Abstract
Carbon nanoparticles attract attention of plant researchers as a possible means of improving crop yield and its quality. There are grounds to believe that the beneficial influence of polyhydroxy fullerene (PHF) on plants is due to its antioxidant activity, but the mechanism of its action on their growth and development remains unclear. Our study shows that PHF added to the nutrient medium accelerates barley roots elongation owing to the increase of their longitudinal extensibility in the growth zone. The impact of PHF on root growth was much more pronounced under the action of stressors inducing the accumulation of reactive oxygen species, such as UV-B radiation, salt stress and the excess of salicylic acid. Dichlorofluorescein assay showed that PHF prevented oxidative stress development and subapical root swelling after UV-B irradiation of roots. The conclusion is drawn that the important reason of root growth acceleration in the presence of PHF is its ability to serve as a scavenger of free radicals. That’s why it may be especially useful for the improvement of plant growth under environmental stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last decade, an array of experiments summarized in reviews (Nair et al. 2010; Rico et al. 2011; Aslani et al. 2014; Husen and Siddigi 2014) has been conducted to determine the possible impact of nanomaterials, mostly metal- and carbon-based, on growth and development of plants. Some of these studies have revealed their non-consequential or negative effect on plant growth and development, whereas others report positive results. Relatively sparse and contradictory are the data concerning the impact of polyhydroxy fullerene (PHF, also named fullerol or fullerenol) used in the current study. PHF induced the damage of onion cells (Chen et al. 2010) but stimulated Arabidopsis hypocotyls elongation by 20–40 % (Gao et al. 2011). PHF-treatment of bitter melon seeds resulted in the increase of biomass yield up to 54 %, fruit yield up to 128 % and phytomedicine content up to 90 % (Kole et al. 2013). Its favourable effect on crop yield is presumed to arise from its antioxidant activity that was reported for fullerene derivatives (Gharbi et al. 2005; Dugan et al. 1997, 2001) and specifically for PHF as for a scavenger of reactive oxygen species (ROS) (Yin et al. 2008). The uptake, translocation and accumulation of PHF in bitter melon is confirmed by bright field imaging and Fourier transform infra-red spectroscopy (Kole et al. 2013). It has been shown also that C60 water-soluble derivatives to which PHF belongs penetrate through zoogenic and phytogenic membranes either like lipophilic ions or in neutral form they take after protonation (Andreev et al. 2008). Up to now, the impact of PHF on plants has been studied under favourable conditions of their growth. But its presumable antioxidant activity challenges us to compare the PHF action in favourable and stressful conditions when enhanced generation of ROS may be expected and the protective function of PHF may be strongly pronounced. The stressors we used were UV-B radiation, salt and salicylic acid (SA), the plant hormone whose excess induced oxidative stress (Rao and Davis 1999; Shim et al. 2003; Rivas-San Vicente and Plasencia 2011). Previously, we got evidence that root growth zone parameters, namely, longitudinal (δl) and transverse (δD/D) extensibility and hydraulic conductivity of rhizoderma membranes (L p) are very sensitive indicators of stress level in root growth zone and that root elongation rate often varies with δl and sometimes with root internal osmotic pressure (Πi) (Ktitorova et al. 2012). That’s why these parameters were chosen to analyze the reasons of root growth responses in the presence of PHF. Studying the influence of PHF on ROS level under stress, we used the oxidant-sensing fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA).
Materials and methods
Chemicals
PHF was synthesized by the hydroxylation of the C60 fullerene in the presence of quaternary ammonium bases using procedures previously described (Semenov et al. 2011). Fullerene C60 of mass fraction purity 99.9 %, with the main detectable impurity C70 was used. The reagent was produced at ZAO “ILIP” (St. Petersburg). Mass-spectrum measurements revealed its molecular weight to be 1128 a.u., which corresponds to C60(OH)24 formula of PHF (Semenov et al. 2011).
Plant material and experimental design
Seeds of barley (Hordeum vulgare L.) or wheat (Triticum aestivum L.) were kept in Petri dishes until radicle protrusion, then spread on porous plates immersed in ceramic beakers containing 10 %-strength Knop’s solution (control solution) and grown for 4 days at 18–22 °C, 16 h/8 h light/dark cycle. PHF and/or chemical stressors were added to the control solutions at 4-days. The final concentration of PHF was 14 mg/l while the chemical stressors were 0.5 mM SA, pH 6.0 or 75 mM NaCl. SA concentration was chosen based on our previous results showing that 1 day exposure of barley roots to 1 mM SA induced sharp retardation of their growth and subapical swelling (Ktitorova et al. 2006b).
