Abstract
Polyhydroxy fullerenes nanoparticles (PHF) are regarded as free radical sponges. Can they mitigate oxidative stress and induce tolerance in plants exposed to salinity? The influence of PHF seed pre-treatment on growth and biochemical attributes of NaCl-stressed wheat is studied. Wheat seeds (cv. Ujala) were pre-treated with control, hydro-priming, 10, 40, 80, and 120 nM PHF doses for 10 h and grown in sand-filled pots under control (0 mM NaCl) and salinity (150 mM NaCl) provided through nutrient solution. Salinity markedly decreased root and shoot growth attributes consistent with the reduction in the chlorophyll contents, whereas it increased the antioxidant activities of superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and ascorbate peroxidase (APX) enzymes. Plants exposed to salinity exhibited increase in malondialdehyde (MDA) and hydrogen peroxide (H2O2) contents which indicated oxidative stress. Further, salinity triggered rise in Na+ uptake while decreased in K+ and Ca2+ contents both in the root and shoot. By contrast, wheat seedlings grown from PHF-treated seeds exhibited recovery in root and shoot growth under salinity. This recovery was linked with lower levels of MDA and H2O2 contents and higher antioxidant activities of CAT, POD, and APX enzymes under salinity stress. The PHF-treated plants had higher chlorophyll, free amino acids, ascorbic acid, and soluble sugars. Moreover, PHF seed pre-treatment resulted in higher K+ and P contents in the root while higher P contents in the shoot. Above all, PHF application mitigated adverse effects of salinity and promoted early seedling growth and establishment in wheat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Osmotic stress followed by specific ion toxicity is the major limiting factor for growth of plants cultivated on saline soils (Munns et al. 2016). Presence of excessive dissolved salts in saline soils reduces soil matric potential that alters plant root water uptake capacity (Sheldon et al. 2017; Tedeschi et al. 2017). Plants therefore experience water stress and ion-disequilibrium under hyper-osmolarity growth conditions (Alemán et al. 2009) which negatively affects turgor pressure and cellular expansion (Taiz and Zeiger 2010). Therefore, salinity-mediated physiological drought inhibits stomatal conductance directly affecting leaf photosynthetic activity (Tavakkoli et al. 2010).
One of the major factors affecting plant growth on saline soils is specific ion toxicity or Na+ toxicity (Zhu 2002). Higher soil Na+ trigger K+ deficiency primarily due to competition through high-affinity K+ transporters in plant root cells (Alemán et al. 2009; Hauser and Horie 2010; Pardo and Rubio 2011). The increased Na+ uptake and its higher concentration inside leaf mesophyll cells initiate specific ion toxicity (Taiz and Zeiger 2010; Hasegawa 2013) disturbing cellular ion homeostasis (Hasegawa et al. 2000; Munns and Tester 2008; Teakle and Tyerman 2010). In addition, redox reactions of photosynthesis and respiration are often impaired due to sodium-mediated enhanced production of reactive oxygen species (ROS) causing oxidative stress (Mittler 2002; Tavakkoli et al. 2010). Considering ion homeostasis, it has been suggested that K+ retention and higher Ca2+ inside cells is an important strategy in order to balance ion-disequilibrium to avoid Na+ build up (Marshner 1995; Shabala and Cuin 2007; Shahbala et al. 2016). Other than this, the ability of certain plants to exclude Na+ is also an important stress tolerance strategy (Colmer et al. 2006; Munns and Tester 2008). Nonetheless, plant responses to counter salinity stress vary greatly depending upon growth stages and stress severity.
Wheat (Triticum aestivum L.) is one of the dominant cereal crops and regarded as a moderately salt-tolerant glycophyte. On the other hand, PHF are carbon nanoparticles which exhibit remarkable electron-scavenging properties (Andrievsky et al. 2005; Sachkova et al. 2017). Recently involvement of PHF in drought tolerance of sugar beet (Borišev et al. 2016) and Brassica napus (Xiong et al. 2018) explained on the basis of improvements in oxidative stress tolerance and abcisic acid. Since high Na+ concentrations ultimately disturb cellular redox status, it will be interesting to investigate how the leaf tissue ion homeostasis responds to external supply of synthetic antioxidant. Accordingly, it was hypothesized that PHF application may improve tissue ion homeostasis through modulation of wheat antioxidant capacity and oxidative stress.
