Abstract
The changes in the structure, such as molecular weight, crosslinking density, and gel content, could influence the stability and the processing performance of natural rubber (NR) during storage. Previous works showed that temperature and humidity influence storage performances. Here, we found different coagulation methods could also affect storage behaviors of natural rubber, since coagulation methods could affect the content of non-rubber components. In this article, natural rubber latex was coagulated by three different coagulation methods. Under storage conditions, the content of some non-rubber components, namely the gel content, Mooney viscosity, Wallace initial plasticity (\(P_{0}\)), plasticity retention index (PRI), molecular weight, tensile strength, and crosslinking density of natural rubber were compared and analyzed. The results showed that properties (PRI, Mooney viscosity, and green strength) of NR were higher than that of NR-Salt and NR-Acid after storage; at the same time, PRI, Mooney viscosity, and green strength of NR-Salt were higher than that of NR-Acid after storage. Mooney viscosity and green strength of NR-Salt and NR-Acid showed a tendency for increase in the begin and then decrease. According to the changes before and after storage, we can choose suitable coagulation methods and rubber based on what we need.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural rubber (NR) is a renewable resource obtained from the Hevea brasiliensis trees. NR consists of not only long-chain branched cis-1,4-polyisoprene but also non-rubber components, such as proteins, lipids, and carbohydrates [1]. The excellent performances of NR, such as high elasticity, high tensile strength, high tear strength, and so on [2, 3], make it widely used in aerospace navigation, automotive, aircraft tires, and damping materials [4,5,6]. These are outstanding properties of NR because of its unique strain-induced crystallization capacity [7,8,9,10,11]. Coagulation of NR latex is important to obtain rubber products with excellent properties. The coagulation methods of natural rubber latex mainly include natural coagulation, microbial coagulation, acid coagulation, and mineral salt coagulation. Different coagulation modes affect the properties of NR [12].Contrasting different coagulation methods during storage could help us choose the suitable coagulation method according to our needs.
Storage hardening deteriorates processability for samples and increases processing energy. During the storage process, end functional groups, which are mainly \(\alpha \)-terminal groups, gather in the rubber matrix through hydrogen bonds, microencapsulation, and ionic bonds [13]. They can also form branched structures and network structures, and then the storage hardening appears [14, 15]. Temperature and humidity can influence storage performances of NR. Temperature promotes the chemical interaction of crosslinking or decomposition of molecular chains in air, which makes rubber products sticky and strength decrease. Humidity also have a crucial role in storage hardening of NR, which is able to affect storage hardening and produce mold. Recently, we found that coagulation can also influence storage properties of NR. Different coagulation methods can affect composition and structure of NR, and the composition and structure can influence properties of NR [16].
The changes in structure and components are able to affect storage hardening properties of samples [17]. Chen et al. [14] researched the comparison on properties of acid and microorganisms coagulated natural rubber during storage by analyzing the molecular weight, gel content, Mooney viscosity, Wallace initial plasticity (\(P_{0}\)), and the plasticity retention index (PRI) of NR in different storage time. Liang et al. [18] analyzed molecular structures, dynamic mechanical properties, glass transition temperatures, and crosslinked network structures. NR latex was coagulated by microorganism and acetic acid, respectively. Wang et al. [19] tested the strain sweep, frequency sweep, and stress relaxation of raw natural rubber coagulated by microorganisms and acid with the use of a rubber process analyzer (RPA). On one hand, there are few researches about the comparison on properties of natural, acid, and mineral salt coagulated NR during storage. On the other hand, many researches focused on the comparison of properties macroscopically, but the mechanisms on how variations of substances and microstructures affect the properties in detail are still not very clear.
In this work, we produced samples of natural, acid, and mineral salt coagulated NR, and compared their properties on storage. We also studied the factors which affected their properties of natural, acid, and mineral salt coagulated NR during storage. From this work, a clearer understanding on the storage and the processing of NR is provided.