UV-B irradiation was another stress tested. Plants were incubated with 14 mg/l PHF for 1 day, then UV-B was applied. The UV-B irradiation was provided with LE-30 lamps (peak emission at 320 nm). The intensity of biologically active radiation was 0.5 W/m2 according to the action spectrum reported by Caldwell (1981).The shoots were irradiated for 6 h under conditions of their growth. The roots to be irradiated were spread on moistened porous plates and exposed to unilateral UV-B light from one side only (0.5 W/m2, 15 min). After irradiation, the seedlings were transferred to previous growing conditions.
Measurements and analysis
Daily length increments of each root (dl r) or shoot (dl sh) were measured.
In order to examine longitudinal extensibility, we employed the modification of the classic method suggested by de Vries (1877) whose experiments were reproduced recently by Peters et al. (2001). We cut 1-cm long root tips containing the root elongation zone, which in control roots is about 6–7 mm long, and measured their lengths by displaying the projection of root segments on the screen of a microfilm reader. Then, root segments were subjected to freezing (at −20 °C) and thawing which resulted in a loss of turgor, and the segment length measurements were repeated. The δl value was determined as the change in root segment length after the elimination of turgor. The method adequacy followed from the distinguished differences in root longitudinal extensibility between the growth zone and the zone where cell elongation ceased. Actually, in the control barley control roots we observed δl = 0.72 ± 0.02 mm in the zone 0–1 cm from the apex and δl = 0.06 ± 0.01 mm in the zone 1–2 cm from the apex (Skobeleva et al. 2011). Thus, δl is determined mainly by the extensibility of cell walls in the elongation zone and by its length.
The osmotic pressure in roots was estimated using microsamples of cell sap obtained by fast freezing of roots in liquid nitrogen with subsequent grinding, thawing, and centrifugation at 400×g for 10 min (Ktitorova et al. 2006a). Supernatant samples were analyzed with a cryoosmometer performing five assays per replicate.
The transverse extensibility of roots was evaluated by normalized changes of root diameter δD/D in response to the increase in external osmotic pressure (ΔΠo) by 0.3 MPa. Root diameter in the growth zone was measured with a sensor of small displacements. The roots were placed into a perfused experimental chamber; the movable plate of the displacement sensor (8 mm in diameter) resided on the horizontally oriented root and covered the growth zone. Changes in root diameter induced by a shift of external osmotic pressure (ΔΠo) were transformed into electric signals and recorded on a chart recorder. The hydraulic conductivity (L p) of root membranes was determined by the “initial flux method” according to the formula:
where t is the time elapsed from the moment of a stepwise change of osmotic pressure.
The δD/D value was determined 10 min after the shift of Πo, when steady-state was achieved. The method of measuring of L p and δD/D was described previously (Ktitorova et al. 2002), where we showed that the parameter L p characterizes hydraulic conductivity of rhizodermal cell membranes.
The experiments were performed in 3–5 replicates, with more than 20 seedlings in each variant. Changes in seedling parameters as compared with the control (untreated seedlings in the control medium) were expressed as percentages.
In order to visualize ROS accumulation in the root growth zone, we used the oxidant-sensing fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA). H2DCF-DA is a nonpolar dye converted by cellular esterases into the polar derivative H2DCF that is nonfluorescent but switches to highly fluorescent DCF when oxidized by intracellular ROS (Schopfer et al. 2001; Naydov et al. 2010). 1-cm root tips were cut at a certain moment of the experiment, drowned in 50 μM H2DCF-DA for 1 min, rinsed in water and examined in a microscope (Axiostar plus, Zeiss, Jena, Germany) with excitation filter BP 450–490 and emission filter LP 520. Images were taken with a video camera (SONY DXC-950P, Sony, Tokyo).