2 Materials and Methods
2.1 PHF Seed Invigoration and Seedling Growth
The PHF nanoparticles [C60(OH)20] were purchased from BuckyUSA (Houston Texas, USA). For seed priming, wheat seeds (cv. Ujala-2016) were soaked in different PHF concentrations viz. control (un-primed), hydro-priming, 10, 40, 80, and 120 nM PHF for 10 h with continuous aeration. After 10 h, the seeds were removed, the surface sterilized with a solution of sodium hypochlorite (0.1%) for 1 min, and then washed five times with de-ionized water. Finally, seeds were dried with blotting paper and sown in pots containing sand under control (0 mM NaCl) and salinity (150 mM NaCl) stress provided through Hoagland’s nutrient solution. The experiment was performed with four replications per treatment under control environmental conditions inside a plant growth chamber (Sanyo, Model MLR-351H) with following growth conditions (PAR, 370 μmol m−2 s−1; photoperiod, 10 h; day/night temperature, 18/23 °C).
2.2 Study of Growth and Biochemical Attributes
After 30 days of seed germination, seedlings were investigated for changes in various growth parameters and biochemical parameters. Photosynthetic pigments were quantified after extraction of fresh leaf tissue (100 mg) with acetone (80%) as described earlier (Arnon 1949; Kirk and Allen 1965). Concentration of oxidative stress indicators was determined after homogenization of fresh plant material in trichloroacetic acid (TCA) (10%) followed by centrifugation at 7000g. The concentration of malondialdehyde (MDA) was determined using thiobarbituric acid (Mukherjee and Choudhuri 1983) while hydrogen peroxide (H2O2) contents were determined using potassium iodide as described (Velikova et al. 2000).
For enzymatic antioxidant analyses, the homogenization of fresh plant material (200 mg) was performed in ice-cold potassium phosphate buffer (100 mM; pH 7.8). The homogenate was centrifuged at 12000g and supernatant was referred as a crude protein extract. The determination of antioxidant enzyme activities of SOD, POD, CAT, and APX were performed from this crude protein extract as described (Beauchamp and Fridovich 1971; Aebi 1984; Chance and Maehly 1955; Nakano and Asada 1981). Total soluble proteins were also quantified as described by Bradford (1976). The antioxidant activities were then presented as U mg−1 protein basis.
Furthermore, plant ethanol extracts were prepared for the determination of non-enzymatic antioxidants and some key osmolytes. For this purpose, 50 mg of plant dry material was homogenized with 10 mL ethanol (80%) and filtered through Whatman No. 41 filter paper. The residue was re-extracted with ethanol and the two extracts were pooled together to a final volume of 20 mL. The determination of flavonoids (Pękal and Pyrzynska 2014), phenolics (Bray and Thorpe 1954), free amino acids (Hamilton and Van Slyke 1943) and total sugars (Dubois et al. 1956) was performed from the extracts.
2.3 Study of Changes in Inorganic Ions
For the determination of various ions, dried plant material of shoot and root was digested using H2SO4/H2O2 mixture (Wolf 1982). Analyses of Na+, K+, and Ca2+ ions were carried out by using a flame photometer (Jenway PFP-7, UK). Moreover, the P contents were determined by using Barton’s reagent as described (Barton 1948). The inorganic ions were finally calculated by using respective calibration curves and ions presented in mg g−1 DW basis. In addition, the analyses of root and shoot Mg2+, Mn2+, Zn2+, and Fe2+ contents was performed using atomic absorption spectrophotometer (Supplementary file).
2.4 Statistical Analyses
Data collected for all the attributes was statistically analyzed by using CoStat software (CoHort Software, Monterey, CA, USA) using completely randomized layout. Differences among means were assessed by Duncan’s multiple range (DMR) test at 95% confidence level.
