Abstract
Salinity is a critical issue impairing the growth and productivity of most crop species through the mediated ionic and osmotic imbalances. As a way forward, the current study was tailored to elucidate the capacity of sulfur nanoparticles (SNPs) to amend salinity consequences on growth and physio-biochemical attributes of wheat. In a controlled experiment, wheat seeds were primed for 12 h with either 100 μM SNPs or deionized water then sown in plastic pots containing 5 kg clay-sand mixture (2:1 w/w). A week later, pots received NaCl (100 or 200 mM) as a sole treatment or in combination with SNPs and after three weeks the data of morph-bio-physiological traits were recorded. Salinity decreased growth rate, pigmentation, protein, amino acids, cysteine, ascorbate, flavonoids and phenolics content in wheat leaves. Plants pre-treated with 100 μM SNPs showed improved growth rate, pigmentation, nitrogen metabolism as well as non-enzymatic antioxidant contents as compared with salinized treatments. Neither salt nor SNP treatments affected photosynthetic performance rate (Fv/fm), however both treatments induced glutathione content. SNP treatment retrieved the undue excessive activities of catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), superoxide dismutase (SOD) and polyphenol oxidase (PPO) besides the increased level of proline caused by salt stress. Likewise, 100 μM SNPs rebalanced the declined nitrogen, phosphorus and potassium contents and decreased sodium uptake caused by salinity. On the whole, priming with 100 μM SNPs improved photosynthetic pigments, nitrogen metabolism, antioxidant status and ionic relations contributing to the enhancement of growth attributes in wheat under salinity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil salinity is a complex stress factor affecting plant growth and productivity. The causes of high salt concentration in agricultural soils are confined to low rainfall rate, high dehydration rate, ineffective drainage, misuse of chemical fertilizers and irrigation with salt-polluted water (Arora et al. 2018). Besides its devastating effect on crop species, soil salinity was reported to be one of the factors causing soil degradation worldwide menacing food security and putting scientists in a great challenge to face this crisis (Tian et al. 2020). The continuous uptake of Na+ along with Cl− ions causes harsh and negative physiological effects on cell and organellar membranes, photo-assimilation, macromolecules, enzymes activity, respiration rate, nutrient availability, in addition to hydration and structural disorders (Semida et al. 2017). Abdel-Fattah and Asrar (2012) reported that high salt concentrations in the rhizosphere resulted in excessive Na+ accumulation in the cytosol altering the ionic balance due to the deficiency of many essential macro and micro-nutrients. As a secondary impact of salt stress, activated oxygen species are (H2O2, OH·, O2·− and 1O2) excessively generated provoking oxidative damage to DNA, proteins and membrane lipids (Saad-Allah 2015).
Several approaches were developed to reclaim salt-affected soils. These techniques included leaching, application of inorganic and organic amendments, developing transgenic plants and microbial inoculation (Moreira et al. 2020). Recently, the world’s attention has turned towars the utilization of nanotechnology in addressing many environmental stresses affecting plants, particularly after the disordered climate change and increased anthropogenic activities. A variety of environmental stresses were relieved by nano-particle application, for instance improving salt tolerance of mung bean using nano-chitosan (Sen et al. 2020), manganese stress in sunflower using sulfur nano-particles (Ragab and Saad-Allah 2020), cadmium stress in wheat using zinc and iron nano-particles (Rizwan et al. 2019), drought stress in wheat using titanium dioxide nano-particles (Faraji and Sepehri 2019) and heat stress in sorghum using selenium nano-particles (Djanaguiraman et al. 2018). In addition to chemical treatments using nanoparticles to boost plant growth and active constituents, many physical treatments have been identified to improve plant growth and phytochemical constituents. Asghar et al. (2017) observed that by soybean seeds pre-exposure to laser and magnetic field the germination, growth and yield were characteristically improved. Low power laser waves have also been documented to boost the germination, growth, and ionic content in moringa seedlings (Urva et al. 2017). The same results were obtained in bottle gourd as pre-sowing irradiation of the seeds with He-Ne continuous wave-laser enhanced biochemical contents, enzymatic activities and seed productivity (Abbas et al. 2017). The growth and cellular activities of peanut were induced by small X-ray doses (Kehinde et al. 2017). Likewise, Abo-Hamad et al. (2013) showed that germination, growth rate, phenolic content and the alkaloidal fraction of turnip were stimulated by low doses of gamma irradiation. Compared to physical treatments, treatment with nanoparticles is considered to be more appropriate because of its safety during treatment and less chances of genetic mutations.
Sulfur (S) is essential for plant growth and development as it is a fundamental constituent of proteins, amino acids and diverse secondary metabolites (Nakajima et al. 2019). Sulfur was reported to be a key player in many plant processes like cellular structure, electron transport and different metabolic pathways (Capaldi et al. 2015). Moreover, S is integrated into the structure of various cofactors, peptides, vitamins, lipids, polysaccharides, glucosinolates (Iqbal et al. 2012), antioxidants, photosynthesis and nitrogen assimilating enzymes (Capaldi et al. 2015). Recent reports showed that S enhanced photosynthetic rate through improving chlorophyll biosynthesis and nitrogen assimilation (Ragab and Saad-Allah 2020). In addition, Al Banna et al. (2020) stated that when introduced as nanoparticles, sulfur displayed a potent anti-nematicidal activity against Meloidogyne javanica invasion.
The use of nano-materials in resisting various plant pathogens attack and mitigating abiotic stress had grown in the last decade. These nano-materials comprise various inorganic metals and metallic oxides such as zinc, titanium, iron, silver, aluminum, gold, copper, iron and lead (Siddiqui et al. 2015). However, these nano-materials considered toxic heavy metals posing severity to soil, plant and human beings particularly in the long run. As a simple, reliable, cost-effective and feasible technique as well as an outstanding substitute for chemical and physical preparation techniques, the biosynthesis of nanoparticles using plant extracts has increasingly gained interest (Singh et al. 2018a). The use of plant extracts in the biosynthesis of sulfur nanoparticles (SNPs) has been reported to be simple, inexpensive, reliable and eco-friendly. The active constituents of these extracts play a key role in the stabilization and the dispersion of SNPs during their biosynthesis (Salem et al. 2015). Sulfur has the ability to be incorporated into organo-sulfur compounds within plant tissues, which is essential for the healthy growth of plants and potent antimicrobial activity (Suleiman et al. 2015), consequently it is considered as the safer among the nano-materials used in the agricultural sector. Different techniques/methods are in practice for the synthesis of nano-materials and among these methods, green synthesis is an eco-benign in nature. This route has been successfully employed for the fabrication of NPs for biological applications (Remya et al. 2017; Igwe and Nwamezie 2018; Awwad et al. 2020a, b; Naseer et al. 2020; Amer and Awwad 2021).
