Abstract
Background
Endoscopic third ventriculostomy (ETV) is a preferred mode of treatment for paediatric obstructive hydrocephalus as a result of lesions in the vicinity of the aqueduct of sylvius and posterior third ventricle. It obviates the need of implanting a foreign body inside the brain and thus reduces hardware-related complications as are seen with ventriculo-peritoneal shunts (VP).
Method
Endoscopic third ventriculostomy was done in nine cases of paediatric hydrocephalus (multiple etiologies) and the patients were followed up for a period of 18 months.
Results
In our series of nine cases, there was one ETV failure in a child of 14 months who required a conversion to VP shunt. Contusions in the fornix were seen in another patient who was of 3 months of age with post fossa tumour, but fortunately the patient did not have any gross memory deficits and was doing well after 6 months of follow-up. Intraventricular bleeding was seen in two cases which could be controlled with generous irrigation. External ventricular drain was not required in any patient. Rest of the eight patients improved slowly over the period of 6 months.
Conclusion
ETV is an attractive mode of treating obstructive hydrocephalus as it avoids the complications which can arise from implanting a foreign body. It requires a learning curve to master the technique. The literature of ETV in the paediatric population is reviewed along with our experience of nine cases of paediatric hydrocephalus with ETV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrocephalus is one of the most common clinical problems encountered in paediatric neurosurgery and management of this entity still remains a challenge for the neurosurgeons around the globe. Shunts, especially ventriculo-peritoneal shunts (VP) have remained the mainstay for the treatment of both communicating and non-communicating hydrocephalus. But shunt procedures are associated with the lifelong risks of shunt infection, shunt malfunction, shunt overdrainage/underdrainage and perforation in the hollow viscus. Approximately 40% of VP shunts in paediatric patients fail within first year of surgery [1]. Endoscopic third ventriculostomy (ETV) presents an alternative option to the shunt procedure. Aqueductal stenosis, posterior fossa tumors, myelomeningocele (MMC) and other midline tumors like tectal gliomas are the most common causes of obstructive hydrocephalus seen in the paediatric age group [2,3,4]. These constitute the most common indications for ETV. The most common causes of communicating pediatric hydrocephalus are intraventricular haemorrhage from premature birth, congenital communicating hydrocephalus, and tubercular meningitis, etc. Here the merits, demerits, indications, contraindications, and technique of ETV as an alternative procedure for CSF diversion in the paediatric population greater than one year with obstructive hydrocephalus have been outlined (Table 1).
What is endoscopic third ventriculostomy?
It is the procedure in which a fenestration is made in the floor of the third ventricle under endoscopic vision. The procedure aims at connecting the third ventricle to the prepontine subarachnoid cistern. The usual site of fenestration is tuber cinereum (a thin transparent area in the floor of the third ventricle posterior to the infundibulum) but rarely other sites like the anteriorly placed lamina terminalis can also be fenestrated. (Fig. 1A)
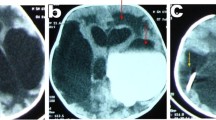
A A schematic depiction of the floor of third ventricle with the square showing the Chiasm, blue star showing the pink or the purple spot (infundibular recess), triangle shows the dorsum sellae, circle in the midline is the target area for the fenestration in the tuber cinereum, red verticle arrow is the basilar artery and its terminal branches. White arrows on both sides show bilateral mammillary bodies. Black hollow stars show the 3rd nerves on both sides. B The mid-sagittal T2 weighted image magnetic resonance imaging (T2W MRI) of a patient with obstructive hydrocephalus (HC) as a result of post fossa pilocytic astrocytoma, demonstrating customization of the position of frontal burr hole. A line is drawn connecting the tuber cinereum and the foramen of Monro and is extended towards the skull; the point where it meets the skull is the site for frontal burr hole
History
Ventriculoscopy was introduced in the early 1900s. Dandy was the first to use a primitive endoscope to perform choroid plexectomy in communicating hydrocephalus [5]. He later introduced the sub-frontal approach for an open third ventriculostomy but this procedure was associated with high mortality. In 1910 L’Espinasse, an urologist used the cystoscope to cauterize the choroid plexus, this was the first attempt at endoscopic management of hydrocephalus [6]. The credit for the first ETV goes to Mixter, another urologist, in 1923, who used a urethroscope to perform the third ventriculostomy in a child with obstructive hydrocephalus [7]. Putnam made the necessary modifications in a urethroscope for the cauterization of the choroid plexus [8]. In 1947, McNickle introduced a percutaneous method of performing the third ventriculostomy that decreased the complication rate and improved the success rate [9]. The start of this century saw many technological advances and this era has seen the advent of better quality endoscopes and immense advancements in diagnostic modalities. Due to these advancements, the neuro-endoscopic procedures for obstructive hydrocephalus have gained popularity. With the advent of thinner endoscopes and frameless guidance devices, endoscopic approaches to lesions with even mild or moderately dilated ventricles are becoming feasible.
