008) Plasticity in reproductive phenotypes reveals status-specific correlations between behavioural, morphological and physiological sexual traits. Evolution 62:1149–1161. https://doi.org/10.1111/j.1558-5646.2008.00346.x
Creel S (2001) Social dominance and stress hormones. Trends Ecol 16:491. https://doi.org/10.1016/S0169-5347(01)02227-3
Creel S (2005) Dominance, aggression and glucocorticoid levels in social carnivores. J Mammal 86:255–264. https://doi.org/10.1644/BHE-002.1
Creel S, Creel NM, Monfort SL (1996) Social stress and dominance. Nature 379:212. https://doi.org/10.1038/379212a0
Creel S, Dantzer B, Goymann W, Rubenstein DR (2013) The ecology of stress: effects of the social environment. Funct Ecol 27:66–80. https://doi.org/10.1111/j.1365-2435.2012.02029.x
Cumming DC, Quigley ME, Yen SSC (1983) Acute suppression of circulating testosterone levels by cortisol in men. J Clin Endocrinol Metab 57:671–673. https://doi.org/10.1210/jcem-57-3-671
de la Peña E, Martín J, Carranza J (2019) The intensity of male-male competition may affect chemical scent constituents in the dark ventral patch of male Iberian red deer. PLoS ONE 14(9):e0221980. https://doi.org/10.1371/journal.pone.0221980
de la Peña E, Martín J, Barja I, Pérez-Caballero R, Acosta I, Carranza J (2020a) a) The immune challenge of mating effort: steroid hormone profile, dark ventral patch and parasite burden in relation to intrasexual competition in male Iberian red deer. Integr Zool. https://doi.org/10.1111/1749-4877.12427
de la Peña E, Martín J, Barja I, Carranza J (2020b) Testosterone and the dark ventral patch of male red deer: the role of the social environment. Sci Nat 107:18. https://doi.org/10.1007/s00114-020-01674-1
Doerr P, Pirke KM (1976) Cortisol-induced suppression of plasma testosterone in normal adult males. J Clin Endocrinol Metab 43:622–629. https://doi.org/10.1530/acta.0.080S055a
Eager CD (2017) Standardize: tools for standardizing variables for regression in R. R package version 0.2.1. https://CRAN.R-project.org/package=standardize.
Escribano-Ávila G, Pettorelli N, Virgós E, Lara-Romero C, Lozao J, Barja I, Cuadra FS, Puerta M (2013) Testing cort-fitness and cort-adaptation hypotheses in a habitat suitability gradient for roe deer. Acta Oecol 53:38–48. https://doi.org/10.1016/j.actao.2013.08.003
Fletcher TJ (1978) The induction of male sexual behavior in red deer (Cervus elaphus) by the administration of testosterone to hinds and estradiol-17β to stags. Horm Behav 11:74–88. https://doi.org/10.1016/0018-506X(78)90059-4
Folstad I, Karter AJ (1992) Parasites, bright males, and the immunocompetence handicap. Am Nat 139:603–622. https://doi.org/10.1086/285346
Gaspar-López E, Landete-Castillejos T, Estevez JA, Ceacero F, Gallego L, García AJ (2010) Biometrics, testosterone, cortisol and antler growth cycle in Iberian red deer stags (Cervus elaphus hispanicus). Reprod Dom Anim 45:243–249. https://doi.org/10.1111/j.1439-0531.2008.01271.x
Goss RJ (1968) Inhibition of growth and shedding of antlers by sex hormones. Nature 220:83–85. https://doi.org/10.1038/220083a0
Goss RJ (1983) Deer antlers: regeneration, function and evolution. Academic Press, Cambridge
Iglesias-Merchán C, Horcajada-Sánchez F, Diaz-Balteiro L, Escribano-Ávila G, Lara-Romero C, Virgós E, Planillo A, Barja I (2018) A new large-scale index (AcED) for assessing traffic noise disturbance on wildlife: stress response in a roe deer (Capreolus capreolus) population. Environ Monit Assess 490:185. https://doi.org/10.1007/s10661-018-6573-y
Ingram JR, Crockford JN, Matthews LR (1999) Ultradian, circadian and seasonal rhythms in cortisol secretion and adrenal responsiveness to ACTH and yarding in unrestrained red deer (Cervus elaphus) stags. J Endocrinol 162:289–300. https://doi.org/10.1677/joe.0.1620289