Statistical analysis
Analysis of variance (ANOVA) for seedling parameters (one-way for PHF action in favourable conditions or two-way for PHF action in the stressful ones) and calculation of correlation coefficients were performed by means of MS-Excel software. Significance of variation among treatments was tested at 5 and 1 % level of significance. The data in the text, tables and figures represent mean values. Bars in figures correspond to confidence intervals calculated at 95 % probability based on the t-test.
Results and discussion
PHF influence upon barley seedlings growth and characteristics in favourable growing conditions
Root growth rate was higher in the presence of 7, 14 and 75 mg/l of PHF than in the control and was lower under 1100 mg/l PHF than in the control (Table 1). Shoot growth rate changed significantly only under 1100 mg/l PHF, when it decreased like the root growth rate. The data of Fig. 1 demonstrate that accelerated root growth rate was already observed on the first day of the exposure with 14 mg/l PHF and persisted later.
Root elongation rate depends on the number of elongating cells and on their extension rate. The number of elongating cells might increase due to accelerated cell production under the action of PHF. However, this would contribute to root elongation only after the cell cycle is completed, which takes the cells of barley meristem about 12 h (Bennett and Finch 1972). Furthermore, if PHF affects only cell production rate, its impact on root elongation will be stronger on the second day of exposure owing to gradual increase of the number of cells that start elongation (Ivanov 2011). The fact that constant growth rate was observed as soon as on the first day of PHF action (Fig. 1) indicates that PHF (14 mg/l) influenced the extension rate. That doesn’t exclude possible alterations in the processes going on in the meristem, which needs further exploration.
Cell extension rate depends on the mechanical properties of cell walls and on their tension that may vary with the internal osmotic pressure. As seen from Table 1, changes of root growth rate correlated strongly with changes of δl (r = 0.96) while did not correlate with changes of Пi (r = −0.56). Therefore, PHF influence on extension root growth is caused by changes of cell wall extensibility and not by changes of osmotic pressure.
The increase of root osmotic pressure observed at PHF concentrations equal to or above 75 mg/l can hardly be attributed to PHF accumulation in cells in view of the tendency of PHF molecules to aggregate at the concentrations over 14 mg/l (Semenov et al. 2011). The maximal gain in Пi was observed with 1100 mg/l PHF in the medium when the increase of Пi value may be explained by the inhibition of root growth with the assimilates influx still in progress. It seems likely that Πi gain is one of the non-specific processes accompanying root growth retardation induced by cell wall stiffening (Skobeleva et al. 2011). An exception is the case of severe stress when non-specific permeability of membranes rises sharply with subsequent electrolyte outflow.
Possible reasons of changes in root growth rate and δl value under the action of PHF in favourable growing conditions
Presumably, the positive effect of PHF on longitudinal extensibility and root growth is due to its ability to serve as a scavenger of free radicals (Yin et al. 2008). It is known that the arrest of cell elongation in the proximal region of the elongation zone is related to the stiffening of cell walls due to the formation of oxidative cross-links between cell wall polymers, with peroxidase and hydrogen peroxide participating in the process (Cosgrove 1997; De Cnodder et al. 2005). It is possible that the lowering of ROS level under the action of PHF retarded the formation of oxidative cross-links in the proximal region of the elongation zone and thus increased the longitudinal extensibility of roots.
The opposite case of growth retardation at 1100 mg/l PHF may be connected with the pro oxidant activity of PHF which can manifest itself at high PHF concentrations (Zha et al. 2012). The decrease in δl and the increase in δD/D as compared with the control values (Table 1) indicated changes in the anisotropy of mechanical properties of cell walls. It might be the sign of the stress that distorts the alignment of cellulose microfibrils due to the disassembly of cortical microtubules in the cytosol (Sivaguru et al. 2003; Takahashi et al. 2003; Ktitorova et al. 2012). The reason of microtubules disorganization is, perhaps, the increase of Ca 2+cyt (Takahashi et al. 2003; Ktitorova et al. 2012) that may be induced by oxidative stress (Mori and Schroeder 2004). It was shown on hippocampus neurons of rats that increasing PHF concentration changes its effect on neurons viability from protective to harmful, which was attributed to the reversion from antioxidant activity that PHF revealed at lower concentrations to pro oxidant activity (Zha et al. 2012). The changing of PHF activity with the increasing of its concentration may be due to the formation of PHF molecule associates. The possibility of similar mechanism working in fullerenes has been discussed (Piotrovsky et al. 2007). So the most probable reason of root and shoot growth retardation by high concentrations of PHF (1100 mg/l) seems to be the development of oxidative stress in the zones of growth.