3 Results
3.1 PHF-Mediated Changes in Growth Attributes of Control and Salt-Stressed Wheat
Non-toxic and growth-promoting influence of PHF seed pre-treatment on shoot growth attributes of wheat seedlings was recorded both under control and salinity (Table 1). Seedlings exposed to 150 mM NaCl exhibited substantial reduction in shoot length, fresh weight and shoot dry weight (P < 0.01). On the other hand, seedlings germinated from PHF pre-treated seeds exhibited recovery in shoot growth attributes compared with untreated control seedlings (P < 0.05; Table-1). Similar to shoot, the root growth of salinity exhibited prominent reduction in terms of root length, root fresh and dry weight respectively (P < 0.05). In contrast, the PHF treated wheat seedlings exhibited better root growth attributes under salinity compared with un-treated ones (P < 0.05; Table-1). The PHF seed pre-treatment also improved the root growth attributes of plant grown without NaCl in the growth medium (P < 0.05).
3.2 PHF-Mediated Changes in Biochemical Attributes of Control and Salt-Stressed Wheat
3.2.1 Photosynthetic Pigments
Prominent reduction (P < 0.01) in chlorophyll a, b and total chlorophyll was evident under salinity. Similarly, reduction in carotenoids was evident among wheat seedlings exposed to salinity stress (P < 0.05; Table 2). However, in contrast, the wheat plants grown from PHF pre-treated seeds had higher chlorophyll a, b and total chlorophyll contents. Maximum improvements (P < 0.05) in the chlorophyll contents were evident in response to 40 and 80 nM PHF seed pre-treatments. Likewise, PHF mediated increase in the carotenoids was also recorded under salinity (Table 2). Overall, the Chl a b−1 and Car Chl−1 relative ratios increased under salinity (P < 0.05).
3.2.2 Oxidative Stress Markers, Antioxidants, and Osmolytes
The 30-day-old wheat plants exhibited prominent (P < 0.01) increase in MDA and H2O2 contents under salinity. Also, prominent increase in the antioxidant activities of SOD and APX was recorded under salinity (P < 0.05). Interestingly, the PHF-treated wheat plants exhibited substantial increase (P < 0.01) in the activities of CAT, POD, and APX antioxidant enzymes. While, PHF-mediated reduction in the SOD activity was also recorded (P < 0.05). In addition, the PHF seed pre-treatment resulted in substantial reduction in the MDA and H2O2 contents among wheat plants under salinity (Table 3; P < 0.05). Apart from this enzymatic antioxidants, wheat plants exposed to 150 mM NaCl exhibited higher values of ascorbic acid, flavonoids, total phenolics, and soluble sugars while reduced free amino acids (Table 4; P < 0.05). The PHF seed pre-treatment resulted in significant improvement (P < 0.01) in free amino acids and soluble sugars. The PHF treatment caused reduction (P < 0.05) in the total phenolics. Moreover, the influence of PHF on ascorbic acid and flavonoids under salinity stress was differential (Table 4).
3.3 PHF-Mediated Changes in Inorganic Ions of Control and Salt-Stressed Wheat
3.3.1 Root Inorganic Ions
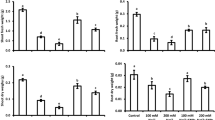
Wheat plants grown under salinity exhibited prominent (P < 0.01) increase in root Na+ concentration which ranged between 5.57 mg g−1 DW for control plants and 12.7 mg g−1 DW under salinity stress (Figure 1a). In contrast, salinity induced reduction (P < 0.05) in the root Ca2+ contents was recorded (Figure 1c), while root K+ and P contents exhibited insignificant differences. On the other hand, plants grown from PHF pre-treated seeds exhibited an increase (P < 0.01) in root K+ uptake under salinity (Fig. 1b). Furthermore, PHF-mediated improvements (P < 0.05) in the root P fraction were evident as well (Fig. 1d). Apart from this, the influence of PHF treatments on root Na+ and Ca2+ remained insignificant (P < 0.05). Reduction in the root Mn2+ contents was also recorded under salinity, while changes in Mg2+, Zn2+, and Fe were insignificant (Fig. S1A–D). Moreover, the influence of PHF on root Mg2+, Mn2+, Zn2+ and Fe contents was differential (Fig. S1A–D).
