Abstract
The expression of male sexual traits, which is stimulated by testosterone, entails significant costs for individuals. Consequently, natural selection is expected to favour the modulation of sexual trait development according to the balance between its costs and benefits. The proportion of rivals in a population may affect this balance by increasing or decreasing the reproductive benefits associated with the development of sex traits. Here, we explore the relationship between testosterone level and sex trait size under two populational conditions of mate competition: fenced (i.e. high male-male competition; all male age groups are present) and unfenced (i.e. low competition; most males present are juveniles). Our model species is the Iberian red deer (Cervus elaphus hispanicus), and the sex trait is the dark ventral patch that males exhibit during the rutting season. Our results showed that the positive relationship between testosterone levels and the size of the dark ventral patch depends on the environmental level of male-male competition. Only in populations where the operational sex ratio was high (i.e. high proportion of rival males), individuals with high levels of testosterone developed the sex trait. Conversely, when mate competition was low, there was no significant relationship between testosterone level and trait size. This result reinforces the idea that the effect of testosterone in promoting the development of sex traits may be mediated by the intensity of mate competition in the population, as well as the role of sexual selection in the evolution of the dark ventral patch in red deer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual signals play a fundamental role in reproductive contexts, but they also have relevant implications for many other aspects of animal lives. Testosterone, for instance, plays a dual role in individuals of numerous species. On the one hand, it promotes the development of sexual characters. On the other hand, it has a suppressant effect on immune function. Thus, only individuals with a high-quality immune system may be able to afford the immunosuppressive costs associated with the development of sexual ornaments. Poor-condition males cannot exhibit well-developed sexual traits without compromising their status health and their future reproductive success. As a result of the trade-offs between investing in ornaments and immune health, sexual signals may be reliable indicators of the immune status of males (Folstad and Karter 1992).
When costs related to trait development outweigh associated benefits, natural selection may favour individual ability to reduce investment in flexible sex traits. The proportion of rivals and mates (i.e. the social environment) may play a central role in the balance between trait costs and associated benefits, thus leading to different optima in trait development. Individuals with a low probability of winning fights due to intrasexual competition are expected to have lower testosterone levels (challenge hypothesis, Wingfield et al. 1990) and reduced expression of sexual traits (McGraw and Ardia 2003; Cornwallis and Birkhead 2008; Karubian et al. 2011). However, since sex traits have many other costs besides those linked to testosterone production, their expression may not be associated with testosterone levels (Vergara and Martínez-Padilla 2012).
Testosterone is an important hormone involved in the regulation of several male reproductive physiological aspects. Secretion of testosterone is especially relevant in competitive contexts during the mating season (Adkins-Regan 2005). It appears to be correlated with intrasexual competition behaviours, such as aggression and territoriality (Fletcher 1978; Lincoln et al. 1972), mate defence (Dloniak et al. 2006), displaying (Mateos and Carranza 1997; Mateos 2005) and susceptibility to being attacked by rivals (Arteaga et al. 2008). At the same time, testosterone plays a role in the development of sexual traits (Atwell et al. 2014). Thus, testosterone level can be crucial for mating competition (Wingfield et al. 1987; Raouf et al. 1997; Peters et al. 2008) and hence reproductive success (Ketterson et al. 2001; Mills et al. 2009; Cain and Pryke 2017). Some studies in several cervid species have shown a positive link between testosterone levels and dominance rank, as well as with male sexual behaviour (Rajagopal 2009; Brockman et al. 2001).
The Iberian red deer (Cervus elaphus hispanicus) is a highly polygynous species in which males compete for females by defending either harems or mating territories (Carranza et al. 1990; Carranza 1995; Carranza et al. 1996). During the rut, males show a conspicuous dark patch in their ventral fur. The dark pigmentation of hairs from this area is due to the excretion of a catecholamine metabolite (DOPEG) in urine that may reveal males’ fighting ability and their capacity to avoid predation (Galván et al. 2019). The expression of this sexual character by each male is variable and flexible. Its size increases during the rutting season (reaching up to 70 cm in length) but also with age, peaking at 6–7 years of age, thus following a similar pattern to that of other traits involved in mate competition such as antler length and body size (Carranza et al. unpublished data). Volatile compounds present in the hair of this area also vary with age (Martín et al. 2014) and the level of mate competition (i.e. the abundance of male rivals in the population) (de la Peña et al. 2019) and are probably involved in intraspecific communication (Martín et al. 2014). Furthermore, the dark ventral patch size shows a bimodal distribution (Carranza et al. unpublished data), revealing two groups of males that differ in reproductive effort and behavioural alternative during the rut (de la Peña et al. unpublished data).
In male Iberian red deer, the role of testosterone has been demonstrated in sperm production and quality, antler strength and the number of broken tines probably as a result of more fighting (Malo et al. 2009). Our preliminary assessments suggest the importance of the social environment regarding the positive relationship between testosterone and cortisol metabolite levels. High testosterone metabolite levels are associated with intrasexual competition-related behaviour but also entails costs in terms of physiological stress (i.e. elevated cortisol metabolite levels). Thus, the higher the mating competition in the population, the more intense the link between both hormone levels (de la Peña et al. unpublished data).