Possible reasons of the changing of transverse root extensibility and of hydraulic conductivity of their membranes under the action of PHF in favourable conditions
As Table 1 indicates, the values of δD/D and L p increase both at low concentrations of PHF, when root growth accelerates, and at higher ones when it is retarded. This supports our earlier observation that the rate of root growth depends on the changing of δl but is independent of L p and δD/D (Ktitorova et al. 2012). It may be suggested that the increase of L p resulted from enhanced expression of aquaporins under the impact of PHF like it was with tobacco cells culture under the impact of multiwalled carbon nanotubes (Khodakovskaya et al. 2009). However, protein expression rose only after 24 h, while in our experiments 20–25 % increase of L p was noted as soon as 1.5 h after the introduction of 14 mg/l PHF into the medium (the data are not shown). The increase of L p under the impact of PHF may be due to the fact that penetration of PHF into the membrane favours the dynamic defects formation in its structure that can serve as routs of water transport (Lawaczek and Fitterich 1988). The sharp rise of L p accompanying root growth retardation in the presence of 1100 mg/l PHF (Table 1) is typical of severe stress in roots (Ktitorova et al. 2012) and may be the effect of increased Ca 2+cyt and Ca2+-dependent phosphorylation of aquaporins (Maurel 1997; Johansson et al. 1998).
The increase of root transverse extensibility in the presence of PHF might be caused by PHF penetration into cell walls, the slackening of interactions between polymers and the loosening of cell wall structure. Another reason of δD/D increase caused by 1100 mg/l PHF may be a severe stress in the root growth zone which distorts cellulose microfibrils packing in cell walls (Sivaguru et al. 2003; Takahashi et al. 2003; Ktitorova et al. 2012).
Thus, the results obtained in the experiments on PHF impact on seedling roots growing in favourable conditions may be explained by the antioxidant activity of PHF in concentrations 7, 14, 75 mg/l and its pro oxidant activity in concentration 1100 mg/l. If this is the case, then the assumed antioxidant activity of PHF must be particularly intense under stress when enhanced generation of ROS is to be expected.
PHF influence on seedling growth and root parameters in the presence of stressors
We compared PHF (14 mg/l) action on barley seedlings in favourable conditions and under the action of stressors provoking oxidative stress in plants. It is known that UV-B radiation induces the accumulation of ROS in irradiated tissues (Frohnmeyer and Staiger 2003). We showed that under UV-B irradiation of shoots oxidative stress develops also in the seedling roots and causes retardation of their growth (Ktitorova et al. 2006a). It is also known that oxidative stress may be induced by the salt stress (Shalata and Neumann 2001; Xu et al. 2011; Grzesiak et al. 2013) and by the excess of SA. Scott et al. (2004) stated that the collective data on SA and oxidative stress have a complex pattern consistent with the model proposed by Rao and Davis (1999) in which SA maintains the cellular redox state and potentiates defenses in ozone-treated plants while excessive SA levels activate an oxidative burst and cell death. SA has been shown to inhibit catalase and ascorbate peroxidase activities or to serve as substrate for ascorbate peroxidase, which may lead to the accumulation of ROS (Chen et al. 1993; Durner and Klessig 1995; Kvaratskhelia et al. 1997).
Under favourable conditions, the addition of 14 mg/l PHF to the medium accelerated root growth 1.2 times and under the impact of stressors 1.5–4.8 times as compared with growth in the same conditions without PHF (Table 2). This indicates that PHF action on root growth may be connected with its antioxidant behaviour which becomes more apparent as ROS accumulate.
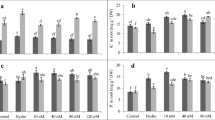
It is known that fast retardation of root growth under stress may be due to the stiffening of cell walls (Neumann 1995; Cosgrove 1997; Ktitorova et al. 2012). Indeed, in our experiments the decrease of root growth rate was accompanied by the decrease of δl, and the acceleration of growth in the presence of PHF was observed when δl increased (Fig. 2a–c).