a–d Polyhydroxy fullerene (PHF) nanoparticles seed pre-treatment mediated changes in root Na+, K+, Ca2+, and P contents of control (0 mM NaCl)  and salinity (150 mM NaCl) stressed
and salinity (150 mM NaCl) stressed  wheat seedlings (n = 4; mean ± SE). Different lowercase letters in each attribute are significantly different at 95% confidence level
wheat seedlings (n = 4; mean ± SE). Different lowercase letters in each attribute are significantly different at 95% confidence level
3.3.2 Shoot Inorganic Ions
Similar to root, marked (P < 0.01) increase in the shoot Na+ contents was recorded under salinity (Fig. 2a). While seedlings grown from PHF-treated seeds exhibited considerable reduction in shoot Na+ (P < 0.05). Salinity also caused reduction (P < 0.05) in the shoot K+ and Ca2+ contents in comparison with control plants (Fig. 2b, c). The application of PHF nanoparticles at 40 nM concentrations improved (P < 0.05) shoot Ca2+ contents and also increased (P < 0.01) shoot P concentration at 40, 80, and 120 nM concentrations of wheat seedlings grown under salinity (Fig. 2d). Salinity induced changes in shoot P contents remained insignificant (P < 0.05). Apart from these, the influence of PHF on the shoot Mg2+, Mn2+, Zn2+, and Fe2+ contents remained insignificant (Fig. S2A–D).
a–d Polyhydroxy fullerene (PHF) nanoparticles seed pre-treatment mediated changes in shoot Na+, K+, Ca2+, and P contents of control (0 mM NaCl)  and salinity (150 mM NaCl) stressed
and salinity (150 mM NaCl) stressed  wheat seedlings (n = 4; mean ± SE). Different lowercase letters in each attribute are significantly different at 95% confidence level
wheat seedlings (n = 4; mean ± SE). Different lowercase letters in each attribute are significantly different at 95% confidence level
4 Discussion
Improvement in salinity tolerance of crops through various exogenous chemicals or biostimulants is an important area of research (Torbaghan et al. 2017; González-Pérez et al. 2018). Exposure of wheat seedlings to salinity reduced plant growth. In agreement, salinity inhibited plant growth through physio-biochemical disturbances (Hasegawa 2013; Geilfus 2018) and is also reported for wheat plants (Raza et al. 2007; Iqbal and Ashraf 2013; Shafiq et al. 2018). Wheat plants germinated from PHF-primed seeds exhibited prominent recovery in root and shoot growth under control and salinity. Consistent with these findings, PHF-application improved the growth attributes of Arabidopsis thaliana (Gao et al. 2011), Momordica charantia (Kole et al. 2013), and Hordeum vulgare (Panova et al. 2016). Moreover, a radio-labeled PHF compound improved wheat root growth under control growth conditions (Wang et al. 2016). In contrast, the evidence of PHF involvement in abiotic stress tolerance of plants is yet to be elucidated. So far, exogenous PHF-mediated drought tolerance in Beta vulgaris L. (Borišev et al. 2016) and Brassica napus L. (Xiong et al. 2018) is reported.
In this study, salinity caused reduction in Chl a, b and total Chl. Furthermore, a decrease in the carotenoid contents was also recorded for seedlings grown under salinity which caused an increase in the Car Chl−1 relative ratio under salinity stress. Salinity-mediated reduction in the photosynthetic pigments is reported earlier in wheat (Iqbal and Ashraf 2013) and is due to Na+ toxicity and oxidative stress (Shu et al. 2012; Raza et al. 2014; Shafiq et al. 2018). The wheat seedlings grown from PHF pre-treated seeds exhibited prominent recovery in the photosynthetic pigments under salinity stress. In this connection, PHF-treated control plants exhibited an increase in photosynthetic pigments (Wang et al. 2016). Our findings of PHF-mediated improvements in the photosynthetic pigments consistent with improvements in the growth attributes can be integrated with mitigation of oxidative stress.