Karubian J, Lindsay WR, Schwabl H, Webster MS (2011) Bill coloration, a flexible signal in a tropical passerine bird, is regulated by social environment and androgens. Anim Behav 81:795–800. https://doi.org/10.1016/j.anbehav.2011.01.012
Lincoln G, Guinness FA, Short RV (1972) The way in which testosterone controls the social and sexual behavior of the red deer stag (Cervus elaphus). Horm Behav 3:375–396. https://doi.org/10.1016/0018-506X(72)90027-X
Ludecke D (2018) ggeffects: Tidy data frames of marginal effects from regression models. J Open Source Softw 3:772. https://doi.org/10.21105/joss.00772
Lynch JW, Ziegler TB, Strier KB (2002) Individual and seasonal variation in fecal testosterone and cortisol levels of wild male tufted capuchin monkeys, Cebus apella nigritus. Horm Behav 41:275–287. https://doi.org/10.1006/hbeh.2002.1772
Malo AF, Roldan ERS, Garde JJ, Soler AJ, Vicente J, Górtazar C, Gomendio M (2009) What does testosterone do for red deer males? P R Soc B Biol Sci 276:971–980. https://doi.org/10.1098/rspb.2008.1367
Martín J, Carranza J, López P, Alarcos S, Pérez-González J (2014) A new sexual signal in rutting male red deer: age related chemical scent constituents in the belly black spot. Mammal Biol 79:362–368. https://doi.org/10.1016/j.mambio.2014.07.005
Mateos C (2005) The subordination stress paradigm and the relation between testosterone and corticosterone in male ring-necked pheasants. Anim Behav 69:249–255. https://doi.org/10.1016/j.anbehav.2004.03.010
McEwen BS, Stellar E (1993) Stress and the individual: mechanisms leading to disease. Arch Intern Med 153:2093–2101. https://doi.org/10.1001/archinte.1993.00410180039004
McGraw KJ, Ardia DR (2003) Carotenoids, immunocompetence, and the information content of sexual colors: an experimental test. Am Nat 162:704–712. https://doi.org/10.1086/378904
Muller MN, Wrangham W (2004) Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii). Behav Ecol Sociobiol 55:332–340. https://doi.org/10.1007/s00265-003-0713-1
Palanza P, Gioiosa L, Parmigiani S (2001) Social stress in mice: gender differences and effects of estrous cycle and social dominance. Physiol Behav 73:411–420. https://doi.org/10.1016/S0031-9384(01)00494-2
Palme R, Robia C, Messmann S, Hofer J, Möstl E (1999) Measurement of faecal cortisol metabolites in ruminants: a non-invasive parameter of adrenocortical function. Wien Tierärztl Monat 86:237–241. https://doi.org/10.1023/A:1014095618125
Pavvitt AT, Walling CA, Möstl E, Pemberton JM, Kruuk LEB (2015) Cortisol but not testosterone is repeatable and varies with reproductive effort in wild red deer stags. Gen Comp Endocrinol 222:62–68. https://doi.org/10.1016/j.ygcen.2015.07.009
Pérez-González J, Carranza J (2009) Female-biased dispersal under conditions of low male mating competition in a polygynous mammal. Mol Ecol 18:4617–4630. https://doi.org/10.1111/j.1365-294X.2009.04386.x
Pérez-González J, Carranza J (2011) Female aggregation interacts with population structure to influence the degree of polygyny in red deer. Anim Behav 82:957–970. https://doi.org/10.1111/j.1365-294X.2009.04386.x
Pérez-González J, Frantz AC, Torres-Porras J, Castillo L, Carranza J (2012) Population structure, habitat features and genetic structure of managed red deer populations. Eur J Wildlife Res 58:933–943. https://doi.org/10.1007/s10344-012-0636-0
Rasmussen HB, Ganswindt A, Douglas-Hamilton I, Vollrath F (2008) Endocrine and behavioral changes in male African elephants: linking hormone changes to sexual state and reproductive tactics. Hormones and Behavior 54(4):539–548
Rosvall KA (2011) Intrasexual competition in females: evidence for sexual selection? Behav Ecol 22:1131–1140. https://doi.org/10.1093/beheco/arr106
RStudio Team (2016) RStudio: integrated development for R. RStudio, Inc., Boston, MA. http://www.rstudio.com/
Sapolsky RM (2005) The influence of social hierarchy on primate health. Science 308:648–652. https://doi.org/10.1126/science.1106477