Indications for ETV
Endoscopic third ventriculostomy is increasingly used in the treatment of paediatric hydrocephalus. Though the results in cases of obstructive hydrocephalus have been far better as compared to communicating hydrocephalus [10,11,12]. Patients with prepontine interval (PPI) > 1 mm and noncommunicating hydrocephalus are considered good candidates for ETV [13].The PPI is measured between the dorsum sellae and the basilar artery on magnetic resonance imaging (MRI). Hydrocephalus, secondary to congenital aqueductal stenosis, posterior third ventricle tumor, cerebellopontine angle tumor, other posterior fossa tumor, Dandy–Waker malformation, syringomyelia with or without Chiari malformation and myelomeningocele (associated chiari 2 malformation) is of obstructive variety and hence can be managed well with ETV.
Prerequisite for a successful ETV
-
Age more than 1 year
-
Triventricular dilatation
-
Dilated foramen Monro
-
Thin transparent floor of the third ventricle
-
Adequate space for making a hole in tuber cinereum (PPI > 1 mm)
Relative contraindications
-
Basilar artery close to the Clivus (PPI < 1 mm)
-
Large massa intermedia as seen in case of Chiari type II malformations
-
Tumors distorting the anatomy in this region
-
Small foramen Magnum
-
Thick floor of the third ventricle
Preoperative imaging
MRI (Fig. 1B) is considered the investigation of choice for providing information regarding the pre-operative evaluation of ETV. In patients, especially the T2 weighed images give valuable information regarding the thickness, bowing and the proximity of the third ventricular floor to the clivus and basilar artery, as well as information regarding thickness of the floor of the third ventricle and the Liliquist membrane, the relative size of the third ventricle and foramen of Monro, presence of any ventricular or external compressive mass, presence of any debris or blood products in the ventricle and finally the thin cut sections also help in visualising the presence of CSF flow through the aqueduct. This last feature can be better visualised using Sagittal Cine Phase contrast sequence MRI, though some multicentric studies have refuted its overall outcome on the procedure [14]. In cases where the coronal suture is not well seen or demarcated on MRI, a 3D reconstructed computed tomography (CT) scan can be obtained to mark the position of the frontal burr hole. The technical feasibility for performing the third ventriculostomy requires an adequate sized third ventricle and foramen of Monro so that the endoscope can be navigated with ease without damaging the adjacent fornix, hypothalamus, and thalamus. The ideal candidates for this procedure, as discussed before, are patients with congenital/acquired aqueductal stenosis, aqueductal obstruction due to posterior fossa tumours, posterior third ventricular tumors and pineal region tumors [15, 16]. The role of ETV in children with myelomeningocele presenting with hydrocephalus has been found to be controversial, as only a few studies have found it useful [17, 18]. Patients with communicating hydrocephalus theoretically would not benefit from the procedure although evidence in this regard has been lacking.