Under this framework, we investigate the role of testosterone and cortisol metabolite levels in the dark ventral patch expression in male Iberian red deer. We expected a positive association between testosterone levels and trait size. We also predicted that this relationship would depend on the age of individuals and the intensity of mate competition in the population due to the differences in mating chances for individuals of different ages in both types of populations. Finally, because of the bimodality nature of the trait, we explore the influence of social environment on patch size within both groups of males showing high and low expression of the trait.
Material and methods
Study area and red deer populations
We studied male Iberian red deer harvested in 14 hunting estates located in Andalucía (Sierra Morena, Córdoba province) and Extremadura (Sierra de San Pedro, Badajoz and Cáceres provinces), in southwestern Spain. The study zone is characterised by the existence of mountain ranges covered by open oak agro-forestry woodland, known as ‘dehesa’, and Mediterranean shrub including Cistus spp., Erica spp., Arbutus unedo, Phyllirea spp., Genista hirsute and Lavandula spp.
Each hunting estate constitutes a different population (average surface = 2347 ha) because of the existing natural (i.e. mountains, geographical distance) and artificial (i.e. fences) barriers between them. Iberian red deer density in these geographical regions is around 0.3 individuals/ha (range = 0.1–1.0 indiv./ha) (Torres-Porras et al. 2014).
Hunting estates in the study area are under two different management regimes. Under one of these regimes, estates are fenced by 2 m high stock-proof wire mesh, while in the other regime, the estates are unfenced and allow deer to move across nearby estates (Pérez-González and Carranza 2009; Torres-Porras et al. 2014). Fenced hunting estates reduce hunting pressure on young male deer, allowing them to reach maturity. By contrast, in unfenced estates, private owners do not want to preserve adult stags as they are at risk of being shot (lost) when crossing to adjacent estates and nearly every male above 2 years of age can be shot (Torres-Porras et al. 2014). As a result of these two different management scenarios, the sex ratio and age structure of red deer populations within unfenced estates differ from that of fenced estates. In unfenced states, the sex ratio is strongly biased towards females and age structure of males is biased towards young individuals (Pérez-González and Carranza 2009; Torres-Porras et al. 2014). Thus, virtually all males can mate in unfenced estates even if they are sub-adult (Pérez-González and Carranza 2009, 2011; Pérez-González et al. 2012). In fenced estates, in contrast, males experience a higher level of mate competition (Pérez-González and Carranza 2009, 2011; Pérez-González et al. 2012). Although fenced states do not allow the free movement of individuals, the age and sex structure of these populations allows intrasexual competition to operate in a natural manner or, at least, very similar to what occurs when there is no hunting pressure. However, differences in management do not translate into different population densities between fenced and unfenced estates (Torres-Porras et al. 2014).
From now on, we refer to the populations of fenced estates as populations with high levels of male-male competition for mating (HC) and to populations of unfenced estates as those with low male-male competition (LC).
Data collection
We sampled 235 males harvested from 14 different hunting estates (161 males from 8 LC populations and 74 males from 6 HC populations) during the hunting season of 2015–2016 or 2016–2017 (between October and February). Only in two estates, we sampled males harvested in both hunting seasons. The sample size for each age category was as follows: 104 individuals were 2-year-old males, 87 were 3- and 4-year-old males, and 44 were males 5 years of age or older.
For each individual, we estimated its age and measured the size of its dark ventral patch and the length of its antlers. All measures were taken from animal carcasses within a few hours after being shot. Age was estimated by counting cementum growth marks at the interradicular pad under the first molar (Carranza et al. 2004). The size of the dark ventral patch was measured longitudinally (in cm) from the penis to the anterior limit of the stained surface. Because sample collection took place after the rutting season, to measure the size of the dark ventral patch, we considered both black and brown hairs (see Appendix 2). We assumed that brown hairs had lost colour intensity due to the time elapsed since the mating season. In this way, we tried to reduce potential errors derived from the timing of data collection. We calculated the average length of both antlers, measuring them from the lowest external edge of the burr over the outer side to the most distant point of the main beam.
We quantified the hormone levels by measuring hormone metabolites in fresh faecal samples. Faeces were collected from the rectum of each individual and frozen at − 20 °C until laboratory analyses. This approach is a widely used and validated procedure (Barja et al. 2007; Escribano-Ávila et al. 2013; Iglesias-Merchán et al. 2018), which is useful to get information on the existing circulating hormone levels 12 to 24 h before sampling (Barja et al. 2012)—serum level quantification was not considered appropriate as hormone levels would be influenced by the hunting action.