Schuett GW, Harlow HJ, Rose JD, Van Kirk EA, Murdoch WJ (1996) Levels of plasma corticosterone and testosterone in male copperheads (Agkistrodon contortrix) following staged fights. Horm Behav 30:60–68. https://doi.org/10.1006/hbeh.1996.0009
Stewart PM (2003) The adrenal cortex. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS (eds) Williams textbook of endocrinology. Saunders, Philadelphia, pp 491–551. doi: https://doi.org/10.1097/00060793-200106000-00001
Strier KB, Ziegler TB, Wittwer DJ (1999) Seasonal and social correlates of fecal testosterone and cortisol levels in wild male muriquis (Brachyteles arachnoides). Horm Behav 35:125–134. https://doi.org/10.1006/hbeh.1998.1505
Suttie JM, Linconl GA, Kay RNB (1984) Endocrine control of antler growth in red deer stags. J Reprod Fer 71:7–15. https://doi.org/10.1530/jrf.0.0710007
Torres-Porras J, Carranza J, Pérez-González J (2009) Combined effects of drought and density on body and antler size of male Iberian red deer (Cervus elaphus hispanicus): climate change implications. Wildlife Biol 15:213–221. https://doi.org/10.2981/08-059
Torres-Porras J, Carranza J, Pérez-González J, Mateos C, Alarcos S (2014) The tragedy of the commons: unsustainable population structure of Iberian red deer in hunting estates. Eur J Wildlife Res 60:351–357. https://doi.org/10.1007/s10344-013-0793-9
Vilela S, Alves da Silva A, Palme R, Ruckstuhl KE, Sousa JP, Alves J (2020) Physiological stress reactions in red deer induced by hunting activities. Animals 10:1003. https://doi.org/10.3390/ani10061003
Wickham H (2016) ggplot2: elegant graphics for data analysis. R Package Version 3.1.1. Springer, New York. ISBN 978–3–319–24277–4. https://ggplot2.tidyverse.org; http://dx.doi.org/https://doi.org/10.18637/jss.v077.b02
Wingfield J, Hegner RE, Dufty AM, Ball GF (1990) The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat 136:829–846. https://doi.org/10.1086/285134
Wingfield JC, Deviche P, Astheimer L, Holberton R, Suydam R, Hun K (1994) Seasonal changes in the adrenocortical responses to stress in common redpolls. J Exp Zool 270:372–380. https://doi.org/10.1002/jez.1402700406
Wingfield JC, Jacobs J, Hillgarth N (1997) Ecological constraints and the evolution of hormone-behavior interrelationships. Ann N Y Acad Sci 807:22–41. https://doi.org/10.1111/j.1749-6632.1997.tb51911.x
Acknowledgements
We thank two anonymous reviewers for their helpful comments and the autonomous governments of Andalucía (Junta de Andalucía) and Extremadura (Junta de Extremadura) and the owners of the hunting estates that provided permissions and facilities for fieldwork. Thanks to Jose Manuel Seoane and students help with field work. To Dr. Álvaro Navarro for revise the hormonal analyses. Financial support came from projects CGL2013-48122-P and CGL2016-77052-P to JC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Yoshiyuki Henning.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
Variance inflation factors (VIFs) for the explanatory variables included in the first GLMM explaining the differences in FCM and FTM including age, antler length and male-male competition. FTM = 1.062; Age = 1.435; antler length = 1.564; Mmate competition = 1.211; antler length × mate competition = 1.065.
Appendix 2
Variance inflation factors (VIFs) for the explanatory variables included in the second GLMM explaining the differences in FCM and FTM controlling by age under two male-male competition scenarios. FTM = 1.910, Age = 1.116; mate competition = 1.120; FTM × mate competition = 1.909.
Rights and permissions
About this article
Cite this article
de la Peña, E., Barja, I. & Carranza, J. Social environment with high intrasexual competition enhances the positive relationship between faecal testosterone and cortisol metabolite levels in red deer. Mamm Biol 101, 207–215 (2021). https://doi.org/10.1007/s42991-021-00100-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-021-00100-x