Experimental
Materials
Fresh natural rubber (NR) latex with 45% dry rubber content was commercially supplied by China Hainan Rubber Industry Group Co., Ltd. (Haikou, China). Sodium dodecyl sulfate (SDS), calcium chloride (\(\hbox {CaCl}_{2}\)), and acetic acid were purchased from Aladdin (Shanghai, China).
Preparation of samples
(a) Natural coagulated NR (NR). About 1500 mL of fresh NR latex was coagulated via forming the film at room temperature environment. The fresh NR latex was poured into a stainless steel plate and left to dry naturally to form film in the open air of the lab. The natural coagulated film is about 2 mm thick. (b) Acid coagulated NR (NR-Acid). About 1500 mL of fresh NR latex was coagulated by adding 300 mL 3% acetic acid. The coagulum was pressed into a crepe by crimping machine into about 2 mm thick, then pressure crepe pieces were soaked and rinsed overnight in running water, and dried in an oven at \(50\,^{\circ }\hbox {C}\) for 6 h, 12 h, 24 h, 36 h, and 48 h, respectively. (c) Mineral salt coagulated NR (NR-Salt). About 1500 mL of fresh NR latex was coagulated by adding 200 mL 0.2% \(\hbox {CaCl}_{2}\) and 40 mL 0.05% SDS. The coagulum was pressed into a crepe by crimping machine into about 2 mm thick. The pressed crepe pieces were soaked and rinsed overnight in running water, and dried in an oven at \(50\,^{\circ }\hbox {C}\) for 6 h, 12 h, 24 h, 36 h, and 48 h, respectively.
Storage experiment
NR, NR-Acid, and NR-Salt samples were put into the programmable constant temperature and humidity testing machine (GT-7005-A2S, Goodtechwill testing instrument company, Qingdao, China) to do accelerated storage experiment. The temperature was \(60\,^{\circ }\hbox {C}\) and the humidity was 30% in the temperature and humidity chamber. Storage times were 0 h, 6 h, 12 h, 24 h, 36 h, and 48 h, respectively. The weight of every type sample with every time point was 170 g when doing accelerated storage experiment.
Characterizations
Wallace initial plasticity (\(P_{0}\)) and the plasticity retention index (PRI)
\(P_{0}\) and PRI were determined according to ISO 2007-1991 and ISO 2930-1995, respectively. The samples were plasticated by roll, and were cut into small discs of \(3.2 \sim 3.6\ \hbox {mm}\). Wallace plastic meter and aging box were used to determine \(P_{0}\) and PRI of samples using the following equation:
\(P_{30}\) is the plasticity value of samples aged at \(140\,^{\circ }\hbox {C}\) for 30 min.
Crosslinking density
The crosslinking density analysis was carried out by nuclear magnetic resonance (VTMR20-010V-T, Shanghai Niumag Co., Ltd., Shanghai, China). The determining temperature was \(100\,^{\circ }\hbox {C}\) and the unvulcanized samples were calculated using XLD-2 model. A sample of appropriate size was placed into a glass tube, stabilized in a magnetic field for a period of time, then tested for its crosslinking density.
Protein content
Measurement of the nitrogen content of NR were performed using a Kjeldahl Auto Analyzer. Protein content was obtained from nitrogen content.
Ester group content
The content of ester group of natural rubber was determined by the intensity ratio of carbonyl group at \(1739\ \hbox {cm}^{-1}\) (C=O) and unsaturated carbon absorbance at \(1664\ \hbox {cm}^{-1}\) (C=C) through FTIR (Spectrum One, PerkinElmer Instrument Co., Ltd., Waltham, MA, USA).
Mooney viscosity
After roll molding, 5 cm round pieces of rubber were cut, and Mooney viscosity was determined according to ISO 289-1-1994 by Mooney viscometer. The temperature of testing was \(100\,^{\circ }\hbox {C}\). The rubber sample was preheated at \(100\,^{\circ }\hbox {C}\) for 1 min, followed by a shear for 4 min to measure the Mooney viscosity.