PHF impact on barley root parameters in stressful conditions: a UV-B irradiation of roots; b root exposure to 75 mM NaCl; c root exposure to 0.5 mM SA. PHF concentration, 14 mg/l; dl r, daily length increment of roots; δl, δD/D: longitudinal and transverse extensibility of roots; L p: hydraulic conductivity of roots, Π i : root osmotic pressure. Root parameters are measured a day after the beginning of stressor action and are presented as percentage of control (untreated seedlings in 10 % strength Knop’s solution). Error bars indicate 95 % confidence intervals (n = 3)
As was shown earlier, UV-B irradiation of barley seedlings roots inhibited sharply root growth first owing to presumable temporal decrease of Πi (0–2 h after irradiation) and then because of nearly two-fold decrease of δl, which led, together with Πi restoration, to radial expansion and subapical swelling of barley roots (4–10 h after irradiation) (Ktitorova et al. 2006b). 24 h after irradiation the value of δl approximated that of the control, and the tendency developed toward the continuation of longitudinal root growth (Ktitorova et al. 2006b). The data of Fig. 2 a confirm the results obtained earlier (Ktitorova et al. 2006b) and demonstrate the protective effect of PHF that manifests itself in the twofold increase in the elongation of irradiated roots during first 24 h after their irradiation as well as in faster reestablishment of their characteristics measured 24 h after irradiation.
The retardation of root growth under salt stress was induced first by the osmotic shock, which was nearly overcome during the first day of adaptation by way of increasing Π i (Fig. 2b). After that the main reason of slowed root elongation under salt stress as well as under the action of SA may be the decline of root longitudinal extensibility (Fig. 2b, c).
Both UV-B irradiation of roots and the action of SA substantially increased the values of δD/D and L p as compared with the control while under salt stress their variance was not significant (Fig. 2a–c). As noted above, the increase of δD/D and L p may be induced by intensified generation of ROS that results in the activation of Ca2+-channels and the increase of Ca 2+cyt (Mori and Schroeder 2004). This is confirmed by the fact that the decrease of δl and the increase of δD/D and L p in barley roots can be induced by Ca-ionophore ionomycin (Ktitorova et al. 2012). Under salt stress the expectable substantial increase of δD/D and L p seems to be obscured by their decrease because of H+-pump activation which is typical for adaption to salt stress (Janicka-Russak et al. 2013). It is shown that H+-pump activation and the acidification of apoplast lead to the decrease of δD/D and L p (Ktitorova et al. 2012).
It should be noted that under the action of PHF parameters δD/D and L p increased in favourable conditions of seedlings growth (Table 1) and decreased in stressful conditions (Fig. 2). It confirms once again that they aren’t related directly to the root growth rate. It testifies also that they are sensitive to various influences and that the ultimate result depends on predominant mechanisms which under stressful conditions were related to ROS level and H+-pump activity.
PHF (14 mg/l) did not influence significantly Π i , but increased δl and decreased δD/D and L p under the action of stressors (Fig. 2). The positive action of PHF on root growth and parameters in these conditions may be attributed to its antioxidant activity and accompanying stabilization of Ca 2+cyt homeostasis. The confirmation of PHF antioxidant activity was received in our experiments with the visualization of ROS level in the roots.
UV-B irradiation of barley roots may cause subapical swelling, probably associated with the oxidation stress resulting from UV-B irradiation (Ktitorova et al. 2006b). The data of Fig. 3c confirm this suggestion and show that subapical swelling was accompanied by a sharp increase of DCF fluorescence indicating the accumulation of ROS in the root tip. DCF fluorescence in unirradiated root tips was observed mainly in the apex (in the quiescent centre) and in dead cells of the root cap (Fig. 3a, b). Addition of 14 mg/l PHF to the medium 24 h before the irradiation prevented subapical swelling and the increase of DCF fluorescence in irradiated roots (Fig. 3d). Therefore, in low concentrations PHF functioned as a free radicals scavenger and decreased oxidation stress level in irradiated roots.