Wheat (Triticum aestivum L.) is a major staple food for about half of the world’s population. Principally, it provides dietary calories and proteins for the growing human population (Agami et al. 2018). Wheat is used in making many foodstuffs like cakes, noodles, biscuits, pasta, bread and wine. Furthermore, wheat straw is used as fodder for livestock with high nutritional value. Beyond this, it can be utilized for biofuels production. Wheat genotype Misr 3 was classified as salt-sensitive (Abd El-Moneim et al. 2020). Thus, the main objective of this study was therefore to assess that whether grain priming with greenly synthesized sulfur nanoparticles (SNPs) could alter pigmentation, stress biomarkers, nitrogen assimilation, antioxidant status and ionic balance to enhance the growth rate and metabolic machinery of wheat exposed to salt stress.
Materials and methods
Chemicals and reagents
All chemicals and reagents were of analytical grade and used without further purification, as obtained. Sodium thiosulfate, methanol, trichloroacetic acid, glacial acetic acid, phosphoric acid, toluene, potassium iodide, Na2HPO4, Na2CO3, H2O2, crystalline iodine, EDTA, HNO3, sodium molybdate, ascorbic acid, potassium acetate and aluminum chloride were purchased from Elnasr company (Cairo, Egypt). However, ammonium molybdate, thiobarbituric acid, stannous chloride, Coomassie brilliant blue G250, H2SO4, K2HPO4, KH2PO4, reduced glutathione, oxidized glutathione, quercetin, NH4Cl, HCl, gallic acid, ethanol, guaiacol, HgCl2, bovine serum albumin, sulfosalicylic acid, methionine, proline, riboflavin, ninhydrin, NADPH, glycerol, sodium nitroprusside, Triton X-100, acetone, 5,5-dithiobis-(2-nitrobenzoic acid), citric acid, Folin-Ciocalteu’s reagent, sodium citrate, nitroblue tetrazolium, glycine, cysteine, tris buffer, perchloric acid, glycine betaine, and 1,2 dichloroethane were purchased from Sigma–Aldrich (St. Louis, MO, USA).
Preparation of sulfur nanoparticles suspension
Sulfur nanoparticles (SNPs) were biosynthesized using sodium thiosulfate and aqueous extract of basil leaves according to Ragab and Saad-Allah (2020). The physico-chemical properties of the obtained SNPs revealed a yellowish-white powder with an average size of 23 nm and spherical polycrystalline nature. A concentration of 100 μM SNPs solution was prepared by suspending the pre-characterized nano-powder in deionized water and sonicated for 30 min instantly before their usage to avoid particle aggregation.
Plant material and experimental setup
Wheat (Triticum aestivum L.) seeds, genotype Misr3, were obtained from the Department of Wheat Research (DWR), Institute of Field Crops (IFC), Egypt. The seeds were superficially disinfected by immersing for 5 min in 0.1% HgCl2 then rinsed twice in deionized water. The sterilized seeds were soaked either in deionized water or 100 μM SNPs suspension for 12 h. The primed seeds were sown in plastic pots (15 cm depth and 20 cm diameter) containing 5 kg clay-sandy soil mixture (2:1 w/w). The pots were adequately supplied with tap water until the full-seedling establishment (6th day) before salt treatments. On the seventh day, the pots were separated into two main groups. The pots were irrigated either with 100 or 200 mM NaCl, apart from the control which received tap water, to 70% of their FC (field capacity). Accordingly, the experiment comprised five treatments (control, 100 mM NaCl, 200 mM NaCl, 100 mM NaCl +100 μM SNPs and 200 mM NaCl +100 μM SNPs) with three replicates each. After 21 days, plants were collected for growth evaluation and biochemical investigation.
Growth characteristics
The harvested plants were separated into roots and shoots, washed with tap water then deionized water. These samples were utilized for evaluating the growth behavior of roots (depth, fresh weight and dry weight) and shoots (length, fresh weight, dry weight and leaf area).
Measurement of photosynthetic pigments and photosynthetic performance (Fv/FM)
The third leaf from the stem base was chosen in each seedling for total photosynthetic pigments spectrophotometric quantification. Definite weight (0.1 g) of fresh leaves was extracted in 85% cold acetone, centrifugated at 5000 rpm for 10 min and the optical density was measured by UV/visible spectrophotometer. The concentration of each pigment (Chl a, Chl b and carotenoids) was assessed based on the procedures prescribed by Metzner et al. (1965) and uttered as mg g−1fw. The photosynthetic performance of photosystem II, as a gauge of photosynthetic performance, was assessed in the fourth dark-adapted leaf using a digital fluorometer (OS-30 P, Hudson, USA).
Stress biomarkers
Electrolytes leakage (EL) of fresh leaves was measured by Sairam et al. (2005) method. Wheat leaves were cut into equal-sized portions. Leaf portions were rinsed for 15 min in deionized water then gently shaken in 25 ml deionized water using an orbital shaker. The electrical conductivity (EC1) was measured after 1 h, but EC2 was measured after 24 h and EL was expressed in percentage.
Malondialdehyde (MDA) assessment was made as specified by Heath and Packer (1968). Fresh leaves were extracted by 5% trichloroacetic acid (TCA), centrifuged for 15 min at 8000 rpm, then equal quantities of the supernatant and thiobarbituric acid (0.67%) were boiled for 25 min. Absorbance was measured at 530 and 600 nm after cooling and the coefficient of 155 mM−1 cm−1 was used in calculating MDA (nmole g−1fw).
Hydrogen peroxide (H2O2) content in 0.1% trichloroacetic acid (TCA) extract of fresh wheat leaves was assessed by Velikova et al. (2000) method. The absorbance of a mixture comprised the supernatant, K-phosphate buffer (pH 7.0) and KI (1 M) was measured at 390 nm and the coefficient of 0.28 μM−1 cm−1 was employed in calculating H2O2 content (μmol g−1fw).
Estimation of nitrogenous compounds
Total soluble proteins in wheat leaves were extracted and quantified using the technique adopted by Bradford (1976). Ethanolic extract (0.1 ml) was mixed with Coomassie brilliant blue G250 (3 ml), measured spectrophotometrically at 695 nm and soluble protein (mg g−1dw) content was assessed using standard curve of BSA (bovine serum albumin) protein.
According to Lee and Takahashi (1966), free amino acids content was measured in the ethanolic extract using glycine as standard and ninhydrin-citrate buffer-glycerol reagent. The extract was well-mixed with the reagent [1% ninhydrin in citrate buffer (0.5 M), 55% glycerol and citrate buffer (pH 5.5)]. After boiling for 12 min, the samples’ optical density was measured at 570 nm and the amino acids were calculated (mg g−1dw).
Proline content in wheat leaf tissues was measured by the procedures ascertained by Bates et al. (1973). Proline was extracted by dry tissue homogenization in sulfosalicylic acid aqueous solution (3%). Leaf homogenates were boiled with acid ninhydrin reagent (prepared by warming ninhydrin in glacial acetic acid and phosphoric acid) for 1 h. The resultant chromatophore was extracted by toluene and the absorbance was determined at 520 nm. Proline was quantified as mg g−1dw based on a standard graph prepared using proline.
Cysteine was extracted from leaf powder using perchloric acid (5%) and the supernatant was incubated for 10 min in a boiling water bath with acid ninhydrin reagent. An aliquot of 1 ml ethanol was added to the mixture and the absorbance was determined at 560 nm. A standard graph prepared by cysteine was used for detecting cysteine concentration (nM g−1dw) in wheat samples (Gaitonde 1967).