Quantification of steroid hormones
We processed faecal samples to quantify the levels of testosterone and cortisol metabolites following a procedure used for other mammal species (roe deer Capreolus, Escribano-Ávila et al. 2013; Iglesias-Merchán et al. 2018; Horcajada-Sánchez et al. 2019; European pine marten Martes, Barja et al. 2007; Iberian wolf Canis lupus signatus, Barja et al. 2008; European badger Meles meles, Barja et al. 2012; wildcat Felis silvestris, Piñeiro et al. 2012). Frozen faecal samples were dried and pulverised. We took 0.5 g of faeces and added 2 ml of phosphate buffer and 2 ml of 100% methanol. The mixture was vortexed. After that, samples were homogenised on the shaker for 16 h. The solvent was decanted, and the supernatant was centrifuged at 4000 rpm for 30 min. Using commercial enzyme immunoassay kits (testosterone, DEMEDITEC DEH3388; cortisol, DEMEDITEC DE1559), we determined the faecal metabolite hormone levels in ng/g of dry faecal matter. The testosterone intra-assay coefficient of variation was 10.8% and inter-assay 10.6%. The cortisol intra-assay coefficient of variation was 9.2% and inter-assay 10.2%. Standard dose-response curves were constructed by plotting the binding percentage (B/Bo × 100) against the standard hormone concentrations added.
Statistical analyses
We used linear mixed models (LMMs) with random intercept fitted by restricted maximum likelihood in the R v.2.14.0 (R Foundation for Statistical Computing, Vienna, Austria) using the package lme4 (Bates et al. 2015).
We used Shapiro-Wilks test to test for normality of the models’ residuals when explaining variation in the dark ventral patch size. Models produced two clusters of residuals that corresponded to males with very different ventral patch sizes: large and small. To account for variation among such groups of males, we added in models a new fixed effect predictor: ‘trait expression’. This variable was a factor with two levels: low trait expression (LTE), for males with a patch size of less than 50 cm; and high trait expression (HTE), for those with patches above 50 cm (Fig. 1; Carranza et al. unpublished data). These two groups were consistent across the three age categories (Fig. 1 a, b and c).
Histogram of the dark ventral patch size (cm) in male Iberian red deer (Cervus elaphus hispanicus) showing the bimodality of the two groups of males attending to the trait expression: grey, low trait expression males (low trait expression, LTE; patch size between 0 and 50 cm); black, high trait expression males (high trait expression, HTE; patch size 50 cm and above). a Overall males. b 2-year-old males. c 3–4-year-old males. d 5-year-old-and-above males. Dotted lines indicate the mean trait size of each group
We included the following explanatory variables: concentration of faecal testosterone metabolites (FTM, in ng), concentration of faecal cortisol metabolites (FCM, in ng), average antler length, intensity of mate competition (LC and HC). The age of males and its quadratic term were also included in the model as covariates because the dark ventral patch and antler length are known to have quadratic relationships with age (Carranza et al. unpublished data).
To show the general relationship between reproductive and stress hormones and the dark ventral patch size, we first analysed the whole dataset. The full model included population (i.e. hunting estate) and hunting year as random terms to see the effects of variation across populations and years.
However, we also categorised age into three groups that tended to differ in trait expression: most 2-year-old males (81%) showed LTE, while 86% of the oldest males (5 years old and above) belonged to HTE. Older males (3- and 4-year-old) were uniformly distributed between the two categories. We ran three subsequent models for each age class to explore the differences in the explanatory variables that may affect trait expression. In the models for juveniles and subadults, we only included the hunting year as a random effect. In the model for adults, we included population (i.e. HTE, LTE) as the only random effect.
To facilitate model convergence, all quantitative covariables were z-transformed over the mean of zero and a standard deviation of one. In all statistical models, we considered two-way interactions, and to avoid risks of over-parameterization, we sequentially removed non-significant interactions (P value > 0.05) following a backward-stepwise selection procedure that took into account the AIC (Akaike’s information criterion).
To avoid multicollinearity between variables, we calculated the variance inflation factors (VIFs; Alin 2010) of each model using the package usdm (Babak 2015). We did not find any evidence of collinearity for any of the models (VIF < 3.19, see Appendix 1). We also checked for normality (using Shapiro-Wilks test) and the lack of heteroscedasticity in the residuals of each LMM. We set statistical significance as P < 0.05.
Results
Relationship between the dark ventral patch size and faecal testosterone and cortisol metabolite levels
Antler length and faecal testosterone metabolite levels showed a positive relationship with the dark ventral patch size in male Iberian red deer after controlling for age and its quadratic term, as well as for the intensity of male-male competition (Table 1). Faecal cortisol metabolite levels had a negative marginal relationship with the trait size.
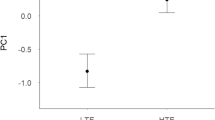
The significant interaction between trait expression and mate competition indicated that only LTE males had larger dark ventral patches in HC than in LC populations, but there were no differences between populations in HTE males (Fig. 2).
Differences in the effect of the level of mate competition (low competition, LC open point; high competition, HC black point) in the dark ventral patch size in the two groups of males of Iberian red deer (Cervus elaphus hispanicus) regarding the trait expression (LTE vs. HTE). Dark ventral patch size refers to predicted values from models (mean ± 95% confidence intervals; Table 1)
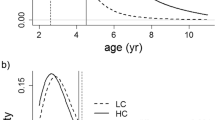
Our results revealed that the link between testosterone and trait size marginally depended on the level of mate competition; in HC populations, this relationship was positive, but there was no relationship in LC populations (Fig. 3).