Relevant anatomy and operative technique
In the operating room (Fig. 2A), the patient is positioned supine under general anesthesia with head-on horseshoe head frame or a donut, with neck kept slightly flexed or neutral (Fig. 2A). The endoscopy tower (Fig. 3) is kept at the patient’s feet facing towards the patient’s head and directly facing the surgeon for unobstructed view during surgery. The source of irrigation is secured as its importance in neuroendoscopy cannot be undermined. The fluid used for irrigation is Ringer lactate and not normal saline that can cause bradycardia and hypertension [19]. It clears the operative field, maintains the ventricular volume and the working space for the neurosurgeon, and lastly it stops the low-pressure venous bleed encountered during the procedure. It also maintains the floor of the third ventricle bowed downwards (Fig. 1B), without irrigation the floor starts moving and bulging upwards like a curtain; thus, making the fenestration difficult (Fig 4C). The light source is adjusted at around 40–50% intensity for proper visualisation and to avoid glare. Generally, the width of the third ventricle and foramen of Monro should be approximately 7 mm or greater [20]. In most cases, aprecoronal burr hole (Fig 2B), 14mm in diameter is placed just anterior to the coronal suture and 2−3 cm away from the midline in the mid-pupillary line, and a curvilinear incision is given to raise a scalp flap (Fig. 2B). The placement of the burr hole is important, as a direct view to the foramen of Monro can be obtained from a properly placed burr hole thus, enabling the usage of only a rigid endoscope. Usually, right-side burr hole is made but a left-sided burr hole may be made if the left lateral ventricle and foramen of Monro are dilated more as compared to the right side. The exact site of the burr hole can be determined by a line, extending from the tuber cinereum and foramen of Monro onto the skull. After the burr hole is made, the dura is coagulated and incised in a curvilinear manner. A VP needle is used for ventricular puncture and to know the direction in which the trocar and cannula should advance. The trocar with the cannula is subsequently introduced into the lateral ventricle. The trocar is removed and a 0° endoscope is introduced into the ventricles through the cannula. Orientation to the ventricular structures is achieved initially by adjusting the camera to make necessary alterations so that the operative view matches the position of the patient. The initial and most important landmark is the foramen of Monro in the lateral ventricles. In cases with developmental anomalies, the location of the foramen of Monro (Fig. 4A) can be identified by locating the choroid plexus and the formation of the internal cerebral veins. The choroid plexus is located at the floor of the lateral ventricles traversing from the lateral to the medial side and enters the foramen of Monro. The thalamostriate and the septal veins are visualised which traverse from the lateral and medial sides, respectively, and join at the foramen of Monro to form the internal cerebral vein. The medial and anterior circumference of the foramen of Monro is formed by the fornices and traction should be avoided in this region as it may lead to short-term memory disturbances in the post-operative period. The posterior and lateral circumferences are formed by thalamus and choroid plexus, respectively. In cases, where the septum is absent, the fornices can be seen as two distinct bundles.