Molecular weight
The molecular weight of the NR samples was determined by gel-permeation chromatography (Waters 1515 GPC, Waters, USA) with three columns in series and equipped with a differential refractive index detector. Tetrahydrofuran (THF, HPLC grade) was used as an eluent with a flow rate of 1.0 mL/min at \(30\,^{\circ }\hbox {C}\). The rubber samples were dissolved in THF at a concentration of 0.05% (w/v) in THF and filtered through a prefilter and \(0.22\,\upmu \hbox {m}\) membrane filter.
Gel content
The gel content of the samples was determined by the swelling method. About 0.1 g (\(\hbox {m}_{1}\)) of the rubber samples were weighed and cut into pieces, and placed into glass bottles of 50 mL capacity. 30 mL of toluene was poured into the glass bottles and sealed before placing them at room temperature for 72 h to completely dissolve uncrosslinked sections of samples. The resulting samples were centrifuged at 17000 r/min for two hours, and the supernatant was extracted and mixed with 20 mL acetone to separate the gel. After drying the gel, the dried mass (\(\hbox {m}_{2}\)) was measured. The gel content was calculated using Eq. 2:
Mechanical property
Mechanical property was performed on dumbbell-shaped samples (\(75\ \hbox {mm} \times 4\hbox {mm} \times 2\ \hbox {mm}\)) with a length of 20 mm using a Gotech AI-3000 instrument at room temperature according to ISO 37-2005. For uniaxial tensile tests, the extension rate was 500 mm/min. Five specimens were measured for each sample, and the average value and standard deviation were calculated.
Ash and metal ions contents
About 5 g (\(\hbox {m}_{1}\)) rubber sample was weighed and placed in the crucible (\(\hbox {m}_{2}\)). The sample was burned in a muffle furnace at \(600\,^{\circ }\hbox {C}\) until the constant weight was reached. After cooling, the weight of ash and crucible (\(\hbox {m}_{3}\)) was measured. The ash was dissolved by concentrated nitric acid in the crucible and then poured in a 25 mL volumetric flask before adding distilled water. The metal ions content data was collected by an Atomic Absorption Spectrometer (TAS-990 Super AFG, Beijing Purkinje General Instrument Co., Ltd., Beijing, China) in combination with flame method.
Results and discussions
Non-rubber components content of NR
NR latex contains a large number of non-rubber constituents, such as proteins, lipids, carbohydrates, metal ions, and so on. These non-rubber components are presumed to have some roles in controlling the structural change of rubber molecules in latex during storage [20]. Table 1 and Table 2 show the comparison of some main non-components of NR, NR-Acid, and NR-Salt. From the tables, we know that the protein content of NR, NR-Salt, and NR-Acid are 3.11%, 1.97%, and 1.77%, respectively. The protein content of NR is highest and the protein content of NR-acid is the least among the samples. At the same time, the ester group content [21] of NR, NR-Salt, and NR-Acid are 233.31 mmol/kg, 91.57 mmol/kg, and 73.69 mmol/kg, respectively. The ester group content reflects the phosphorus content, therefore, we can conclude that the phosphorus content of NR is maximum and the phosphorus content of NR-Acid is the least among three. From the metal ions content in Table 2, the different kinds of metal ions contents of NR, NR-Acid, and NR-Salt showed a similar tendency, except for the calcium ions (\(\hbox {Ca}^{2+}\)) content. As we used calcium chloride (\(\hbox {CaCl}_{2}\)) solution to coagulate NR, the calcium ions (\(\hbox {Ca}^{2+}\)) content of NR-Salt was the highest.
As we all know, when coagulating NR latex, samples of natural coagulation method need not to be soaked and rinsed, so proteins, lipids, and some metal ions are retained. However, samples of NR-Acid and NR-Salt methods need to be creeped, soaked and washed, resulting in the loss of some non-rubber components, especially proteins and phosphorus. The higher protein and phosphorus content in NR-Salt compared to NR-Acid was due to ionic complex formation between the metal ions and the non-rubber components.