Estimation of non-enzymatic antioxidant molecules
The content of ascorbic acid in wheat leaves was estimated using the method of Oser (1979). Leaf powder was crushed with 5% aqueous sulfosalicylic acid and centrifugated for 15 min at 8000 rpm. A sample of 1 ml extract was mixed with 5 ml reaction mixture [2% Na-molybdate, 0.15 N H2SO4 and 1.5 mM Na2HPO4 (2:2:1 v/v)] and incubated for 40 min at 60 °C. Ascorbic acid concentration was estimated based on absorbance at 660 nm using ascorbic acid as a standard (μmol g−1dw).
Glycine betaine (GB) level in wheat leaves was extracted by shaking in deionized water for 24 h and estimated by the method ascertained by Grieve and Grattan (1983). The extract was mixed with 2 N HCl (1:1 v/v) then allowed to react with pre-chilled KI-I2 reagent. The mixture was stirred constantly and 1,2 dichloroethane was then used to dissolve the produced periodate crystals and the color intensity was measured at 365 nm. GB content was calculated and expressed as μg g−1dw using a calibration curve constructed with GB.
Total glutathione content was assessed by Sedlak and Lindsay (1968) method. Briefly, 0.1 g fresh wheat leaves were homogenized in 5 ml of 5% sulfosalicylic acid. The reaction mixture comprised 1 ml extract, 1 ml of 200 mM tris buffer (pH 8.2) and 0.1 ml of 100 mM 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB). Methanol (3.9 ml) was added and the reaction vessels were shaken at room temperature for 20 min and the absorbance was determined at 412 nm against standard reduced glutathione (GSH). The total glutathione concentration was represented as nmol g−1fw using the standard curve plotted with GSH.
Total flavonoids were assessed in the alcoholic extract by adding 0.1 ml of 10% AlCl3, 0.1 ml of 1 M K-acetate and 1.5 ml of ethanol (95%) to 0.5 ml extract. The mixture absorbance was read at 417 nm after 30 min against quercetin as a standard flavonoid and total flavonoids were represented as mg g−1dw (Chang et al. 2002).
Total polyphenols in wheat leaves ethanolic extract were quantified by mixing 0.1ml extract with 0.1 ml Folin-Ciocalteu’s reagent and 1 ml 20% Na2CO3 (Jindali and Singh 1975). The mixture absorbance was assessed at 650 nm after 1 h against gallic acid as a standard phenol and the phenolic content was expressed as mg g−1dw.
Antioxidant enzymes assay
Enzyme extract was performed by homogenizing 0.5 fresh leaves in 8 ml of 0.1 M K-phosphate buffer (pH 7). The resultant extract was centrifugated at 10,000 rpm for 30 min using a cooling centrifuge at 4 °C and the supernatant was utilized for six enzymes assay. All the assayed enzyme activity was expressed as μM g−1fw min−1.
Catalase (CAT, EC1.11.1.6) activity was assayed by monitoring H2O2 decomposition rate at 240 nm (Kato and Shimizu 1987). The reaction mixture comprised 0.15 mM H2O2 in 50 mM K-phosphate buffer (pH 7). The initiation of the reaction was made by adding 0.1 ml enzyme extract to the reaction mixture and the absorbance decline was monitored at 240. The activity of CAT was calculated using the extinction coefficient of 40 mM−1 cm−1.
The increased absorption following guaiacol oxidation by guaiacol peroxidase (POD, EC 1.11.1.7) in the presence of H2O2 and the subsequent formation of tetraguaiacol was the basis of POD assay using Kato and Shimizu (1987) method. Initiation of the reaction was performed by adding 0.1 ml enzyme extract to the reaction mixture (11.8 mM H2O2, 7.2 mM guaiacol and100 mM K-phosphate buffer pH 5.8). The activity of POD was calculated by using the extinction coefficient of 26.6 mM−1 cm−1 at 470 nm.
The formation of formazan resulting from the photochemical reduction of nitroblue tetrazolium (NBT) in the existence of superoxide dismutase (SOD, EC 1.15.1.1) was followed to assess the activity of SOD (Beyer and Fridovich 1987). The reaction was initiated by adding the enzyme extract into the reaction mixture (9.9 mM l-methionine, 0.057 mM NBT, 50 mM K-phosphate buffer pH 7.8, 0.025% Triton X-100, and 0.0044% riboflavin) then illumination for 15 min with 30 W fluorescent lamp. The absorbance was measured at 560 nm after switching the light off and the activity of SOD was calculated by the aid of the extinction coefficient of 21.1 mM−1 cm−1.
The technique prescribed by Nakano and Asada (1981) was employed for assaying the activity of ascorbate peroxidase (APX, EC 1.11.1.11). The decline in H2O2 absorption in the reaction mixture due to ascorbate-dependent oxidation was monitored at 290 nm. An aliquot of the enzyme extract was added to 3 ml of the reaction mixture (0.5 mM ascorbic acid, 0.2 mM EDTA in 5 mM K-phosphate buffer pH 7 and 0.25 mM H2O2). The absorbance was observed at 290 nm and APX activity was calculated by using 2.8 mM−1 cm−1 as an extinction coefficient.
Total glutathione reductase (GR, EC 1.6.4.2) activity was assayed via monitoring the rate of NADPH oxidation in the existence of oxidized glutathione (GSSG) based on the procedures of Halliwell and Foyer (1978). The reaction was initiated by adding the enzyme extract to the reaction mixture (0.5 mM NADPH, 0.5 mM GSSG, 2 mM EDTA and 20 mM K-phosphate buffer pH 7.5). The decrease in NADPH absorbance at 340 nm was recorded and the extinction coefficient of 6.2 mM−1 cm−1 was used in calculating GR activity.
Polyphenol oxidase (PPO, EC1.10.3.1) catalyzes the oxidation of gallic acid into purpurogallin. The method of Kumar and Khan (1982) was employed for PPO assay by adding 0.5 ml of the enzyme extract into the reaction mixture (2 mM pyrogallol in 0.1 M K-phosphate buffer pH 6). The mixture then incubated at 25 °C for 5 min and the reaction was terminated by adding sulfuric acid (2.5 N). Purpurogallin absorbance was monitored at 420 nm and the activity of PPO was determined from the extinction coefficient of 26.40 M−1 cm−1.
Mineral analysis
A mixture of 70% HNO3 and 30% H2O2 (5:3 v/v) was used in the wet digestion of wheat leaf samples. The concentration of K, Ca, Mg and Na in the digested samples was quantified at Tanta University Central Lab by an inductively coupled plasma-optical spectrophotometer (Polyscan 61 E, Thermo Jarrell-Ash Corp., Franklin, MA, USA). Nitrogen was assayed calorimetrically by Rochelle reagent using NH4Cl as a standard and phosphorus was assayed by molybdenum blue reagent using KH2PO4 as a standard according to Allen et al. (1974).
Statistical analysis
The experimental data were represented as mean ± SD (standard deviation) of three independent replications. One-way analysis of variance (ANOVA) was applied onto the results using SPSS software (V 20). The least significant difference (LSD) at 5% level was used to determine the significant differences between means. P < 0.05 was considered significant unless otherwise specified.