The operating room set up. No. 1 depicts the anaesthesia station, no. 2 depicts the operating surgeon standing towards the end of the patient, with no. 3 endoscopic monitor directly in front, no. 4 depicts the first assistant, no. 5 the instrument trolley, no. 6 the scrub nurse, no. 7 the overhead light source and no. 8 the view box
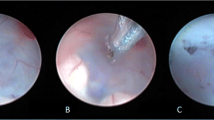
A The right Foramen of Monro (black star) with anterior septal vein on the medial side and the thalamo-striate vein on the lateral side. Choroid plexus can be followed and it will lead to the foramen of Monro. B Light blue star in the purple spot (infundibulum), hollow arrow is the dorsum sellae. Red star shows the thinnest and transparent tuber cinereum, favourable for fenestration. C The upward bulging tuber cinereum instead of a flat and firm floor seen in 4B. This occurs when there is inadequate irrigation used during the procedure. It makes it difficult for the fenestration to be performed. D Yellow dot demonstrates the floor of third ventricle. Black dot is the opening in the second membrane, the membrane of Liliquist, vertical red arrow is the basilar artery after making holes in second membrane. E In long standing hydrocephalus, the whole of the third ventricular floor becomes very thin and transparent, even the basilar artery is visible clearly though it. However, to perform a successful ETV both the floor and the second membrane should be fenestrated. Blue stars are B/l 3rd cranial nerves, blue triangle is dorsum sellae and red vertical arrow is the basilar artery
The ventriculoscope is navigated into the third ventricle. The paired mammillary bodies are evident midway along the floor of the third ventricle and the thinned out floor (tuber cinereum) of the ventricle can be seen just anterior to it. The basilar artery and its branches pulsations are often seen through the thinned out floor just anterior to the mammillary bodies in the midline (Fig 4B). The infundibular recess can be seen anteriorly midline in the floor of the third ventricle as a pinkish/purple spot. Just slightly posterior to this recess is a white rectangular band of dorsum sellae. The ventriculoscope may be taken out at this stage and a fibreoptic scope or a 30°/45° angled scope may be introduced to inspect the third ventricular anatomy and even the inlet of the stenosed aqueduct can be visualised. The structures in the floor of the third ventricle from anterior to posterior are; chiasm, infundibular stalk (pink spot), dorsum sellae, tuberculum, basilar artery, and branches, paired mammillary bodies. The 0° scope is now reintroduced for the rest of the procedure. An endoscope holder should be used at this step to provide steadiness and to make the hands of the surgeon free for manoeuvring the embolectomy catheter. The ideal spot of fenestration is a point midway between the basilar complex and dorsum sellae in the midline. Sometimes, the space is very narrow (due to an ectatic basilar artery), and also in patients with the thick and opaque floor (e.g. patients with previous ependymitis, intraventricular hemorrhage etc.) the recess, clivus and basilar pulsations are not visible so the procedure may be abandoned and VP shunt should be done. Blunt fenestration is made with a 3F Fogarty catheter (embolectomy catheter) with the stylet in situ and dilated to a diameter of 4–6 mm. This is the most critical step of the surgery and a properly performed fenestration is essential in avoiding injury to the basilar artery, which is the most serious complication of the procedure, so as far as possible sharp dissection using monopolar or bipolar coagulation should be avoided. Presence of the stylet inside the catheter renders adequate firmness to the tip to fenestrate the floor in a single attempt; thus, preventing multiple attempts which can injure the hypothalamic nuclei. Though the ideal fenestration is considered to be in the midline, it is not uncommon to see that the fenestration wavers slightly to the side opposite the burr hole (i.e. to the left side in the right burr hole approach) due to the trajectory. Visualisation and identifying the basilar bifurcation and its branches is highly essential and is the key to prevent an intra-operative catastrophe of injuring the vessels. Several techniques have been proposed by various authors for adequate dilatation of the fenestration but the most common technique is dilatation with a 3F Fogarty catheter. Alternatively, the passage of the endoscope itself has been used to achieve the required dilation. There is often another thin membrane below the floor which is described as the “second membrane” or the “Liliequist’s membrane” (Fig 4D). Perforation of the membrane is essential for a successful and functioning third ventriculostomy. The scope is then gently advanced to visualise the basilar artery and its branches. Continuous irrigation of the ventricular cavity is carried out with Ringer’s lactate solution at body temperature to clear the minor bleeding, which might occur during the procedure. The floor of the ventricle should pulsate with respiration and heartbeat and the flap movement should be visualised, this is a predictor of a successful ETV. The track through the brain should be inspected for any bleeding, a small gelatin sponge is inserted in the track, and the wound closed in layers.