Influence of storage on gel content, crosslinking density, and molecular weight of NR
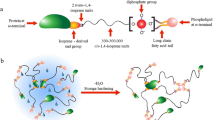
According to previous researches, the \(\omega \)-terminal group and the \(\alpha \)-terminal group of Hevea rubber connected with protein and phospholipid, respectively, and these terminal groups could form supermolecular structure, such as branched structure and gel structure [22,23,24]. Generally, Hevea rubber molecules have 2-4 branched points which form starlike molecular structure. If these Hevea rubber molecules with branched points connected with each other, a spatial network structure will result in formation of a gel. To some extent, the gel content can reflect the crosslinking density. Previous research showed that the formation of gel points and branched points may depend on hydrogen bonds and ionic bonds[25].
Figure 1a shows the comparison of gel content of NR, NR-Acid, and NR-Salt. It is observed that the values of gel content of three coagulation methods increased during storage. This is attributed to storage hardening. To be specific, groups of rubber molecules can be involved by physical and chemical reactions during storage, forming intermolecular or intramolecular crosslinking, resulting in spatial network structures in Hevea rubber [26, 27]. The gel content values of NR-Salt were the highest, whereas the gel contents of NR-Acid were the lowest, and they were reflected in their crosslinking density values. The reasons for this phenomenon are as follows: non-rubber components can act as branched points and crosslinking points with metal ions. Even though the nitrogen content, phosphorus content, acetone extract, and water-soluble substances of NR are higher than that of NR-Acid and NR-Salt, but calcium ions (\(\hbox {Ca}^{2+}\)) of NR-Salt are still much more than other two. Furthermore, calcium ions (\(\hbox {Ca}^{2+}\)) can also act as branched points and crosslinking points, resulting in the gel content of NR-Salt to be the highest.
Figure 2 shows the molecular weights of NR, NR-Acid, and NR-Salt. The molecular weight of NR was observed to increase, going from \(5.10\times 10^{5}\) to \(8.00\times 10^{5}\) Da. This is mainly because of molecular chains crosslink formation with other molecular chains, making the molecular weight larger[28]. The molecular weights of NR-Acid and NR-Salt both show a tendency to increase from 0 to 36 h before decreasing from 36 to 48 h. The molecular weight of NR-Salt was observed to increase from \(2.95\times 10^{5}\) to \(6.45\times 10^{5}\) Da, then decreasing to \(6.02\times 10^{5}\) Da. The molecular weight of NR-Acid was observed to increase from \(2.75\times 10^{5}\) to \(3.97\times 10^{5}\) Da, then decreased to \(3.52\times 10^{5}\) Da. That is because proteins, lipids, metal ions, and some other non-rubber components can help to form gel and some other supermolecule structures which make the molecular weight higher, but the molecular weight of NR-Salt and NR-Acid decrease in the end because of aging.
Influence of storage on the properties of NR
The \(P_{0}\) is the index of plasticity of rubber. As the smelting power consumption of low plasticity rubber increases, operation time is prolonged. The \(P_{0}\) of NR is determined by the bulk viscosity of the material. The higher molecular weight, the higher the viscosity. From Fig. 3a, we can see that \(P_{0}\) of NR, NR-Acid, and NR-Salt increase as the storage time increases, which reveals that the viscosity and the molecular weight increase. This is because of the effects of cross-linking between molecules. The \(\omega \)-terminal groups of NR can interact with proteins through intermolecular hydrogen or ionic bonds [29,30,31,32] and the \(\alpha \)-terminal groups form fulcrum through the micelle or the polar end of the lipids molecular, which increase the molecular weight of NR [25, 33, 34].