Results
Growth characteristics
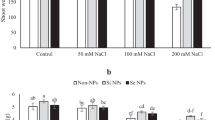
The decreased growth rate in wheat as a repercussion of salt exposure and the promoting effects of sulfur nanoparticles (SNPs) are prescribed in Fig. 1. The data showed that shoot system traits (height, fresh weight, dry weight and leaf area) and the root system traits (depth, fresh weight and dry weight) were progressively declined with increasing salt level, as the high dose of salt (200 mM) caused more deteriorations than the low one (100 mM) in all measured growth parameters, contrasted with the control treatment. Meanwhile, the results illustrated that the priming of wheat grains in 100 μM SNPs resulted in a significant enhancement in the growth rate of salt-stressed wheat plants. The alleviatory effect of SNPs was more evident with the low salt concentration, as in many cases it restored the growth rate to values close to those of the control treatment.
Photosynthetic pigments and photosynthetic performance (Fv/fm)
Data in Fig. 2 illustrated that salt treatments significantly decreased chlorophylls and carotenoids contents in wheat leaves. Chlorophyll a was more sensitive to the higher dose of NaCl, however chlorophyll b and carotenoids were significantly affected by the lower dose of NaCl, as compared to the control. Nevertheless, priming wheat seeds in 100 μM SNPs enhanced the photosynthetic pigments content of salt-stressed wheat. The improvement in pigments content due to SNPs was more apparent with the low concentration (100 mM) of NaCl, as it retrieved pigments level into values close to that of the control. Regarding photosynthetic performance (Fv/fm) of dark-adapted leaves, no significant change was monitored between salt and SNPs treatments, compared to the control, where all treatments slightly decreased Fv/fm ratio, except 100 mM NaCl treatment, which gave a value close to that of the control.
Stress biomarkers
Electrolyte leakage (as a measure of membrane integrity), MDA (as a measure of lipid peroxidation) and H2O2 (as a measure of oxidative stress) were significantly increased in the leaves of wheat exposed to salt stress, compared to control values. The detrimental effect of salt was more apparent at the high salt dosage (Fig. 3). The results with SNPs revealed a discernible recovery impact and lessened the stress biomarkers level in salt-stressed wheat leaves. This treatment declined membrane leakage, MDA and H2O2 to values close to the un-stressed control, particularly MDA at the lower NaCl dose (100 mM).
Nitrogenous compounds
The contents of total soluble protein, free amino acids and free cysteine of wheat leaves were incredibly declined by salt treatments. Supplying the low (100 mM) NaCl dosage showed a marked decrease (30.8%) in protein content, meanwhile the higher dose (200 mM) resulted in 14.6 and 43.3% shrinkage in the pool of amino acids and cysteine, respectively (Fig. 4). However, stressed plants grown under SNPs showed higher protein and cysteine accumulation, though amino acids level was less or more affected by the applied SNPs, compared to single stress treatments. On the other hand, proline showed higher levels as a function of salt concentration, compared to the control treatment. Nonetheless, free proline showed a reliable decrease following the combination of SNPs and salt treatments.
Non-enzymatic antioxidants
Analysis of non-enzymatic antioxidant compounds content of wheat leaves showed highly significant variations in response to experimental treatments. Application of NaCl significantly decreased ascorbic acid, flavonoids and total phenolic compounds levels, while seed priming in 100 μM greenly synthesized SNPs restored the level of the aforesaid compounds to values relatively close to that of the control, particularly total phenols (Fig. 5). On the other side, glycine betaine and total glutathiones were markedly increased in the leaves of salt-stressed wheat. Interestingly, priming with SNPs promoted higher levels of glutathiones, compared to control and stress treatments; while glycine betaine remained unchanged.
Antioxidant enzymes
The activities of catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR) and polyphenol oxidase (PPO) were significantly affected by salt stress and the priming in SNPs solution (Fig. 6). The activities of CAT, POD, SOD, APX and PPO were induced by salt application as compared to the control treatment, but their activities were significantly reduced under the influence of priming in SNPs. Conversely, GR activity was reduced because of the adverse effect of salt stress but significantly increased by SNPs pre-treatment comparable with control activity. Accordingly, the activities of all enzymes, except GR, were increased as affected by NaCl, but an evident decline was attained in their activities as a result of priming with SNPs.
Elemental content of wheat leaves
The contents of N, P, K, Ca, Mg and Na, as well as K/Na ratio in the leaves of wheat grown under salt stress and SNPs were estimated and expressed as mg g−1dw (Table 1). Treatment with NaCl resulted in an extensive accumulation of Na ions in wheat leaves as compared to the control. Comparable with salt treatments, priming in 100 μM SNPs distinctly declined Na level in the wheat leaves to values fairly close to that of the control. Likewise, the levels of Ca and Mg were increased as affected by salt treatments, but the application of SNPs lowered their accumulation to values more or less close to the control. Contrastingly, N, P and K contents, as well as the ratio of K/Na, were obviously decreased after wheat exposure to salinity. Wheat priming with SNPs significantly increased the uptake of N, P and K into the leaves of Na-stressed wheat, promoting better ionic homeostasis along with enhanced growth.
Discussion
Commonly, after wheat exposure to salt stress, the growth pattern is harshly reduced (Shafiq et al. 2020). This response could be attributed to the ionic and osmotic disturbances imposed as a consequence of salinization (Ilangumaran and Smith 2017). In accord with this, we recorded growth inhibition of wheat as affected by salt treatments throughout this study. Moreira et al. (2020) ascribed growth decline by salinity to the increased toxicity caused by Na+ ions which disrupt the ionic balance by competing with the uptake of essential ions like K+. In this regard, Kasim et al. (2016) clarified that salinity disrupts the photosynthesis process, cellular turgidity, stomatal opening and hydraulic status causing reduced growth rate. Despite the detrimental consequences of NaCl stress on wheat growth, the tested concentration of sulfur nanoparticles (SNPs) was capable of alleviating these effects. Because of their microscopic size and larger surface area, SNPs are swiftly absorbed and assimilated into organo-sulfur compounds guaranteeing effective growth (Ragab and Saad-Allah 2020). Earlier, we proclaimed that seed priming with sulfur nanoparticles (SNPs) decreased Mn toxicity in sunflower. Owing to its role in plant growth, sulfur was reported to be integrated into the structure of many metabolically active compounds, comprising methionine, cysteine, 5-adenylyl sulfate, glutathione, coenzyme A and sulfur-containing proteins (Marschner 2011). Riffat and Ahmad (2018) elucidated the role of sulfur in salt-stressed plants by osmolyte homeostasis in addition to modulating the antioxidative machinery and signaling pathways, offering anti-stress defense, permitting better growth under salt stress.