Complications
Transient arrhythmias can occur during fenestration, that usually settle down by itself. In case the perforation is performed away from the midline hypothalamic damage leading to endocrinopathy may be seen in 2–9% of the patients resulting in diabetes insipidus (DI), amenorrhoea, precocious puberty, and pathological weight gain [21]. Transient paresis of the III and VI cranial nerves has also been observed in 1–2% of cases [22], as they travel laterally along the third ventricular floor. Bleeding from the edges of the perforation is usually self-limiting and repeated irrigation is sufficient. However, damage to the basilar artery seen in < 0.2% of cases may occur and may be fatal during the procedure or lead to formation of a pseudoaneurysm [23]. Hence, every attempt should be made to identify the basilar artery through the thinned out floor and avoiding the site of perforation too close to it. Haemorrhages due to vascular injury other than basilar artery maybe seen in 1–8.5% of cases. Short-term memory disturbances can be seen due to traction injury to the fornices. Subdural effusions and subdural haematoma can occur due to the sudden release of cerebrospinal fluid (CSF). Mortality has been reported in 1%, permanent morbidity in 1.6%, and a transient morbidity in 7.8%, CSF leak was seen in 1–6% cases, meningitis in 1–5% and seizures were reported in around 1% cases. The overall complication rate is around 5.8–16% [23, 24].
Assessment of effectiveness
Post-operative MRI is usually done after 6–8 weeks as the ventricular size reduction seen after ETV is slower than after a shunt procedure. On MRI there is improvement in the pre-operative downwards bulging of the third ventricular floor as well as resolution of the periventricular leucencies is seen, the symptoms resolve and the head size enlargement arrests (Fig. 5). The CSF cisterns become prominent suggesting a patent third ventriculostomy. T2-weighted fast spin-echo images or 2D phase contrast images can be used to assess the flow through the third ventricular floor. The overall success rate of 76% has been reported [3, 25, 26]. Success rates of 70−81% in patients older than 2 years and between 45% and 50% in children below 1 year of age have been reported [27,28,29,30,31].In a study by Baldauf et al., the results showed an overall success rate of 43% in children less than 2 years and a success rate of 37.5% in children younger than 1 year of age [32]. Drake, in a large (n = 368) multicentre Canadian study, found that younger age is a significant predictor of increased failure after ETV, but he found no correlation with hydrocephalus etiology and surgeon/center volume [27, 28]. Warf et al. in their study found that both older age and certain etiologies like obstructive hydrocephalus were associated with a higher chance of ETV success [30, 31]. The success rate also varies with the underlying pathology. In the case of myelomeningocele the success rate has varied from 27% in patients who had ETV as their initial procedure to 77% in patients where a previous shunt has been inserted, and the ETV was performed as a subsequent procedure [33]. An overall success rate of 72% has been reported, though this rate improves if the patient is above 6 months of age [7]. In the case of tumours success rate of 95% has been reported in posterior fossa tumours, though 16% of patients developed post-operative hydrocephalus requiring a VP shunt. In pineal region tumours, a success rate as high as 91% has been demonstrated. Tectal plate tumours have similarly been managed with ETV and biopsy and have shown a success rate of around 88% [28,29,30,31,32,33,34].
A, B Preoperative MRI and post-operative CT scan, respectively, of a patient of obstructive hydrocephalus as a result of pilocytic astrocytoma, an ETV was performed as an emergency procedure and definitive surgery was done after 7 days. The post-operative CT done after 15 days of ETV and after 8 days definitive surgery shows substantial reduction n the ventricular size along with thin subdural effusion on the left fronto-parietal region which resolved completely after 45 days. The patient is doing well at 5-month follow-up. C, D pre- and post-operative MRI scans, respectively, of another patient of congenital hydrocephalus presented with shunt malfunction. Successful ETV as patients are doing well after 1-year follow-up; significant decrease in the ventricular size can be appreciated
ETV failure is defined as any subsequent surgery for definitive treatment of hydrocephalus after the initial ETV surgery (i.e., repeat ETV or placement of a VP shunt). ETV failure is usually indicated by the development of delayed CSF leak at the operative site with the formation of wound pseudomeningocoele. The patient may present with the appearance of symptoms like irritability, increase in head circumference, headache, new-onset seizures, nausea, vomiting, lethargy, etc. the symptoms may vary according to the age of the patient. MRI may demonstrate an increase in ventricular size when compared to previous scans. Treatment depends on whether there is CSF flow through the ostomy or not. If on MRI CSF flow is observed, lumbar puncture can be attempted 2–3 times to initiate flow through the ostomy; if this fails then shunt procedure should be performed. If on MRI no flow is seen, ETV can be repeated. If on intraoperative direct visualisation ostomy is found open with sufficient width, a shunt procedure is warranted and if the ostomy is found occluded by debris or blood a new ostomy is performed.