Relationships between faecal testosterone metabolite levels (FTM) and dark ventral patch size in male Iberian red deer (Cervus elaphus hispanicus) under two situations of mate competition (low competition, LC open points and discontinuous line; high competition, HC black points and continuous line). Dark ventral patch size refers to predicted values from the model (Table 1)
Two-year-old males: faecal cortisol metabolite levels and the dark ventral patch size
For 2-year-old males, we found a significant negative relationship between faecal cortisol metabolite concentrations and trait size (Table 2). At this age, only males with low levels of physiological stress (cortisol) had large dark ventral patches. We did not find any significant relationship between testosterone, antler length and levels of mate competition on the expression of the dark ventral patch.
Three to 4-year-old males: antler length, mate competition and the dark ventral patch size
Neither faecal testosterone nor cortisol metabolite levels were significantly related to the patch size in 3–4-year-old males. However, antler length was positively correlated with trait size (Table 3). Even though mate competition did not have a significant effect on patch size, we found that LTE males had larger ventral patches in HC than in LC populations, whereas there were no differences in HTE males.
Five-year-old and above males: mate competition and the dark ventral patch size
In males of 5 years or older, we found a marginal effect of the antler length on the dark ventral patch size (Table 4). However, there was an effect of mate competition on trait size. Thus, individuals in HC populations had larger dark ventral patches than in LC populations (Fig. 4). No significant interactions were found in this age category of males.
Effect of the level of mate competition (low competition, LC open point; high competition, HC black point) on the dark ventral patch size in ≥ 5-year-old male Iberian red deer (Cervus elaphus hispanicus). Dark ventral patch size refers to predicted values from models (mean ± 95% confidence intervals; Table 4)
Discussion
Our results showed a positive relationship between testosterone metabolite levels and the expression of the dark ventral patch in male Iberian red deer. However, our results also indicated that this relationship is modulated by the level of mate competition in the social environment.
Thus, faecal testosterone metabolite levels were positively related to the size of the dark ventral patch only for males exposed to high intrasexual competition. Under strong male-male competition, the intensity of selection is high (Shuster and Wade 2003). Under high intrasexual competition, the expression of characters informing about the social rank and body condition, as well as the male’s reproductive effort, is crucial for achieving mating success despite the associated costs involved (Rubenstein and Shen 2009). These costs may promote that only those males with enough skills and condition (e.g. antler size), and associated chances for winning in the competition, develop the trait, leading to the variation in trait size among HC males. Due to these associated costs, which may include impaired immune function (de la Peña et al. 2020), reduced survival (Wingfield et al. 2001) or increased stress (de la Peña et al. unpublished data), males are expected to reduce their testosterone levels either when their chances for winning are low or when competition in the population is not intense (Wingfield et al. 2001). However, our results show that they reduced the expression of the sexual trait even if testosterone remained high. This result suggests that trait development and associated male-male competition may entail higher costs than testosterone level itself. This is in agreement with previous research in the same red deer populations showing that the relationship between testosterone and cortisol, as a proxy of some associated cost, was more intense under conditions of high male-male competition (de la Peña et al. unpublished).
Interestingly, for the effect of intrasexual competition on the dark ventral patch size, we also found differences in between the two groups of trait expression males (LTE vs. HTE). In LTE males, the intensity of intrasexual competition in the population positively affected the size of the sexual trait. In contrast, we did not find an effect of mate competition on the size of the dark ventral patch in HTE males. One possible explanation of this result is that most HTE males may have reached the maximum, asymptotic size of the trait and cannot develop it further.
The differences between LTE and HTE were especially noticeable for males 3 and 4 years old. These males before reaching prime age are good candidates for using alternative mating strategies such as sneaking and defending harems (Carranza et al. 1990) depending on their condition (e.g. antler size) and chances relative to rivals. The differences between LTE and HTE males concerning the effect of the social environment on the trait size agree with the hypothesis that the dark ventral patch may reveal two reproductive alternatives regarding mating effort (Carranza et al. unpublished data). Our results suggested that this differentiation may be evident at the age of 3 and 4.
When analysing the relationship between the dark ventral patch size and the testosterone metabolite levels according to age classes of males, we also found that, in the case of 2-year-old males, only individuals with low levels of cortisol metabolite or low-stress levels expressed the dark ventral patch. This suggests a role for early condition in the expression of this sexual trait in male Iberian red deer. Only those juvenile males with low levels of physiological stress (i.e. faecal cortisol metabolite levels), and therefore well-body-condition males, may invest in the development of the dark ventral patch. Thus, this result may indicate the separation of condition-depending traits from the testosterone-mediated physiological effects (e.g. Mougent et al. 2005; Wyman et al. 2010), as well as the individual flexibility to invest in early and late-life performance (Lemaître et al. 2015; Hartl et al. 1995).