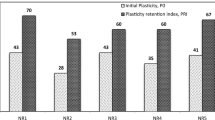
The PRI is an indicator of oxidation resistance and high temperature resistance of raw rubber. Figure 3b shows the relationship between PRI and storage time. The PRI of NR, NR-Acid, and NR-salt decreased with increasing storage time. The PRI of NR shows the highest result, followed by NR-Salt and NR-Acid. Different coagulation methods affect the content of non-rubber components. By comparing nitrogen and phosphorus content, we know that non-rubber components containing proteins and lipids can affect the aging of NR.
The Mooney viscosity reflects the processing properties of rubber, and reflected by the molecular weight (wide and narrow distribution range). Rubber with high Mooney viscosity is not easily mixed evenly and extruded, if its molecular weight is high with a wide distribution range. On the other hand, the resulting plasticity is low. However, if the Mooney viscosity is too low, the physical and mechanical properties of vulcanized products are poor. From the Fig. 4, we can see that with the extension of storage time, the Mooney viscosity of NR shows an increasing trend. The Mooney viscosity of NR goes from 76 to 83 with storage time from 0 to 48 h with a rate increase of 9.21%. The main factor is that the molecular chains are chemically crosslinked to form a new molecular crosslinking network, such as the aldol condensation of crosslinking which occurs in the aldehyde groups of different NR molecular chains, and the fluidity of the molecular chains become worse so that the Mooney viscosity increases. From 0 to 36 h, the Mooney viscosity of NR-Salt increased, and it reduced slightly after 36 h. From 0 to 24 h, the Mooney viscosity of NR-Acid increased, and it is decreased slightly after 36 h. Hardening occurs when NR is stored, causing the increase in Mooney viscosity, but on prolonged storage NR molecular structure will rupture. Therefore, the proteins and lipids are able to improve the degree of storage hardening.
Stress-strain curves of natural, acid, and mineral salt coagulated NR during storage with 0 h, 6 h, 12 h, 24 h, 36 h, and 48 h are shown in Fig. 5. With increasing storage time, the green strength of NR exhibits an increasing trend, as shown in Fig. 5a, whereas for NR-Acid and NR-Salt, their trends are similar whereby from 0 h to 24 h, the green strength increased but decreased from 24 h to 48 h, as shown in Fig. 5b and c. Comparing these three figures, we can see that NR shows higher green strength than NR-Acid and NR-Salt at the same storage time, with the green strength of NR-Salt being higher than NR-Acid in Fig. 5d. As a whole, the green strength increases because of the storage hardening which induces the crosslinking of molecular chains in the beginning, then decreases because of breaks of molecular chains by aging with the presence of temperature, oxygen, and metal ions. As for each corresponding point in time, the green strength of NR was higher than that of NR-Salt because protein content, lipids content and the content of \(\hbox {Mg}^{2+}\), \(\hbox {Cu}^{2+}\), \(\hbox {Zn}^{2+}\), \(\hbox {Mn}^{2+}\), and \(\hbox {Fe}^{2+}\) of NR were higher, although the content of \(\hbox {Ca}^{2+}\) of NR-Salt was much more, we can easily note that proteins and lipids show a more important effect on the structure network than metal ions.
Conclusions
In summary, as storage time prolongs, the gel content and \(P_{0}\) increase, whereas PRI decreases. The molecular weight and Mooney viscosity of NR-Acid and NR-Salt show the similar tendency for increasing from 0 to 36 h, and decreasing from 36 to 48 h, but NR generally increases from 0 to 48 h. As for stress-strain curves, green strength of NR increased from 0 to 48 h, whereas the green strength of NR-Acid and NR-Salt increased from 0 to 24 h, before decreasing from 24 to 48 h. Not only temperature and humidity affect storage properties of NR, but also coagulation methods can affect storage properties of NR. Different coagulation methods influence the content of non-rubber components. Non-rubber components (such as proteins, lipids, and metal ions) play an important role on structures which then affect storage properties. The PRI, Mooney viscosity, and green strength of NR are higher than NR-Salt and NR-Acid. Mooney viscosity and green strength of NR-Salt and NR-Acid showed a tendency for increase in the early stage, and before decreasing. In totally, the research provides a clearer understanding on the storage and processing of NR.