Regarding chlorophyll and carotenoids, our results reveal an intensive decline in their content by salt stress. This result could be explicated by the stimulated-pigment breakdown and surplus production of ROS (Shafiq et al. 2020). Although chlorophyll content is reduced by salt exposure, the salt tolerance of the plant species is the criterion determining the reduction extent. In this context, chlorophyll has been considered as a biochemical marker of salt tolerance in different plants (Akram and Ashraf 2011). Carotenoids have been found to participate significantly in protecting against stress injury. Aside from opposing photooxidative damage of chlorophyll, carotenoids offer signaling mission under abiotic stress and protect cellular membranes against oxidative damage (Verma and Mishra 2005). The salt-induced decrease in carotenoids content was explained by the impairment of thylakoid membranes; the site of photosynthetic pigments attachment, and chloroplast membrane by ROS-induced lipid peroxidation (Sayyad-Amin et al. 2016). Based on the results of this study, carotenoids were not one of the main protection techniques offered by wheat under salinity conditions.
The usage of SNPs substantially increased photosynthetic pigments content in the salt-stressed wheat leaves, reflecting improvement in the growth rate as previously reported in sunflower subjected to Mn stress (Ragab and Saad-Allah 2020). The restorative effect of SNPs on photosynthetic pigments was ascribed to their interaction with the other organic molecules forming organo-sulfur compounds, inducing chlorophyll biosynthesis by the leaves (Salem et al. 2016). Lu et al. (2019) declared that SNPs restore water balance in the stressed plants allowing them to avoid osmotic and ionic imbalances leading to improved photosynthetic capacity. Furthermore, Ragab and Saad-Allah (2020) confirmed that SNPs sustained a high level of Mg in the stressed plants lessening the deleterious impact on chlorophyll biosynthesis.
The loss of membrane integrity and the increase in H2O2 and MDA concentrations as a common response to salt stress is a marker of oxidative stress. In the current experiment, salt stress increased electrolytes leakage and induced the overproduction of H2O2 and MDA in wheat leaves. Similar results have been reported in various crop species (Baniasadi et al. 2018; Sen et al. 2020; Shafiq et al. 2020). The substantial accumulation of MDA and H2O2 upon exposure to salinity was explained by the inadequate detoxification and antioxidant systems offered by the plant (Hossain et al. 2011). Likewise, the induced accumulation of H2O2 and MDA could be explained by the obtained deficiency in the non-enzymatic antioxidant molecules (ascorbate, flavonoids, phenolics and carotenoids) and the increasing cascade of ROS upon salt exposure. Our study demonstrates that priming with SNPs significantly augmented oxidative damage caused by salt in wheat and then decreased electrolytes leakage, H2O2 and MDA concentration in salt-stressed plants. In an analogous study on lettuce, Najafi et al. (2020) reported a distinct decline in H2O2 and MDA concentration, decreased oxidative damage and improved growth by using SNPs.
The incorporation of nitrogen into the metabolic pathways is directly affected by stress conditions. The current study results showed a marked decrease in protein, amino acids and cysteine contents in salt-stressed wheat leaves. The decreased protein level as affected by salt stress was attributed to the disturbance in the main pathways offering carbon skeleton for their biosyntheses like glycolysis, Krebs cycle and pentose shunt pathway (Li et al. 2015). Reduced protein level might be correlated with depleting gene regulation and signal transduction pathways, consequently impaired biosynthetic pathway (Muneer et al. 2014) and/or protein degradation via incorrect protein folding and assembly (Nam et al. 2012). The decreased content of free amino acids could be explained by the deprived nitrogen supply and carbon skeleton required for amino acids biosynthesis as affected by salinity. Moreover, the decline in cysteine content upon salt stress might be ascribed to the involvement of sulfur in non-enzymatic antioxidants like glutathiones to cope with the excessive ROS accumulation.
An increase was observed upon priming with SNPs in the concentration of proteins, amino acids and cysteine in salt-stressed wheat leaves. The increased nitrogen metabolism by SNPs might be explained by their role in balancing nitrogen uptake, sustaining carbon skeleton, protecting nitrogen assimilating enzymes and upregulating sulfur metabolism (Ragab and Saad-Allah 2020). The increased protein, amino acids and cysteine contents provide osmoregulation and antioxidant defense to the stressed plants (Wu et al. 2004). The high cysteine level was reported to amend growth inhibition caused by salinity by modulating redox homeostasis (Genisel et al. 2014).
Proline is a main osmolyte that effectively counteracts the oxidative damage generated by salt stress. The salt-induced increase in the level of proline reported in this study was proposed in several crop species (Saad-Allah 2015; Kasim et al. 2016; Sen et al. 2020). Proline act as a low molecular weight osmolyte, performing vital roles in stressed plants as stabilizing proteins and enzymes structure, scavenging of ROS, sustaining membrane integrity and stabilizing PSII complex (Szabados and Savouré 2010). Controversially, SNPs decreased proline accumulation in salt-stressed wheat. This effect could be ascribed to the additional osmoprotectant and antioxidant potentials offered by SNPs, hence there is no need for further proline production in the presence of SNPs.
Our results revealed a decreased level of ascorbic acid, flavonoids, and phenolic compounds under salt stress; but following SNPs application retrieved their normal levels. These antioxidant molecules fulfill imperative roles in plant growth and development. Ascorbic acid performs various roles in plants, as it stimulates ROS scavenging, cell division, cell elongation and signal transduction (Pignocchi and Foyer 2003). Flavonoids are complex polyphenols that perform a wide range of biological tasks as inhibiting lipid peroxidation, ROS scavenging, metals chelation and protection against UV (Di Ferdinando et al. 2012). Also, phenolic compounds perform several roles in plants including contribution in the cell wall structure, protection against oxidative damage and regulation of growth (Cheynier et al. 2013). The decrease in ascorbic acid, flavonoids, and phenolic compounds content next to salinity exposure reveal salt-induced inhibition in their biosynthesis and point to the dependence of wheat on the enzymatic antioxidants along with other non-enzymatic molecules. Nonetheless, SNPs eliminated salt-induced inhibition and restored the level of the above-mentioned molecules to more or less their normal values. Elicitation of nanoparticles to the non-enzymatic antioxidants has been previously reported by silver in marigold (Ghanati and Bakhtiarian 2014), by copper in ashwagandha (Singh et al. 2018b) and by selenium in lemon balm (Babajani et al. 2019). The enhanced production in phenolic compounds, flavonoids and ascorbic acid in salt-stressed wheat through priming with SNPs could be attributed to its involvement in the upregulation of their biosynthetic pathway (Babajani et al. 2019). However, more research is needed on the molecular level to give better understanding of SNPs involvement in the alleviation of salinity in wheat plants.
Wheat leaves exhibited a significant increase in the activity of the antioxidant enzymes (CAT, POD, APX, SOD and PPO) with salt exposure, only GR showed reduced activity with salinity. Enhanced antioxidant enzymes activity enable plants to eliminate the toxic levels of ROS and lower lipid peroxidation of cellular membranes under salinity. CAT, APX and GR substantially decrease superoxide and hydrogen peroxide levels in salt-exposed plants (Kaymakanova and Stoeva 2008). de Azevedo-Neto et al. (2006) reported that CAT and APX are the key enzymes regulating H2O2 cellular levels preventing cellular damage. Also, SOD is the main scavenger for superoxide radical, while the formed H2O2 is then scavenged by CAT, POD and APX.