For individuals of 5 years or older, who are close to prime age (Carranza et al. 2004), our results showed that antler length and the intensity of intrasexual competition were positively associated with the size of the dark ventral patch. These results are consistent with the hypothesis that male investment in sex traits and reproductive effort depends on the chances of mating success and to which extent the expression of the trait is needed to defeat rivals, i.e. the level of intrasexual competition in the population. Thus, these mature males only develop larger ventral patches under conditions of high competition for mates (HC), while in LC populations, the number of females is relatively high and the number of rival males is low, allowing prime-age males to mate with almost no competition (Pérez-González and Carranza 2011).
Previous studies have shown that, due to the females’ availability, under a low intrasexual competition populations, males are not forced to disperse in order to achieve mating, which leads to a decrease in genetic diversity in LC males in relation to HC males (Pérez-González et al. 2012). Future studies should examine the potential relationship between genetic diversity and the dark ventral patch size under low and high mate competition. The dark ventral patch may be revealing the selective process by which successful males achieve mating.
Besides the level of male-male competition, differences in other factors such as habitat quality and food availability (which influence the nutritional status of individuals) could also influence different investment in sex traits in the two populations. Research on the potential effect of the resource availability and quality on the dark ventral patch size and testosterone levels is strongly recommended.
When it comes to sex ornaments, animals with better nutritional, health and oxidative status tend to show more intense colouration, as they can afford to produce substantial amounts of costly pigments (Faivre et al. 2003; McGraw 2006). The relationship between the expression of both carotenoid- and melanin-based colouration signals and glucocorticoid levels remains unclear (negative relationship: Saino et al. 2002; Roulin et al. 2008; Douglas et al. 2009; Lobato et al. 2010; positive relationship: Lendvai et al. 2013). However, the dark ventral patch is neither a melanin- nor a carotenoid-based ornament. Instead, colouration results from the excretion of a catechol metabolite in the urine, DOPEG, that oxidises with air contact-generating alomelanin (Galván et al. 2019). Even though the dark colouration is not endogenously produced, it may still reveal the competitive quality of a male (Galván et al. 2019).
Up to date, there are no studies on the link between catecholamines and cortisol circulating levels conducted in wildlife populations. However, some studies have demonstrated the role of catecholamines in the stress-induced reduction of testosterone in the plasma of adult mammals (i.e. rat, Rattus norvegicus; golden hamster, Mesocricetus auratus) under controlled laboratory conditions (Damber and Janson 1978; Götz et al. 1983; Gatenbeck et al. 1987). Nevertheless, catecholamines are also involved in the regulation of some reproductive aspects such as testes development and are thus potent physiological stimulators of testosterone production (Mayerhofer et al. 1992). Due to the close relationship between testosterone levels and males’ aggressiveness, it might be possible that the investment in mate competition and reproduction implies costs in terms of physiological stress, related to the exposure to agonistic encounters. Moreover, testosterone production entails costs related to increased male susceptibility to parasitism (de la Peña et al. 2020). Considering all the above, we speculate that the release of catecholamines related to the dark ventral patch expression might be leading to costs derived from exposure to physiological stress in the medium to long term, which may ultimately lead to decreased testosterone levels (Gatenbeck et al. 1987).
Sexual signals that reveal males’ body condition and are related to the individual dominance rank usually vary in a unimodal manner (Tibbetts et al. 2017). Phenotypic variation is expected by the cost to produce and maintain sexual traits, and because of the differences between individuals in their capacity to afford the signal-related (Tibbetts et al. 2017). In contrast to most signals of quality, the dark ventral patch size is bimodal (Gadgil 1972; Senar et al. 1993; Tibbetts and Dale 2004). The dark ventral patch size is not well explained by testosterone and cortisol metabolite levels. This study suggests that the intensity of intrasexual competition in the population and the associated behaviours, which involve moderate to high levels of testosterone, are key to understanding the differential investment in the dark ventral patch size during the rut.
A preliminary study has shown that the expression level of this trait may also convey information about the males’ behavioural strategy during the mating season, revealing their reproductive effort regarding territorial and intrasexual competition-related behaviours (de la Peña et al. unpublished data). The dark ventral patch seems to be a quality signal that conveys information about the dominance rank and body condition of stags, as well as about their mating behaviour effort during the rut. However, why should a behavioural strategy be signalled by particular phenotypic trait discernible by conspecifics or at least correlated with it? This is an intriguing and overlooked question in the literature, typically examined in the case of colour morphs (Sinervo and Zamudio 2001; Lamichhaney et al. 2016; Ábalos et al. 2016).
Overall, we found that there is a positive association between faecal testosterone metabolite levels and the dark ventral patch size. Both this association and the sex trait size in LTE are mediated by the social environment, more specifically by the intensity of mate competition. In contrast, HTE males do not modulate their investment in the dark ventral patch according to their mate competition context. The intrasexual competition has a different effect on different types of males when they are 3 and 4 years old. From the age of 5, the mate competition level directly influences the size of the dark ventral patch, displaying their option between two types of strategies when facing the rutting season. The social environment is crucial in the expression of the dark ventral patch in all males when they reach the age of 5 years. Future studies should explain how the cost-benefit balance conditioned by population structure, associated with individual glucocorticoid variation, health status and body condition, is related to trait expression level individually, as well as the relationship between current reproductive behavioural alternative and both present and future fitness consequences.