Pearls for safe and uncomplicated ETV
-
A customized frontal burr hole
-
Absolute haemostasis should be achieved before inserting the cannula, as seepage of even a small amount of blood can obscure the field of vision.
-
A VP needle should be used to puncture the ventricle, so as to avoid the cannula or trocar going into and injuring the basal ganglia.
-
The ventriculoscope should be withdrawn into the sheath while negotiating the foramen of Monro so as to avoid injury to the fornix in case of the relatively small foramen of Monro.
-
An endoscope holder may be used to fix the endoscope while it is advanced through the foramen Monro.
-
If the ventricular floor is too thick and basilar artery pulsations are not appreciated, the procedure should be abandoned and a VP shunt should be inserted. However if the operating surgeon is quite experienced then a safe attempt can be made by making the fenestration right over the arachnoid of the dorsum sellae.
-
Small bleeding from the floor stops by continuous and patient irrigation. If in doubt an EVD can be left for a day or two in the lateral ventricle.
Review of literature
Endoscopic third ventriculostomy in the pediatric population is a promising alternative to VP shunt as it lacks any implanted hardware in the brain thus reducing the incidence of hardware-related failure or malfunction, complications avoid lengthening procedure at a later stage even if it is functioning well. The success of ETV has found to be dependent on many factors like age of the patient, etiolgy. Due to the immaturity of the infant brain and its reduced capacity to absorb CSF the success rate of ETV has been found better in adults as compared to children. The ETV success score (Table 2) given by Kulkarni et al. in a multinational series [6]; has over 50% of the weighted score attributable to age alone. According to this model, age, cause of hydrocephalus, and previous history of shunt procedure were all important and independent predictors of ETV success, with age being the strongest predictor. Durnford et al. [35] and Naftel et al., [36] have also validated this algorithm for estimating ETV success, though these were single institution studies. Though this score has not been validated in the adult population. The highest success rate has been seen in patients with aqueductal stenosis, tectal gliomas and posterior fossa tumors, with success rates as high as 88% with tectal gliomas. Poor success rates have been seen in neonates suffering from post-haemorrhagic ventricular dilatation due to intraventricular haemorrhage from prematurity, with success rates ranging from 0 to 33%. Drake reported 1- and 5-year success rates of 65% and 52%, respectively [27, 28]. Kulkarni et al. reported a 6-month success rate of 66.3% in 618 patients analyzed from 3 countries [6]. Kadrian et al. reported an 89% overall long-term success rate in a patient population that included both children and adults. Success at 1 year for the < 1-year age group was 48.7%, while it was 75.1% for the 10- to 19-year age group [29]. The most common factors included in these studies were age, sex, etiology of hydrocephalus, previous surgery, center volume, and surgeon volume. The success rate of secondary ETV done after failed VP shunt is around 70%. The complication rate after secondary ETV (31%) is higher as compared to ETV done as primary the procedure (8%) [37].
In our series of nine cases, there was one ETV failure in a child (14 months old), who had obstructive hydrocephalus due to pilocytic astrocytoma of the posterior fossa. This patient had CSF leak from the frontal wound on the 5th post-operative day and a VP Shunt was performed before subjecting him for the definitive surgery.
Contusions in the fornix was encountered in another patient with post fossa tumour (3 months old), but fortunately the patient did not have any gross memory deficits and was doing fine after 6 months of follow-up. We have starting using holding arm for the stabilization of the scope during the procedure after this complication. It also frees the surgeon’s hands for negotiating the embolectomy catheter with ease, through the tuber cinereum.