Increased SOD activity as a consequence of salt stress was attributed to the accelerating dismutation of superoxide anions generated upon salt-treatment (Desingh and Kanagaraj 2007). Moreover, the induction of CAT, POD and APX activities was ascribed to their role in detoxifying H2O2 (Gupta and Gupta 2005). The recorded decrease in GR activity is in accordance with those obtained by Khan and Patra (2007); Nagesh Babu and Devaraj (2008) and Chawla et al. (2013). The increased activity of antioxidant enzymes has been ascribed to either increased activity of already present enzymes or upregulation of genes encoding these enzymes (Chawla et al. 2013). However, these induced antioxidant enzymes considered with no computable and effective role, as the oxidative stress (membrane leakage, MDA and H2O2) remained at a strict level influencing plant normal growth and homeostasis.
In the present study, SNPs significantly declined antioxidant enzymes activity, except for GR, in salt-stressed wheat leaves. The decrease in antioxidant enzymes activity by nanoparticles had been early reported by ZnNPs in chickpea (Burman et al. 2013) and AgNPs in water hyssop (Krishnaraj et al. 2012). The decreased antioxidant enzymes activity by SNPs treatment reflects enhanced growth with reduced toxicity under saline conditions. The role and mechanism of action of SNPs in regulating enzymatic activities of plants under stress conditions are complex and remain controversial. More research is required owing this issue to elucidate their role at various conditions including stress type, severity and duration, in addition to crop type, developmental stage and mode of their application. Further studies would map out the role involved in growth endorsement mediated by SNPs.
The decreased N, P and K contents reported in wheat leaves after salt exposure was formerly reported by Zhu (2001) and Tavakkoli et al. (2011). The lower N content was proposed to be a consequence of the antagonism between Na+ and NH4+ or between Cl− and NO3− (Bar et al. 1997). Also, the salt-induced decline in P content was reported to be associated with the decreased PO43− solubility and activity in saline medium (Qadir and Schubert 2002). Suhayda et al. (1990) explained the cause of K+ decrease under saline conditions by the antagonistic effect of Na+ on K+ sites in the root. Unexpectedly, Ca and Mg concentrations were increased in salinized wheat leaves. Dogan et al. (2010) stated that salt-tolerant tomato accumulated more Ca by salt exposure. Such episode provides salinity tolerance via sustaining membrane integrity, wall stabilization and activation of wall enzymes. Likewise, Talaat and Shawky (2014) explored the effect of salt stress on different wheat genotypes and affirmed salinity induced Mg accumulation in all genotypes. Zheng et al. (2010) explained the increased Mg magnitude in saline conditions to its imperative role in osmotic balance. Nevertheless, the increased Na uptake impaired other essential ions uptake, particularly K, lead to reduced K/Na ratio and disrupt osmotic adjustment (Hashem et al. 2016).
Salt-stressed and pre-treated with SNPs wheat showed enhanced levels of N, P and K, however the accumulation of Ca, Mg and Na was diminished to values close to those of the control. Ragab and Saad-Allah (2020) proposed that SNPs retrieved the stress-mediated damage to the cellular membranes, consequently improved mineral content under stress conditions through increasing K/Na ratio, allowing the subservient uptake of other beneficial minerals. Moreover, Rais and Masood (2013) concluded that nitrate reductase activity and N uptake were regulated by the increased S availability.
Conclusion
In conclusion, salt promoted a decrease in the growth of wheat is correlated with increased oxidative stress, reduced photosynthetic pigments and loss of membrane integrity. Salt exposure increased H2O2 accumulation, lipid peroxidation (MDA) and solute efflux through cellular membranes. Besides, salt stress resulted in decreased nitrogen assimilation and non-enzymatic antioxidant molecules accumulation but increased the activity of the antioxidant enzymes. These consequences are directly correlated with ionic disturbances in wheat. The study results exposed that SNPs amended NaCl detrimental consequences on wheat through decreasing oxidative stress injury, sustaining membrane fluidity, triggering antioxidant status and inducing ionic homeostasis, contributing to enhanced growth. However, the conclusion needs to be validated by further experimental studies for comparing the alleviatory effect of the bulk S (Na2SO3) used in the preparation process and SNPs.
References
Abbas M, Arshad M, Nisar N, Ghaffar A, Nazir A, Asif Tahir M, Iqbal M (2017) Muscilage characterization, biochemical and enzymatic activities of laser irradiated Lagenaria siceraria seedlings. J Photochem Photobiol B Biol 173:344–352
Abd El-Moneim D, Alqahtani MM, Abdein MA, Germoush MO (2020) Drought and salinity stress response in wheat: physiological and TaNAC gene expression analysis in contrasting Egyptian wheat genotypes. J Plant Biotechnol 47:1–14
Abdel-Fattah GM, Asrar A-WA (2012) Arbuscular mycorrhizal fungal application to improve growth and tolerance of wheat (Triticum aestivum L.) plants grown in saline soil. Acta Physiol Plant 34:267–277
Abo-Hamad S, Saad-Allah K, Abo-Kassem E (2013) Effect of gamma irradiation or potassium on some primary and secondary metabolites of Brassica rapa (L.) root under cadmium stress. Int Res J Agric Sci Soil Sci 3:408–415
Agami RA, Alamri SAM, Abd El-Mageed TAA, Abousekken MSM, Hashem M (2018) Role of exogenous nitrogen supply in alleviating the deficit irrigation stress in wheat plants. Agric Water Manag 210:261–270
Akram MS, Ashraf M (2011) Exogenous application of potassium dihydrogen phosphate can alleviate the adverse effects of salt stress on sunflower. J Plant Nutr 34:1041–1057
Al Banna LS, Salem NM, Jaleel GA, Awwad AM (2020) Green synthesis of sulfur nanoparticles using Rosmarinus officinalis leaves extract and nematicidal activity against Meloidogyne javanica. Chem Int 6:137–143
Allen SE, Grimshaw HM, Parkinson JA, Quarmby C (1974) Chemical analysis of ecological materials. Blackwell Scientific, New York
Amer MW, Awwad AM (2021) Green synthesis of copper nanoparticles by Citrus limon fruits extract , characterization and antibacterial activity. Chem Int 7:1–8
Arora NK, Fatima T, Mishra I, Verma M, Mishra J, Mishra V (2018) Environmental sustainability: challenges and viable solutions. Environ Sustain 1:309–340
Asghar T, Iqbal M, Jamil Y, Zia-ul-Haq NJ, Shahid M (2017) Comparison of He-Ne laser and sinusoidal non-uniform magnetic field seed pre-sowing treatment effect on Glycine max (Var 90-I) germination, growth and yield. J Photochem Photobiol B Biol 166:212–219