Effect of PHF on the fluorescence of dichlorfluoroscein in root tips after UV-B irradiation of barley roots (0.5 W/m2, 15 min). a unirradiated root in 10 % strength Knop’s solution; b unirradiated root in 10 % strength Knop solution + 14 mg/l PHF; c irradiated root in 10 % strength Knop’s solution, 4 h after UV-B irradiation; d irradiated root in 10 % strength Knop’s solution + 14 mg/l PHF, 4 h after UV-B irradiation
Possible reasons of PHF influence on shoot growth
Since there is good reason to believe that PHF spreads along the shoots and comes into contact with the growth zones, the question arises, why shoot growth is not accelerated under the impact of PHF in favourable growing conditions like it happens with the root growth (Table 1; Fig. 1). In some of the experiments we even observed the tendency towards the decrease of shoot growth rate with simultaneous acceleration of root growth (Table 1; Fig. 1). Gao et al. (2011) reported inverse but in certain respects similar results obtained on Arabidopsis seedlings: after 4 days of germination and growing in Petri dishes with 100 mg/l PHF their hypocotyl length increased by 20 % compared to controls, whereas root length did not change significantly. When the stimulation of hypocotyl growth was maximal (the increase by 40 %, with 200 mg/l PHF), root growth showed a tendency toward retardation. It could be assumed that in these cases there was redistribution of nutrients in favour of the fast-growing organ that might restrict the growth of the other one. In our experiments, the prevailing acceleration of root growth was provoked by the experimental scheme adopted when PHF penetrated primarily the roots with accelerating their growth, increasing the influx of assimilates and energy sources to them and thus possibly restricting shoot growth. It should be noted that in the presence of stressors when root growth was restrained shoot growth was significantly accelerated under the impact of PHF (Table 3). Since we explain favourable influence of PHF on plant growth by its antioxidant activity, we think it possible that this activity may develop both in the root growth zone and in that of the shoots.
PHF significantly decreased shoot growth under UV-B irradiation of shoots (Table 3). This may be connected with the accompanying acceleration of root growth above the control level (Table 2). Growth retardation under stress is considered to be a defensive mechanism that allows the plant to redistribute energy flows for the repair after injury (Neumann 1995). So, root growth retardation may encourage the growth of irradiated shoots. Conversely, root growth increase under the action of PHF may cause delayed shoot repair.
The results obtained attest to the feasibility of stimulating root growth with low concentrations of PHF warning at the same time of possible harmful effect of PHF in case of its excessive accumulation in tissues. It is shown that the stimulation of root growth by PHF is due mainly to increased extensibility of cell walls in the growth zones. Most efficiently PHF acted under stress, which is explained by its antioxidant activity.
References
Andreev I, Petrukhina A, Garmanova A, Babakhin A, Andreev S, Romanova V, Troshin P, Troshina O, DuBuske L (2008) Penetration of fullerene C60 derivatives through biological membranes. Fuller Nanotub Carbon Nanostruct 16:89–102
Aslani F, Bagheri S, Julkapli NM, Juraimi ASh, Hashemi FSG, Baghdadi A (2014) Effects of engineered nanomaterials on plants growth. Sci World J ID. doi:10.1155/2014/641759
Bennett MD, Finch RA (1972) The mitotic cycle time of root meristem cells of Hordeum vulgare. Caryologia Int J Cytol Cytosyst Cytogen 25:439–444
Caldwell MM (1981) Plant response to solar ultraviolet radiation. In: Lange OL et al (eds) Encyclopedia of plant physiology, physiological plant ecology 1: Responses to the Physical Environment, vol 12A. Springer, Heidelberg, pp 169–197
Chen Z, Silva H, Klessig DF (1993) Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262:1883–1886
Chen R, Ratnikova TA, Stone MB, Lin S, Lard M, Huang G, Hudson JS, Ke PC (2010) Differential uptake of carbon nanoparticles by plant and mammalian cells. Small 6:612–617