References
Ábalos J, Pérez I de Lanuza G, Carazo P, Font E (2016) The role of male colouration in the outcome of staged contests in the European common wall lizard (Podarcis muralis). Behaviour 153:607–631
Adkins-Regan E (2005) Hormones and animal social behaviour. Princeton University Press, Princeton
Alin A (2010) Multicollinearity. Wiley Interdiscip Rev Comput Stat 3:370–374
Arteaga L, Bautista A, Martínez-Gómez M, Nicolás L, Hudson R (2008) Scent marking, dominance and serum testosterone levels in male domestic rabbits. Physiol Behav 94:510–515
Atwell J, Cardoso GC, Whittaker CJ, Price TD, Ketterson ED (2014) Hormonal, behavioral, and life-history traits exhibit correlated shifts in relation to population establishment in a novel environment. Am Nat 184:147–160
Babak N (2015) Uncertainty analysis for species distribution models. R package version 1.1–15
Barja I, Silván G, Rosellini S, Piñeiro A, González-Gil A, Camacho L, Illera JC (2007) Stress physiological responses to tourist pressure in a wild population of European pine marten. J Steroid Biochem Mol Biol 104:136–142
Barja I, Silvá F, Rosellini S, Piñeiro A, Illera MJ, Illera JC (2008) Quantification of sexual steroid hormones in faeces of Iberian wolf (Canis lupus signatus): a non-invasive sex typing method. Reprod Domest Anim 43:701–707
Barja I, Escribano-Ávila G, Lara-Romero C, Virgós E, Benito J, Rafart E (2012) Non-invasive monitoring of adrenocortical activity in European badgers (Meles meles) and effects of sample collection and storage on faecal cortisol metabolite concentrations. Anim Biol 62:419–432
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Statist Softw 61:1–48
Brockman DK, Whitten PL, Richard AF, Benander B (2001) Birth season testosterone levels in male Verreaux’s sifaka, Propithecus verreauxi: insights into socio-demographic factors mediating seasonal testicular function. Behav Ecol Sociobiol 49:117–127
Cain KE, Pryke SR (2017) Testosterone production ability predicts breeding success and tracks breeding stage in male finches. J Evol Biol 30:430–436
Carranza J (1995) Female attraction by males versus sites in territorial rutting red deer. Anim Behav 50:445–453
Carranza J, Alvarez F, Redondo T (1990) Territoriality as a mating strategy in red deer. Anim Behav 40:79–88
Carranza J, Fernandez-Lario P, Gomendio M (1996) Correlates of territoriality in rutting red deer. Ethology 102:793–805
Carranza J, Alarcos S, Sánchez-Prieto C, Valencia J, Mateos C (2004) Disposable-soma senescence mediated by sexual selection in an ungulate. Nature 432:215–218
Cornwallis CK, Birkhead TR (2008) Plasticity in reproductive phenotypes reveals status-specific correlations between behavioural, morphological and physiological sexual traits. Evolution 62:1149–1161
Damber JE, Janson PO (1978) The effects of LH, adrenaline and noradrenaline on testicular blood flow and plasma testosterone concentrations in anaesthetized rats. Acta Endrocrinol Buch 88:390–396
de la Peña E, Martín J, Carranza J (2019) The intensity of male-male competition may affect chemical scent constituents in the dark ventral patch of male Iberian red deer. PLoS One 14(9):e0221980
de la Peña E, Martín J, Barja I, Pérez-Caballero R, Acosta I, Carranza J (2020) The immune challenge of mating effort: steroid hormone profile, dark ventral patch and parasite burden in relation to intrasexual competition in male Iberian red deer. Integr Zool. https://doi.org/10.1111/1749-4877.12427
Dloniak SF, French JA, Holekamp KE (2006) Faecal androgen concentrations in adult male spotted hyaenas, Crocuta crocuta, reflect interactions with socially dominant females. Anim Behav 71:27–37
Douglas HD, Kitaysky AS, Kitaiskaia EV, Maccomick A, Kelly A (2009) Size of ornament is negatively correlated with baseline corticosterone in males of a socially monogamous colonial seabird. J Comp Physiol B 179:297–304
Escribano-Ávila G, Pettorelli N, Virgós E, Lara-Romero C, Lozano J, Barja I, Cuadra FS, Puerta M (2013) Testing Cort-Fitness and Cort-Adaptation hypotheses in a habitat suitability gradient for roe deer. Acta Oecol 53:38–48
Faivre B, Grégoire A, Préault M, Cézilly F, Sorci G (2003) Immune activation rapidly mirrored in a secondary sexual trait. Science 300:103–103
Fletcher TJ (1978) The induction of male sexual behavior in red deer (Cervus elaphus) by the administration of testosterone to hinds and estradiol-17β to stags. Horm Behav 11:74–88
Folstad I, Karter AJ (1992) Parasites, bright males, and the immunocompetence handicap. Am Nat 139:603–622