Intraventricular bleeding was encountered in two cases which could be controlled with generous irrigation. No external ventricular drain was used in any of the cases.
Conclusions
ETV is an attractive mode of treating obstructive hydrocephalus as it avoids the complications which can arise from implanting a foreign body. It requires a learning curve to master the technique. Thorough history and a scrupulous radiological workup are required to know the feasibility of a safe ETV. Unlike a VP shunt, this procedure is not apt for any hydrocephalus irrespective of its cause. There are special indications that should be adhered to. The equipment is another limiting factor in a cost-constrained environment in developing countries. An initial attempt of ETV should always be given to the candidates who qualify for it as the option of performing a VP shunt would always be there with the surgeon at a later date.
References
Choudhary A, Sobti S, Zambre S, Bhaskar S (2020) Endoscopic third ventriculostomy in failed ventriculoperitoneal shunt in pediatric population. Asian J Neurosurg 15:937–940. https://doi.org/10.4103/ajns.AJNS_117_20
Elbaba S, Steinmetz M, Ross J, Moon D, Luciano M (2001) Endoscopic third ventriculostomy for objective hydrocephalus in the pediatric population: evaluation of outcome. Eur J Pediatr Surg 11:S52–S54
Yadav YR, Jaiswal S, Adam N (2006) Endoscopic third ventriculostomy in infants. Neurol (India) 54:161–163
Ogiwara H, Dipatri AJ Jr, Alden TD, Bowman RM, Tomita T (2010) Endoscopic third ventriculostomy for obstructive hydrocephalus in children younger than 6 months of age. Childs Nerve Syst 26:343–347
Dandy WE (1918) Extirpation of the choroid plexus of the lateral ventricles in communicating hydrocephalus. Ann Surg 68:569–579. https://doi.org/10.1097/00000658-191812000-00001
Oluigbo C, Keating R (2017) Endoscopic management of hydrocephalus and choroid plexus cauterization. In: Ammar A (ed) Hydrocephalus, Springer, Cham, pp 201–208. https://doi.org/10.1007/978-3-319-61304-8_15
Mixter WJ (1923) Ventriculoscopy and puncture of the floor of the third ventricle. Boston Med Surg J 188:277–278
Putnam TJ (1934) Treatment of hydrocephalus by endoscopic coagulation of the choroid plexus. N Engl J Med 210:1373–1376
McNickle HF (1947) The surgical treatment of hydrocephalus. A simple method of performing third ventriculostomy. Br J Surg 34:302–307
Koch-Wiewrodt D, Wagner W (2006) Success and failure of endoscopic third ventriculostomy in young infants: are there different age distributions? Childs Nerv Syst 22:1537–1541
Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S (2010) Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. Clinical article. J Neurosurg Pediatr 6:310–315
Kulkarni AV, Drake JM, Mallucci CL, Sgouros S, Roth J, Constantini S et al (2009) Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Pediatr 155:254-259.e1
Souweidane MM, Morgenstern PF, Kang S, Tsiouris AJ, Roth J (2010) Endoscopic third ventriculostomy in patients with a diminished prepontine interval. J Neurosurg Pediatr 5:250–254. https://doi.org/10.3171/2009.10.PEDS09187
Fukuhara T, Vorster SJ, Luciano MG (2000) Risk factors for failure of endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurgery 46:1100–1109
Cinalli G, Sainte-Rose C, Chumas P et al (1999) Failure of third ventriculostomy in the treatment of aqueductal stenosis in children. Neurosurg Focus 6:e3
Grant JA, McLone DG (1997) Third ventriculostomy: a review. Surg Neurol 47:210–212
Venkataramana NK (2011) Hydrocephalus Indian scenario—a review. J Pediatr Neurosci 6:S11–S22. https://doi.org/10.4103/1817-1745.85704