Awwad AM, Amer MW, Salem NM, Abdeen AO (2020a) Green synthesis of zinc oxide nanoparticles (ZnO-NPs) using Ailanthus altissima fruit extracts and antibacterial activity. Chem Int 6:151–159
Awwad AM, Salem NM, Aqarbeh MM, Abdulaziz FM (2020b) Green synthesis, characterization of silver sulfide nanoparticles and antibacterial activity evaluation. Chem Int 6:42–48
Babajani A, Iranbakhsh A, Ardebili ZO, Eslami B (2019) Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ Sci Pollut Res 26:24430–24444
Baniasadi F, Saffari VR, Maghsoudi Moud AA (2018) Physiological and growth responses of Calendula officinalis L. plants to the interaction effects of polyamines and salt stress. Sci Hortic 234:312–317
Bar Y, Apelbaum A, Kafkafi U, Goren R (1997) Relationship between chloride and nitrate and its effect on growth and mineral composition of avocado and citrus plants. J Plant Nutr 20:715–731
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burman U, Saini M, Kumar P (2013) Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol Environ Chem 95:605–612
Capaldi FR, Gratão PL, Reis AR, Lima LW, Azevedo RA (2015) Sulfur metabolism and stress defense responses in plants. Trop Plant Biol 8:60–73
Chang CC, Yang MH, Wen HM, Chern JC (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182
Chawla S, Jain S, Jain V (2013) Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.). J Plant Biochem Biotechnol 22:27–34
Cheynier V, Comte G, Davies K, Martens L, Martens S (2013) Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol Biochem 72:1–20
de Azevedo-Neto AD, Prisco JT, Enéas-Filho J, Abreu CE, Gomes-Filho E (2006) Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot 56:87–94
Desingh R, Kanagaraj G (2007) Influence of salinity stress on photosynthesis and antioxidative systems in two cotton varieties. Gen Appl Plant Physiol 33:221–234
Di Ferdinando M, Brunetti C, Fini A, Tattini M (2012) Flavonoids as antioxidants in plants under abiotic stresses. In: Abiotic stress responses in plants. Springer, New York, pp 159–179
Djanaguiraman M, Belliraj N, Bossmann SH, Prasad PVV (2018) High-temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS Omega 3:2479–2491
Dogan M, Tipirdamaz R, Demir Y (2010) Salt resistance of tomato species grown in sand culture. Plant Soil Environ 56:499–507
Faraji J, Sepehri A (2019) Ameliorative effects of TiO2 nanoparticles and sodium nitroprusside on seed germination and seedling growth of wheat under PEG-stimulated drought stress. J Seed Sci 41:309–317
Gaitonde MK (1967) A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J 104:627–633
Genisel M, Erdal S, Kizilkaya M (2014) The mitigating effect of cysteine on growth inhibition in salt-stressed barley seeds is related to its own reducing capacity rather than its effects on antioxidant system. Plant Growth Regul 75:187–197
Ghanati F, Bakhtiarian S (2014) Effect of methyl jasmonate and silver nanoparticles on production of secondary metabolites by Calendula officinalis L (Asteraceae). Trop J Pharm Res 13:1783–1789
Grieve CMM, Grattan SRR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Gupta S, Gupta NK (2005) High temperature induced antioxidative defense mechanism in seedlings of contrasting wheat genotypes. Indian J Plant Physiol 10:73–75
Halliwell B, Foyer CH (1978) Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography. Planta 139:9–17
Hashem A, Abd-Allah EF, Alqarawi AA, Egamberdieva D (2016) Bioremediation of adverse impact of cadmium toxicity on Cassia italica Mill by arbuscular mycorrhizal fungi. Saudi J Biol Sci 23:39–47
Heath RLRL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hossain MA, Hasanuzzaman M, Fujita M (2011) Coordinate induction of antioxidant defense and glyoxalase system by exogenous proline and glycinebetaine is correlated with salt tolerance in mung bean. Front Agric China 5:1–14
Igwe OU, Nwamezie F (2018) Green synthesis of iron nanoparticles using flower extract of Piliostigma thonningii and their antibacterial activity evaluation. Chem Int 4:60–66
Ilangumaran G, Smith DL (2017) Plant growth promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front Plant Sci 8:1768
Iqbal N, Khan NA, Nazar R, da Silva JAT (2012) Ethylene-stimulated photosynthesis results from increased nitrogen and sulfur assimilation in mustard types that differ in photosynthetic capacity. Environ Exp Bot 78:84–90
Jindali KK, Singh RN (1975) Phenolic content in male and female Carica papaya: a possible physiological marker for sex identification of vegetative seedlings. Physiol Plant 33:104–107
Kasim WA, Saad-Allah KM, Hamouda M (2016) Seed priming with extracts of two seaweeds alleviates the physiological and molecular impacts of salinity stress on radish (Raphanus sativus). Int J Agric Biol 18:653–660
Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves; phenolic-dependent peroxidative degradation. Can J Bot 65:729–735
Kaymakanova M, Stoeva N (2008) Physiological reaction of bean plants (Phaseolus vulg. L.) to salt stress. Gen Appl Plant Physiol 34:177–188
Kehinde DO, Ogunwenmo KO, Ajeniya B, Ogunowo AA, Onigbinde AO (2017) Effect of X-ray irradiation on growth physiology of Arachis hypogaea (Var. Kampala). Chem Int 3:296–300
Khan MH, Patra HK (2007) Sodium chloride and cadmium induced oxidative stress and antioxidant response in Calamus tenuis leaves. Indian J Plant Physiol 12:34–40
Krishnaraj C, Jagan EG, Ramachandran R, Abirami SM, Mohan N, Kalaichelvan PT (2012) Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochem 47:651–658
Kumar KB, Khan PA (1982) Peroxidase and polyphenol oxidase in excised ragi (Eleusine corocana cv PR 202) leaves during senescence. Indian J Exp Biol 20:412–416
Lee YYP, Takahashi T (1966) An improved colorimetric determination of amino acids with the use of ninhydrin. Anal Biochem 14:71–77
Li W, Zhao F, Fang W, Xie D, Hou J, Yang X, Zhao Y, Tang Z, Nie L, Lv S (2015) Identification of early salt stress responsive proteins in seedling roots of upland cotton (Gossypium hirsutum L.) employing iTRAQ-based proteomic technique. Front Plant Sci 6:732
Lu Y, Wang Q, Li J, Xiong J, Zhou L, He S, Zhang J, Chen Z, He S, Liu H (2019) Effects of exogenous sulfur on alleviating cadmium stress in tartary buckwheat. Sci Rep 9:1–12
Marschner H (2011) Marschner’s mineral nutrition of higher plants. Academic Press, Amsterdam
Metzner H, Rau H, Senger H, Sieprawska A, Filek M, Tobiasz A, Walas S, Dudek-Adamska D, Grygo-Szymanko E (1965) Trace elements’ uptake and antioxidant response to excess of manganese in in vitro cells of sensitive and tolerant wheat. Planta 65:186–194
Moreira H, Pereira SIA, Vega A, Castro PML, Marques APG (2020) Synergistic effects of arbuscular mycorrhizal fungi and plant growth-promoting bacteria benefit maize growth under increasing soil salinity. J Environ Manag 257:109982–109993