Here, substantial increase in the MDA and H2O2 contents of wheat seedlings grown under salinity was recorded. Furthermore, an increase in the antioxidant activities of SOD, CAT, POD, and APX was also evident under salinity. While, PHF-treated plants exhibited marked reduction in oxidative stress under salinity, indicated by lesser contents of MDA and H2O2. The PHF-induced decrease in MDA and H2O2 contents in maize was attributed to better antioxidant capacity (Assemi et al. 2010). Here as well, PHF seed invigoration also improved the enzymatic antioxidant activities of CAT, POD, and APX. Consistent with these findings, the application of PHF as foliar spray increased the activities of CAT, APX, GPX, and GST antioxidant enzymes linked with H2O2 detoxification (Borišev et al. 2016). Further, PHF-mediated improvements in SOD and CAT activities among maize seedlings were also reported (Liu et al. 2016).
Interestingly, salinity stress increased the SOD activity among seedlings while the PHF application decreased SOD activity in comparison with untreated plants. The SOD serves as the first line of antioxidant defense system to dismutase O2− to H2O2 (Cavalcanti et al. 2007; Mittler 2006) which in turn is scavenged by H2O2 neutralizing enzymes (Apel and Hirt 2004). In this study, we recorded PHF-mediated increase in the activities of H2O2 neutralizing enzymes in the absence of any increase in the SOD activity. These findings can be explained on the basis of O2− radical scavenging ability of PHF molecule which has been regarded as radical sponges and O2− scavenging activity of PHF mimics SOD activity (Foley et al. 2002). Above all, PHF conferred oxidative stress tolerance in wheat plants under salinity through the upregulation of H2O2- neutralizing enzymes. The mitigation of oxidative stress in PHF-treated plants is consistent with the improvements in growth and recovery in the photosynthetic pigments.
Considering enzymatic antioxidants, PHF seed pre-treatment resulted in significant improvements in free amino acids, soluble sugars, and ascorbic acid contents under salinity. Salinity also caused increase in ascorbic acid and soluble sugars while reduced amino acids. Of limited reports, PHF-mediated accumulation of soluble sugars has been reported in Beta vulgaris under water-limited growth conditions (Borišev et al. 2016). The retention of compatible osmolytes like soluble sugars, proline, and other free amino acids is linked with better osmotic adjustments under salinity (Flowers and Colmer 2008; Shafiq et al. 2018). Other than osmolytes, another important component of osmoregulation is inorganic ions primarily Na+, K+, Ca2+, and P.
In the present study, marked increase in the root Na+ concentration while, a reduction in the K+ and Ca2+ contents was recorded. On the other hand, plants grown from PHF pre-treated seeds exhibited increased K+ uptake in the roots under salinity. Furthermore, PHF-mediated improvements in the root P contents were also recorded. In general, plants regulate cellular osmotic potential through effective adjustments of Na+/K+ ratio via increased K+ uptake and its retention (Cuin et al. 2009; Barragán et al. 2012). Both Ca2+ and K+ are actively involved in modulation of plant responses to salinity (Shabala 2017; Nedjimi 2017). The tissue-specific salinity tolerance in halophytes is also linked with K+ retention in mesophyll cells (Percey et al. 2016). Here PHF-mediated increase in the root K+ and P contents might have contributed to better cellular osmotic adjustments. For leaves, an increase in the shoot Na+ contents while reduction in K+ and Ca2+ contents was recorded. The increase in shoot Na+ fraction can be due to enhanced translocation of Na+ from root to shoot in order to achieve better osmotic adjustment through a process called xylem loading (Shabala et al. 2010; Zhang et al. 2017). Therefore, controlled Na+ transport effectively regulated Na+ accumulation in the metabolically active cells (Läuchli et al. 2008; Cuin et al. 2011). Other than Na+, PHF at 10 and 40 nM concentrations reduced Na+ uptake in the shoot while increased P at 40, 80, and 120 nM concentrations under salinity.