Cosgrove DJ (1997) Relaxation in a high-stress environment: the molecular basis of extensible cell walls and cell enlargement. Plant Cell 9:1031–1041
De Cnodder T, Vissenberg K, Van Der Straeten D, Verbelen JP (2005) Regulation of cell length in the Arabidopsis thaliana root by the ethylene precursor 1-aminocyclopropane-1-carboxylic acid: a matter of apoplastic reactions. New Phytol 168:541–550
de Vries H (1877) Untersuchungen über die mechanischen Ursachen der Zellstreckung, ausgehend von der Einwirkung von Salzlösungen auf den Turgor wachsender Pflanzenzellen. Wilhelm Engelmann, Leipzig
Dugan LL, Turetsky DM, Du C, Lobner D, Wheeler M, Almli CR, Shen CK-F, Luh T-Y, Choi DW, Lin T-S (1997) Carboxyfullerenes as neuroprotective agents. Proc Natl Acad Sci USA 94:9434–9439
Dugan LL, Lovett EG, Quick KL, Lotharius J, Lin TT, O’Malley KL (2001) Fullerene-based antioxidants and neurodegenerative disorders. Parkinsonism Relat Disord 7:243–246
Durner J, Klessig DF (1995) Inhibition of ascorbate peroxidase by salicylic acid and 2,6-dichloroisonicotinic acid, two inducers of plant defense responses. Proc Natl Acad Sci USA 92:11312–11316
Frohnmeyer H, Staiger D (2003) Ultraviolet-B radiation-meditated responses in plants. Balancing Damage and protection. Plant Physiol 133:1420–1428
Gao J, Wang Y, Folta KM, Krishna V, Bai W, Ingeglia P, Georgieva A, Nakamura H, Koopman B, Moudgil B (2011) Polyhydroxy fullerenes (PHFs or fullerenols): beneficial effects on growth and lifespan in diverse biological models. PLoS ONE 6(5):e19976. doi:10.1371/journal.pone.0019976
Gharbi N, Pressac M, Hadchouel M, Szwarc H, Wilson SR, Moussa F (2005) Fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett 5:2578–2585
Grzesiak M, Filek M, Barbasz A, Kreczmer B, Hartikainen H (2013) Relationships between polyamines, ethylene, osmoprotectants and antioxidant enzymes activities in wheat seedlings after short-term PEG- and NaCl-induced stresses. Plant Growth Regul 69:177–189. doi:10.1007/s10725-012-9760-9
Husen A, Siddigi KS (2014) Carbon and fullerene nanomaterials in plant system. J Nanobiotechnol 12:16–26
Ivanov VB (2011) Using the roots as test objects for the assessment of biological action of chemical substances. Russ J Plant Physiol 58:1082–1089
Janicka-Russak M, Kabała K, Wdowikowska A, Kłobus G (2013) Modification of plasma membrane proton pumps in cucumber roots as an adaptation mechanism to salt stress. J Plant Physiol 170:915–922
Johansson I, Karisson M, Shulka VK, Chrispeels MJ, Larsson C, Kjellbom P (1998) Water transport activity of the plasma memrane aquaporin PM28A is regulated by puosphorylation. Plant Cell 10:451–459
Khodakovskaya M, Dervishi E, Mahmood M, Yang X, Li Z, Fumiya W, Biris A (2009) Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 3:3221–3227
Kole C, Kole P, Randunu KM, Choudhary P, Podila R, Ke PC, Rao AM, Marcus RK (2013) Nanobiotechnology can boost crop production and quality: first evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia). BMC Biotechnol 13:37–58
Ktitorova IN, Skobeleva OV, Sharova EI, Ermakov EI (2002) Hydrogen peroxide appears to mediate a decrease in hydraulic conductivity in wheat roots under salt stress. Russ J Plant Physiol 49:369–380
Ktitorova IN, Skobeleva OV, Kanash EV, Bilova TE, Sharova EI (2006a) Causes of root growth retardation induced by ultraviolet-B irradiation of shoots of barley seedlings. Russ J Plant Physiol 53:85–95
Ktitorova IN, Demchenko NP, Kalimova IB, Demchenko KN, Skobeleva OV (2006b) Cellular analysis of UV-B-induced barley root subapical swelling. Russ J Plant Physiol 53:824–836
Ktitorova IN, Skobeleva OV, Agaltsov KG (2012) Biophysical parameters as informative tools for elucidating the causes of root growth retardation under stressful conditions. Russ J Plant Physiol 59:118–126
Kvaratskhelia M, George SJ, Thorneley RN (1997) Salicylic acid is a reducing substrate and not an effective inhibitor of ascorbate peroxidase. J Biol Chem 272:20998–21001
Lawaczek R, Fitterich H (1988) Pathways of water through erythrocyte membranes. Routes along defect structures. J Theor Biol 135:401–407