Muneer S, Park YG, Manivannan A, Soundararajan P, Jeong BR (2014) Physiological and proteomic analysis in chloroplasts of Solanum lycopersicum L. under silicon efficiency and salinity stress. Int J Mol Sci 15:21803–21824
Nagesh Babu R, Devaraj VR (2008) High temperature and salt stress response in French bean (Phaseolus vulgaris). Aust J Crop Sci 2:40–48
Najafi S, Razavi SM, Khoshkam M, Asadi A (2020) Effects of green synthesis of sulfur nanoparticles from Cinnamomum zeylanicum barks on physiological and biochemical factors of lettuce (Lactuca sativa). Physiol Mol Biol Plants 26:1055–1066
Nakajima T, Kawano Y, Ohtsu I, Maruyuama-Nakashita A, Allahham A, Sato M, Sawada Y, Hirai MY, Yokoyama T, Ohkama-Ohtsu N (2019) Effects of thiosulfate as a sulfur source on plant growth, metabolites accumulation and gene expression in arabidopsis and rice. Plant Cell Physiol 60:1683–1701
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nam MH, Huh SM, Kim KM, Park WW, Seo JB, Cho K, Kim DY, Kim BG, Yoon I (2012) Comparative proteomic analysis of early salt stress-responsive proteins in roots of SnRK2 transgenic rice. Proteome Sci 10:25
Naseer A, Ali A, Ali S, Mahmood A, Kusuma HS, Nazir A, Yaseen M, Khan MI, Ghaffar A, Abbas M, Iqbal M (2020) Biogenic and eco-benign synthesis of platinum nanoparticles (Pt NPs) using plants aqueous extracts and biological derivatives: environmental, biological and catalytic applications. J Mater Res Technol 9:9093–9107
Oser BL (1979) Hawk’s physiological chemistry, 14th edn. McGraw-Hills, New York
Pignocchi C, Foyer CH (2003) Apoplastic ascorbate metabolism and its role in the regulation of cell signalling. Curr Opin Plant Biol 6:379–389
Qadir M, Schubert S (2002) Degradation processes and nutrient constraints in sodic soils. Land Degrad Dev 13:275–294
Ragab GA, Saad-Allah KM (2020) Green synthesis of sulfur nanoparticles using Ocimum basilicum leaves and its prospective effect on manganese-stressed Helianthus annuus (L.) seedlings. Ecotoxicol Environ Saf 191:110242
Rais L, Masood A (2013) Sulfur and nitrogen co-ordinately improve photosynthetic efficiency, growth and proline accumulation in two cultivars of mustard under salt stress. J Plant Biochem Physiol 1:1–6
Remya V, Abitha V, Rajput P, Rane AV, Dutta A (2017) Silver nanoparticles green synthesis: a mini review. Chem Int 3:165–171
Riffat A, Ahmad MSA (2018) Changes in organic and inorganic osmolytes of maize (Zea mays L.) by sulfur application under salt stress conditions. J Agric Sci 10:543–561
Rizwan M, Ali S, Ali B, Adrees M, Arshad M, Hussain A, ur Rehman M, Waris A (2019) Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214:269–277
Saad-Allah KM (2015) Impact of sea salt stress on growth and some physiological attributes of some soybean (Glycine max L.) varieties. Iran J Plant Physiol 6:1559–1571
Sairam RKR, Srivastava GC, Agarwal S, Meena RCR (2005) Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol Plant 49:85–91
Salem NM, Albanna LS, Awwad AM, Ibrahim QM, Abdeen AO (2015) Green synthesis of nano-sized sulfur and its effect on plant growth. J Agric Sci 8:188–194
Salem NM, Albanna LS, Awwad AM (2016) Green synthesis of sulfur nanoparticles using Punica granatum peels and the effects on the growth of tomato by foliar spray applications. Environ Nanotechnol Monit Manag 6:83–87
Sayyad-Amin P, Jahansooz MR, Borzouei A, Ajili F (2016) Changes in photosynthetic pigments and chlorophyll-a fluorescence attributes of sweet-forage and grain sorghum cultivars under salt stress. J Biol Phys 42:601–620
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Semida WM, Abd El-Mageed TA, Mohamed SE, El-Sawah NA (2017) Combined effect of deficit irrigation and foliar-applied salicylic acid on physiological responses, yield, and water-use efficiency of onion plants in saline calcareous soil. Arch Agron Soil Sci 63:1227–1239
Sen SK, Chouhan D, Das D, Ghosh R, Mandal P (2020) Improvisation of salinity stress response in mung bean through solid matrix priming with normal and nano-sized chitosan. Int J Biol Macromol 145:108–123
Shafiq F, Iqbal M, Ashraf MA, Ali M (2020) Foliar applied fullerol differentially improves salt tolerance in wheat through ion compartmentalization, osmotic adjustments and regulation of enzymatic antioxidants. Physiol Mol Biol Plants 26:475–487
Siddiqui MH, Al-Whaibi MH, Firoz M, Al-Khaishany MY (2015) Role of nanoparticles in plants. In: Nanotechnology and plant sciences. Springer, New York, pp 19–35
Singh J, Dutta T, Kim K-H, Rawat M, Samddar P, Kumar P (2018a) Green synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol 16:84
Singh OS, Pant NC, Laishram ML, Tewari M, Dhoundiyal R, Joshi K, Pandey CS (2018b) Effect of CuO nanoparticles on polyphenols content and antioxidant activity in ashwagandha (Withania somnifera L. Dunal). J Pharmacogn Phytochem 7:3433–3439
Suhayda CG, Giannini JL, Briskin DP, Shannon MC (1990) Electrostatic changes in Lycopersicon esculentum root plasma membrane resulting from salt stress. Plant Physiol 93:471–478
Suleiman M, Al-Masri M, Al Ali A, Aref D, Hussein A, Saadeddin I, Warad I (2015) Synthesis of nano-sized sulfur nanoparticles and their antibacterial activities. J Mater Environ Sci 6:513–518
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Talaat NB, Shawky BT (2014) Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ Exp Bot 98:20–31
Tavakkoli E, Fatehi F, Coventry S, Rengasamy P, McDonald GK (2011) Additive effects of Na+ and Cl− ions on barley growth under salinity stress. J Exp Bot 62:2189–2203
Tian F, Hou M, Qiu Y, Zhang T, Yuan Y (2020) Salinity stress effects on transpiration and plant growth under different salinity soil levels based on thermal infrared remote (TIR) technique. Geoderma 357:113961
Urva SH, Jamil Y, Haq Z, Mujahid T, Khan A, Iqbal M, Abbas M (2017) Low power continuous wave-laser seed irradiation effect on Moringa oleifera germination, seedling growth and biochemical attributes. J Photochem Photobiol B Biol 170:314–323
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci 151:59–66
Verma S, Mishra SN (2005) Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J Pant Physiol 162:669–677
Wu FB, Chen F, Wei K, Zhang GP (2004) Effect of cadmium on free amino acid, glutathione and ascorbic acid concentrations in two barley genotypes (Hordeum vulgare L.) differing in cadmium tolerance. Chemosphere 57:447–454
Zheng Q, Liu Z, Chen G, Gao Y, Li Q, Wang J (2010) Comparison of osmotic regulation in dehydration- and salinity-stressed sunflower seedlings. J Plant Nutr 33:966–981
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saad-Allah, K.M., Ragab, G.A. Sulfur nanoparticles mediated improvement of salt tolerance in wheat relates to decreasing oxidative stress and regulating metabolic activity. Physiol Mol Biol Plants 26, 2209–2223 (2020). https://doi.org/10.1007/s12298-020-00899-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-020-00899-8