5 Conclusions
Salt stress reduced photosynthetic pigments and generated oxidative stress which negatively affected wheat growth parameters. In addition, salinity caused increased accumulation of sodium in root and shoot while it reduced potassium and calcium contents. By contrast, the plants grown from polyhydroxy fullerene nanoparticles–treated seeds exhibited salinity tolerance linked with increased antioxidant activities of catalase, peroxidase, and ascorbate peroxidase enzymes. Furthermore under salinity stress, increased root and shoot phosphorus was recorded for wheat plants in response to polyhydroxy fullerene. Above all, these biochemical modifications contributed to recovery in chlorophyll contents, osmolytes accumulation, and ultimately growth improvement under salt stress. Therefore, polyhydroxy fullerene nanoparticles seed pre-treatment promoted early seedling growth and establishment in wheat exposed to salt stress.
References
Aebi H (1984) Catalase in vitro (In: L. Pac). Academic Press, Orlando
Alemán F, Nieves-Cordones M, Martínez V, Rubio F (2009) Potassium/sodium steady-state homeostasis in Thellungiella halophila and Arabidopsis thaliana under long-term salinity conditions. Plant Sci 176:768–774
Andrievsky G, Klochkov V, Derevyanchenko L (2005) Is the C60 fullerene molecule toxic?! Fuller Nanotub Car N 13(4):363–376
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Arnon DI (1949) Copper enzymes in isolated chlorlasts: polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1–15
Assemi S, Tadjiki S, Donose BC, Nguyen AV, Miller JD (2010) Aggregation of fullerol C60(OH)24 nanoparticles as revealed using flow field-flow fractionation and atomic force microscopy. Langmuir 26(20):16063–16070
Barragán V, Leidi EO, Andrés Z, Rubio L, De Luca A, Fernández JA, Cubero B, Pardo JM (2012) Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 24:1127–1142
Barton CJ (1948) Photometric analysis of phosphate rock. Anal Chem 20(11):1068–1073
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Borišev M, Borišev I Župunski M, Arsenov D, Pajević S, Ćurčić Ž, Vasin J, Djordjevic A (2016) Drought impact is alleviated in sugar beets (Beta vulgaris L.) by foliar application of fullerenol nanoparticles. PLoS One 11(11):263 1-20
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Bray HG, Thorpe WV (1954) Analysis of phenolic compounds of interest in metabolism. Methods Biochem Anal 1:27–52
Cavalcanti FR, Lima JPMS, Ferreira-Silva SL, Viegas RA, Silveira JAG (2007) Roots and leaves display contrasting oxidative response during salt stress and recovery in cowpea. J Plant Physiol 164:591–600
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Colmer TD, Munns R, Flowers TJ (2006) Improving salt tolerance of wheat and barley: future prospects. Aust J Exp Agric 45(11):425–1443
Cuin TA, Bose J, Stefano G, Jha D, Tester M, Mancuso S, Shabala S (2011) Assessing the role of root plasma membrane and tonoplast Na+/H+ exchanger in salinity tolerance in wheat: in planta quantification methods. Plant Cell Environ 34:947–961
Cuin TA, Tian Y, Betts SA, Chalmandrier R, Shabala S (2009) Ionic relations and osmotic adjustment in durum and bread wheat under saline conditions. Funct Plant Biol 36:1110–1119
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–431
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytol 179:945–963
Foley S, Crowley C, Smaihi M, Bonfils C, Erlanger BF, Seta P, Larroque C (2002) Cellular localisation of a water-soluble fullerene derivative. Biochem Biophys Res Commun 294(1):116–119
Gao J, Wang Y, Folta KM, Krishna V, Bai W, Indeglia P, Georgieva A, Nakamura H, Koopman B, Moudgil B (2011) Polyhydroxy fullerenes (fullerols or fullerenols): beneficial effects on growth and lifespan in diverse biological models. PLoS One 6(5):1–8