Maurel C (1997) Aquaporins and water permeability of plant membranes. Annu Rev Plant Physiol Plant Mol Biol 48:399–429
Mori IC, Schroeder JI (2004) Reactive Oxygen Species Activation of Plant Ca2+ Channels. A signalling mechanism in polar growth, hormon transduction, stress signalling, and hypothetically mechanotransduction. Plant Physiol 135:702–708
Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar DS (2010) Nanoparticulate material delivery to plants. Plant Sci 179:154–163
Naydov IA, Mudrik VA, Ivanov BN (2010) Light-induced hydrogen peroxide dynamics in protoplasts from leaves of both wild-type Arabidopsis and its mutant deficient in ascorbate biosynthesis. Dokl Biochem Biophys 432:137–140
Neumann PM (1995) Inhibition of root growth by salinity stress: toxicity or an adaptive biophysical response? In: Baluška F et al (eds) Structure and function of roots. Kluwer Academic Publishers, Dordrecht, pp 299–304
Peters WS, Farm MS, Kopf AJ (2001) Does growth correlate with turgor-induced elastic strain in stems? a reevaluation of de Vries’ classical experiments. Plant Physiol 125:2173–2179
Piotrovsky LB, Eropkin MYu, Eropkina EM, Dumpis MA, Kiselev OI (2007) Mechanisms of biologic action of fullerenes—dependence on aggregate state. Psychopharmacol Biol Narcol 7:1548–1554
Rao MV, Davis KR (1999) Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J 17:603–614
Rico CM, Majumdar S, Duarte-Gardea M, Peralta-Videa JR, Gardea-Torresdey JL (2011) Interaction of nanoparticles with edible plants and their possible implications in the food chain. J Agric Food Chem 59:3485–3498
Rivas-San Vicente M, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62:3321–3338. doi:10.1093/jxb/err031
Schopfer P, Plachy C, Frahry G (2001) Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol 125:1591–1602
Scott IM, Clarke SM, Wood JE, Mur LA (2004) Salicylate accumulation inhibits growth at chilling temperature in Arabidopsis. Plant Physiol 135:1040–1049. doi:10.1104/pp.104.041293
Semenov KN, Charykov NA, Keskinov VN (2011) Fullerenol synthesis and identification. Properties of the fullerenol water solutions. J Chem Eng Data 56:230–239
Shalata A, Neumann PM (2001) Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J Exp Bot 52:2207–2211
Shim IS, Momose Y, Yamamoto A, Kim DW, Usui K (2003) Inhibition of catalase activity by oxidative stress and its relationship to salicylic acid accumulation in plants. Plant Growth Regul 39:285–292
Sivaguru M, Pike Sh, Gassmann W, Baskin TI (2003) Aluminium rapidly depolymerizes cortical microtubules and depolarizes the plasma membrane: evidence that these responses are mediated by a glutamate receptor. Plant Cell Physiol 44:667–675
Skobeleva OV, Ktitorova IN, Agal’tsov KG (2011) The causes for barley root growth retardation in the presence of ammonium and glutamate. Russ J Plant Physiol 58:307–315
Takahashi H, Kawahara A, Inoue Y (2003) Ethylene promotes the induction by auxin of the cortical microtubule randomization required for low-pH-induced root hair initiation in lettuce (Lactica sativa L.) seedlings. Plant Cell Physiol 44:932–940
Xu S, Hu J, Li Y, Ma W, Zheng Y, Zhu S (2011) Chilling tolerance in Nicotiana tabacum induced by seed priming with putrescine. Plant Growth Regul 63:279–290
Yin JJ, Lao F, Vehg J, Fu PP, Zhao YL et al (2008) Inhibition of tumor growth by endohedral metallofullerenol nanoparticles optimized as reactive oxygen species scavenger. Mol Pharmacol 74:1132–1140
Zha YY, Yang B, Tang ML, Guo QC, Chen JT, Wen LP, Wang M (2012) Concentration-dependent effects of fullerenol on cultured hippocampal neuron viability. Int J Nanomed 7:3099–3109. doi:10.2147/IJN.S30934
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panova, G.G., Ktitorova, I.N., Skobeleva, O.V. et al. Impact of polyhydroxy fullerene (fullerol or fullerenol) on growth and biophysical characteristics of barley seedlings in favourable and stressful conditions. Plant Growth Regul 79, 309–317 (2016). https://doi.org/10.1007/s10725-015-0135-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-015-0135-x