Gadgil M (1972) Male dimorphism as a consequence of sexual selection. Am Nat 106:574–580
Galván I, Solano F, Zougagh M, Andrés F, Murtada K, Ríos A, de la Peña E, Carranza J (2019) Unprecedented high catecholamine production causing hair pigmentation after urinary excretion in red deer. Cell Mol Life Sci 76:397–404
Gatenbeck L, Eneroth P, Johansson B, Strömberg L (1987) Plasma testosterone concentrations in male rats during short and long-term stress stimulation. Scand J Urol Nephrol 21:139–142
Hartl GB, Klein F, Willing R, Apollonio M, Lang G (1995) Allozymes and the genetics of antler development in red deer (Cervus elaphus). J Zool 237:83–100
Götz F, Stahl F, Rohde W, Dörner G (1983) The influence of adrenaline on plasma testosterone in adult and newborn male rats. Exp Clin Endocrinol Diabetes 81:239–244
Horcajada-Sánchez F, Escribano-Ávila G, Lara-Romero C, Virgós E, Barja I (2019) The effect of livestock on the physiological condition of roe deer (Capreolus capreolus) is modulated by habitat quality. Sci Rep 9:15953
Iglesias-Merchán C, Horcajada-Sánchez F, Diaz-Balteiro L, Escribano-Ávila G, Lara-Romero C, Virgós E, Planillo A, Barja I (2018) A new large-scale index (AcED) for assessing traffic noise disturbance on wildlife: stress response in a roe deer (Capreolus capreolus) population. Environ Monit Assess 490:185
Karubian J, Lindsay WR, Schwabl H, Webster MS (2011) Bill coloration, a flexible signal in a tropical passerine bird, is regulated by social environment and androgens. Anim Behav 81:795–800
Ketterson ED, Nolan V Jr, Casto JM, Buerkle CA, Clotfelter E, Grindstaff JL, Jones KJ, Lipar JL, McNabb FMA, Neudorf DL, Parker-Renga I, Schoech SJ, Snajdr E (2001) Testosterone, phenotype and fitness: a research program in evolutionary behavioral endocrinology. Avian Endocrinology. Narosa Publishing House, New Delhi, pp 19–40
Lamichhaney S, Fan G, Widemo F, Gunnarsson U, Thalmann DS, Hoeppner MP, Chen W (2016) Structural genomic changes underlie alternative reproductive strategies in the ruff (Philomachus pugnax). Nat Genet 48:84–88
Lemaître JF, Berger V, Bonenfant C, Douhard M, Gamelon M, Plard F, Gaillard JM (2015) Early-late life trade-offs and the evolution of ageing in the wild. Proc Biol Sci 282(1806):20150209
Lendvai AZ, Giraudeau M, Németh J, Bakó V, McGraw KJ (2013) Carotenoid-based plumage colouration reflects feather corticosterone levels in male house finches (Haemorhous mexicanus). Behav Ecol Sociobiol 67:1817–1824
Lincoln G, Guinness FA, Short RV (1972) The way in which testosterone controls the social and sexual behavior of the red deer stag (Cervus elaphus). Horm Behav 3:375–396
Lobato E, Moreno J, Merino S, Morales J, Tomas G, Martinez J, Vasquez RA, Kuchar A, Mostl E, Osorno JL (2010) Arrival date and territorial behaviour are associated with corticosterone metabolite levels in a migratory songbird. J Ornithol 151:587–597
Malo AF, Roldan ERS, Garde JJ, Soler AJ, Vicente J, Górtazar C, Gomendio M (2009) What does testosterone do for red deer males? Proc R Soc Lond B 276:971–980
Martín J, Carranza J, López P, Alarcos S, Pérez-González J (2014) A new sexual signal in rutting male red deer: age related chemical scent constituents in the belly black spot. Mamm Biol 79:362–368
Mateos C (2005) The subordination stress paradigm and the relation between testosterone and corticosterone in male ring-necked pheasants. Anim Behav 69:249–255
Mateos C, Carranza J (1997) Signals in intrasexual competition between ring-necked pheasant males. Anim Behav 53:471–485
Mayerhofer A, Steger RW, Gow G, Bartke A (1992) Catecholamines stimulate testicular testosterone release of the immature golden hamster via interaction with alpha- and beta-adrenergic receptors. Acta Endocrinol Buch 127:526–530
McGraw KJ (2006) Dietary mineral content influences the expression of melanin-based ornamental colouration. Behav Ecol 18:137–142
McGraw KJ, Ardia DR (2003) Carotenoids, immunocompetence, and the information content of sexual colors: an experimental test. Am Nat 162:704–712
Mills SC, Grapputo A, Jokinen I, Koskela E, Mappes T, Oksanen TA, Poikonen T (2009) Testosterone-mediated effects on fitness-related phenotypic traits and fitness. Am Nat 173:475–487
Mougent F, Reader BF, Piertney SB (2005) Separating behavioural and physiological mechanisms in testosterone-mediated trade-offs. Am Nat 166:158–168
Pérez-González J, Carranza J (2009) Female-biased dispersal under conditions of low male mating competition in a polygynous mammal. Mol Ecol 18:4617–4630
Pérez-González J, Carranza J (2011) Female aggregation interacts with population structure to influence the degree of polygyny in red deer. Anim Behav 82:957–970