Geilfus CM (2018) Chloride: from nutrient to toxicant. Plant Cell Physiol 59(5):877–886
González-Pérez L, Páez-Watson T, Álvarez-Suarez JM, Obando-Rojas MC, Bonifaz-Arcos E, Viteri G, Rivas-Romero F, Tejera E, Rogers HJ, Cabrera JC (2018) Application of exogenous xyloglucan oligosaccharides affects molecular responses to salt stress in Arabidopsis thaliana seedlings. J Soil Sci Plant Nutr 18(4):1187–1205
Hamilton PB, Van Slyke DD (1943) The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. J Biol Chem 150(1):231–250
Hasegawa PM (2013) Sodium (Na+) homeostasis and salt tolerance of plants. Environ Exp Bot 92:19–31
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Biol 51:463–499
Hauser F, Horie T (2010) A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ 33:552–565
Iqbal M, Ashraf M (2013) Gibberellic acid mediated induction of salt tolerance in wheat plants: growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environ Exp Bot 86:76–85
Kirk JT, Allen RL (1965) Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun 21(6):523–530
Kole C, Kole P, Randunu KM, Choudhary P, Podila R, Ke PC, Rao AM, Marcus RK (2013) Nanobiotechnology can boost crop production and quality: first evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia). BMC Biotechnol 13(1):37
Läuchli A, James RA, Huang CX, McCully M, Munns R (2008) Cell-specific localization of Na+ in roots of durum wheat and possible control points for salt exclusion. Plant Cell Environ 31:1565–1574
Liu F, Xiong F, Fan Y, Li J, Wang H, Xing G, Yan F, Tai F, He R (2016) Facile and scalable fabrication engineering of fullerenol nanoparticles by improved alkaline-oxidation approach and its antioxidant potential in maize. J Nanopart Res 483(18):338
Marshner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, New York
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19
Mukherjee SP, Choudhuri MA (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58(2):166–170
Munns R, James RA, Gilliham M, Flowers TJ, Colmer TD (2016) Tissue tolerance: an essential but elusive trait for salt-tolerant crops. Funct Plant Biol 43(498):1103–1113
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880
Nedjimi B (2017) Calcium application enhances plant salt tolerance: a review. In: Naeem M, Ansari A, Gill S (eds) Essential plant nutrients. Springer, Cham
Panova GG, Ktitorova IN, Skobeleva OV, Sinjavina NG, Charykov NA, Semenov KN (2016) Impact of polyhydroxy fullerene (fullerol or fullerenol) on growth and biophysical characteristics of barley seedlings in favourable and stressful conditions. Plant Growth Regul 79(3):309–317
Pardo JM, Rubio F (2011) Na+ and K+ transporters in plant signaling. In: Transporters and pumps in plant signaling. Springer, Berlin Heidelberg, pp 65–98
Pękal A, Pyrzynska K (2014) Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal Methods 7(9):1776–1782
Percey WJ, Shabala L, Wu Q, Su N, Breadmore MC, Guijt RM, Bose J, Shabala S (2016) Potassium retention in leaf mesophyll as an element of salinity tissue tolerance in halophytes. Plant Physiol Biochem 109:346–354
Raza SH, Athar HR, Ashraf M, Hameed A (2007) Glycinebetaine-induced modulation of antioxidant enzymes activities and ion accumulation in two wheat cultivars differing in salt tolerance. Environ Exp Bot 60(3):368–376
Raza SH, Ahmad MB, Ashraf MA, Shafiq F (2014) Time-course changes in growth and biochemical indices of mung bean [Vigna radiata (L.) Wilczek] genotypes under salinity. Braz J Bot 37(4):429–439