Mohanty A, Das BS, Sastry Kolluri VR et al (1996) Neuroendoscopic fenestration of occluded foramen of Monro causing unilateral hydrocephalus. Pediatr Neurosurg 25:248–251
Haldar R, Singh Bajwa SJ (2019) Potential neuroendoscopic complications: an anesthesiologist’s perspective. Asian J Neurosurg 14:621–625. https://doi.org/10.4103/ajns.AJNS_37_17
Yadav YR, Parihar V, Pande S, Namdev H, Agarwal M (2012) Endoscopic third ventriculostomy. J Neurosci Rural Pract 3:163–173. https://doi.org/10.4103/0976-3147.98222
Woodworth G, McGirt MJ, Thomas G et al (2007) Prior CSF shunting increases the risk of endoscopic third ventriculostomy failure in the treatment of obstructive hydrocephalus in adults. Neurol Res 29:27–31
Teo C, Rahman S, Boop FA et al (1996) Complications of endoscopic neurosurgery. Child Nerv Syst 12:248–253
Bouras T, Sgouros S (2013) Complications of endoscopic third ventriculostomy. World Neurosurg 79:S22.e9–S22.e12
Amini A, Schmidt RH (2005) Endoscopic third ventriculostomy in a series of 36 adult patients. Neurosurg Focus 19:E9
Gorayeb RP, Cavalheiro S, Zymberg ST (2004) Endoscopic third ventriculostomy in children younger than 1 year of age. J Neurosurg 100:427–429
Etus V, Ceylan S (2005) Success of endoscopic third ventriculostomy in children less than 2 years of age. Neurosurg Rev 28:284–288
Drake J, Chumas P, Kestle J, Pierre-Kahn A, Vinchon M, Brown J et al (2006) Late rapid deterioration after endoscopic third ventriculostomy: additional cases and review of the literature. J Neurosurg 105:118–126
Drake JM, Canadian Pediatric Neurosurgery Study Group (2007) Endoscopic third ventriculostomy in pediatric patients: the Canadian experience. Neurosurgery 60:881–886
Kadrian D, van Gelder J, Florida D, Jones R, Vonau M, Teo C et al (2008) Long-term reliability of endoscopic third ventriculostomy. Neurosurgery 62:614–621
Warf BC (2005) Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J Neurosurg 103:475–481
Warf BC (2005) Hydrocephalus in Uganda: the predominance of infectious origin and primary management with endoscopic third ventriculostomy. J Neurosurg 102:1–15
Baldauf J, Oertel J, Gaab MR, Schroeder HW (2007) Endoscopic third ventriculostomy in children younger than 2 years of age. Childs Nerv Syst 23:623–626. https://doi.org/10.1007/s00381-007-0335-4
Teo C, Jones R (1996) Management of hydrocephalus by endoscopic third ventriculostomy in patients with myelomeningocele. Pediatr Neurosurg 25:57–63
Jallo GI, Kothbauer KF, Abbott IR (2005) Endoscopic third ventriculostomy. Neurosurg Focus 19:E11
Durnford AJ, Kirkham FJ, Mathad N, Sparrow OC (2011) Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus: validation of a success score that predicts long-term outcome. Clinical article. J Neurosurg Pediatr 8:489–493
Naftel RP, Reed GT, Kulkarni AV, Wellons JC (2011) Evaluating the Children’s Hospital of Alabama endoscopic third ventriculostomy experience using the Endoscopic Third Ventriculostomy Success Score: an external validation study. Clinical article. J Neurosurg Pediatr 8:494–501
Hader WJ, Walker RL, Myles ST et al (2008) Complications of endoscopic third ventriculostomy in previously shunted patients. Neurosurgery 63:ONS168–ONS174
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kataria, R., Mehrotra, M., Purohit, D.K. et al. Endoscopic third ventriculostomy in the paediatric population. J Ped Endosc Surg 3, 183–192 (2021). https://doi.org/10.1007/s42804-021-00117-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42804-021-00117-6