Pérez-González J, Frantz AC, Torres-Porras J, Castillo L, Carranza J (2012) Population structure, habitat features and genetic structure of managed red deer populations. Eur J Wildl Res 58:933–943
Peters M, Simmons LW, Rhodes G (2008) Testosterone is associated with mating success but not attractiveness or masculinity in human males. Anim Behav 76:297–303
Piñeiro A, Barja I, Silván G, Illera JC (2012) Effects of tourist pressure and reproduction on physiological stress response in wildcats: management implications for species conservation. Wildl Res 39:532–539
Rajagopal T (2009) A study on the reproductive behaviour and pheromones of an endangered Indian Blackbuck (Antelope cervicapra L.) to enhance captive breeding and conservation. Ph.D. Thesis. Bharathidasan University, Tiruchirappalli, Tamil Nadu, India
Raouf SA, Parker PG, Ketterson ED, Nolan V, Ziegenfus C (1997) Testosterone affects reproductive success by influencing extra–pair fertilizations in male dark–eyed juncos (Aves: Junco hyemalis). Proc R Soc Lond B 264:1599–1603
Roulin A, Wink A, Salamin N (2008) Selection on a eumelanic ornament is stronger in the tropics than in temperate zones in the worldwide-distributed barn owl. J Evol Biol 22:345–354
Rubenstein DR, Shen SF (2009) Reproductive conflict and the costs of social status in cooperatively breeding vertebrates. Am Nat 173:650–662
Saino N, Incagli M, Martinelli R, Møller AP (2002) Immune response of male barn swallows in relation to parental effort, corticosterone plasma levels, and sexual ornamentation. Behav Ecol 13:169–174
Senar JC, Camerino M, Copete JL, Metcalfe NB (1993) Variation in black bib of the Eurasian siskin (Carduelis spinus) and its role as a reliable badge of dominance. Auk 110:924–927
Shuster SM, Wade MJ (2003) Mating systems and strategies. Princeton University Press, Princeton
Sinervo B, Zamudio KR (2001) The evolution of alternative reproductive strategies: fitness differential, heritability, and genetic correlation between the sexes. J Hered 92:198–205
Tibbetts EA, Dale J (2004) A socially enforced signal of quality in a paper wasp. Nature 432:218–222
Tibbetts EA, Mullen SP, Dale J (2017) Signal function drives phenotypic and genetic diversity: the effects of signaling individual identity, quality or behavioural strategy. Philos Trans R Soc B 372:20160347
Torres-Porras J, Carranza J, Pérez-González J, Mateos C, Alarcos S (2014) The tragedy of the commons: unsustainable population structure of Iberian red deer in hunting estates. Eur J Wildl Res 60:351–357
Vergara P, Martínez-Padilla J (2012) Social context decouples the relationship between a sexual ornament and testosterone levels in a male wild bird. Horm Behav 62:407–412
Wingfield J, Ball G, Dufty A, Hegner R, Ramenofsky M (1987) Testosterone and aggression in birds. Am Sci 75:602–608
Wingfield J, Hegner RE, Dufty AM, Ball GF (1990) The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat 136:829–846
Wingfield JC, Lynn S, Soma KK (2001) Avoiding the ‘costs’ of testosterone: ecological bases of hormone-behavior interactions. Brain Behav Evol 57:239–251
Wyman MJ, Agrawal AF, Rowe L (2010) Condition-dependence of the sexually dimorphic transcriptome in Drosophila melanogaster. Evolution 64:1836–1848
Acknowledgements
We thank Marco Apollonio and an anonymous reviewer for helpful comments. We thank the autonomous governments of Andalucía (Junta de Andalucía) and Extremadura (Junta de Extremadura) and the owners of the hunting estates that provided permissions and facilities for fieldwork. Jose Manuel Seoane and students from the University of Córdoba helped in fieldwork and samples collection. We are indebted to P. Capilla-Lasheras for his advice on the statistical approach.
Funding
Financial support came from projects CGL2013-48122-P and CGL2016-77052-P to JC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
No animal was harvested for the purpose of this study. The relevant permits were obtained for the samples and data collection after hunting actions by the government of Andalusia.
Additional information
Communicated by: Paula Roig Boixeda
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1615 kb).
Rights and permissions
About this article
Cite this article
de la Peña, E., Martín, J., Barja, I. et al. Testosterone and the dark ventral patch of male red deer: the role of the social environment. Sci Nat 107, 18 (2020). https://doi.org/10.1007/s00114-020-01674-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-020-01674-1