Sachkova AS, Kovel ES, Vorobeva AA, Kudryasheva NS (2017) Antioxidant activity of fullerenols. Bioluminescent monitoring in vitro. Procedia Tech 27:230–231
Shabala S, Shabala S, Cuin TA, Pang J, Percey W, Chen Z, Conn S, Eing C, Wegner LH (2010) Xylem ionic relations and salinity tolerance in barley. Plant J 61:839–853
Shabala L, Zhang J, Pottosin I, Bose J, Zhu M, Fuglsang AT, Velarde-Buendia A, Massart A, Hill CB, Roessner U, Shabala S (2016) Cell-type-specific H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare) to salinity stress. Plant Physiol 172(4):2445–2458
Shabala S (2017) Signalling by potassium: another second messenger to add to the list? J Exp Bot 68(15):4003–4007
Shabala S, Cuin T (2007) Potassium transport and plant salt tolerance. Physiol Plant 133:651–669
Shafiq F, Raza SH, Bibi A, Khan I, Iqbal M (2018) Inluence of proline priming on antioxidative potential and ionic distribution and its relationship with salt tolerance of wheat. Cereal Res Commun 46(2):286–299
Sheldon AR, Dalal RC, Kirchhof G, Kopittke PM, Menzies NW (2017) The effect of salinity on plant-available water. Plant Soil 418(1-2):477–491
Shu S, Guo R, Sun J, Yuan Y (2012) Effects of salt stress on the structure and function of the photosynthetic apparatus in Cucumis sativus and its protection by exogenous putrescine. Physiol Plant 146:285–296
Taiz L, Zeiger E (2010) Plant physiology, fifth edn. Sinauer Associates, Sunderland
Tavakkoli E, Fatehi F, Coventry S, Rengasamy P, McDonald GK (2010) Additive effects of Na+ and Cl− ions on barley growth under salinity stress. J Exp Bot 62:2189–2203
Teakle NL, Tyerman SD (2010) Mechanisms of Cl− transport contributing to salt tolerance. Plant Cell Environ 33:566–589
Tedeschi A, Zong L, Huang CH, Vitale L, Volpe MG, Xue X (2017) Effect of salinity on growth parameters, soil water potential and ion composition in Cucumis melo cv. Huanghemi in north‐western China. J Agron Crop Sci 203(1): 41–55
Torbaghan ME, Lakzian A, Astaraei AR, Fotovat A, Besharati H (2017) Salt and alkali stresses reduction in wheat by plant growth promoting haloalkaliphilic bacteria. J Soil Sci Plant Nutr 17(4):1058–1087
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci 151(1):59–66
Wang C, Zhang H, Ruan L, Chen L, Li H, Chang XL, Zhang X, Yang ST (2016) Bioaccumulation of 13C-fullerenol nanomaterials in wheat. Environ Sci Nano 3(4):799–805
Wolf B (1982) A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun Soil Sci Plant Anal 13(12):1035–1059
Xiong JL, Li J, Wang HC, Zhang CL, Naeem MS (2018) Fullerol improves seed germination, biomass accumulation, photosynthesis and antioxidant system in Brassica napus L. under water stress. Plant Physiol Biochem 129:130–140
Zhang WD, Wang P, Bao Z, Ma Q, Duan LJ, Bao AK, Zhang JL, Wang SM (2017) SOS1, HKT1; 5, and NHX1 synergistically modulate Na+ homeostasis in the halophytic grass Puccinellia tenuiflora. Front Plant Sci 8:576
Zhu J (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Funding
The study was partially funded by Higher Education Commission (HEC), Islamabad, Pakistan through Project grants no. 20-1522/R&D/09 and 20-1523/R&D/10 to Prof. Dr. Muhammad Iqbal.
Author information
Authors and Affiliations
Contributions
All the authors contributed equally to this manuscript.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 46 kb)
Rights and permissions
About this article
Cite this article
Shafiq, F., Iqbal, M., Ali, M. et al. Seed Pre-treatment with Polyhydroxy Fullerene Nanoparticles Confer Salt Tolerance in Wheat Through Upregulation of H2O2 Neutralizing Enzymes and Phosphorus Uptake. J Soil Sci Plant Nutr 19, 734–742 (2019). https://doi.org/10.1007/s42729-019-00073-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-019-00073-4