References
Bawn CEH (1963) The chemistry and physics of rubber-like substances. Polymer 5:311
He SJ, Wang YQ, Wu YP, Wu XH, Lu YL, Zhang LQ (2013) Preparation, structure, performance, industrialisation and application of advanced rubber/clay nanocomposites based on latex compounding method. Plast Rubber Compos 39(1):33–42
Wei YC, Liu GX, Zhang HF, Zhao F, Luo MC, Liao S (2019) Non-rubber components tuning mechanical properties of natural rubber from vulcanization kinetics. Polymer 183:121911
Liu Y, Fan Z, Ma H, Tan Y, Qiao J (2006) Application of nano powdered rubber in friction materials. Wear 261(2):225–229
Kookarinrat C, Paoprasert P (2015) Versatile one-pot synthesis of grafted-hydrogenated natural rubber. Iran Polymer J 24(2):123–133
Sato S, Honda Y, Kuwahara M, Kishimoto H, Yagi N, Muraoka K (2004) Microbial scission of sulfide linkages in vulcanized natural rubber by a white rot basidiomycete, ceriporiopsis subvermispora. Biomacromolecules 5(2):511–515
Toki S, Sics I, Ran SF, Liu LZ, Hsiao BS, Murakami S, Senoo K, Kohjiya S (2002) New insights into structural development in natural rubber during uniaxial deformation by in situ synchrotron X-ray diffraction. Macromolecules 35(17):67–77
Huang C, Huang G, Li S, Luo M, Liu H, Fu X, Qu W, Xie Z, Wu J (2018) Research on architecture and composition of natural network in natural rubber. Polymer 154:90–100
Le Gac PY, Albouy PA, Sotta P (2019) Strain-induced crystallization in a carbon-black filled polychloroprene rubber: kinetics and mechanical cycling. Polymer 173:158–165
Chen P, Zhao J, Lin Y, Chang J, Meng L, Wang D, Chen W, Chen L, Li L (2019) In situ characterization of strain-induced crystallization of natural rubber by synchrotron radiation wide-angle X-ray diffraction: construction of a crystal network at low temperatures. Soft Matter 15(4):734–743
Wu X (2015) Natural rubber/graphene oxide composites: effect of sheet size on mechanical properties and strain-induced crystallization behavior. Express Polymer Lett 9(8):672–685
Intapun J, Sainte-Beuve J, Bonfils F, Tanrattanakul V, Dubreucq E, Vaysse L (2010) Effect of microorganisms during the initial coagulum maturation of hevea natural rubber. J Appl Polymer Sci 118(3):1341–1348
Zhang X, Liu J, Zhang Z, Wu S, Zhang L (2018) Toughening elastomers using mussel-inspired multiphase design. ACS Appl Mater Inter 10(28):23485–23489
Chen J, Li SD, Li LF, Wang ZF, Yang L, Zhong JP (2018) Contrastive study on properties of acid and microorganisms coagulated natural rubber during accelerated storage. J Rubber Res 21(1):17–29
Yunyongwattanakorn J, Tanaka Y, Kawahara S, Klinklai W, Sakdapipanich J (2003) Effect of non-rubber components on storage hardening and gel formation of natural rubber during accelerated storage under various conditions. Rubber Chem Technol 76(5):1228–1240
Liu J, Wu S, Tang Z, Lin T, Guo B, Huang G (2015) New evidence disclosed for networking in natural rubber by dielectric relaxation spectroscopy. Soft Matter 11(11):2290–2299
Yunyongwattanakorn J, Sakdapipanich JT, Kawahara S, Hikosaka M, Tanaka Y (2007) Effect of gel on crystallization behavior of natural rubber after accelerated storage hardening test. J Appl Polym Sci 106(1):455–461
Liang Y, Huang MF, Zeng ZQ (2011) Molecular structures and mechanical properties of microbe rapid coagulation natural rubber. Chin J Struct Chem 30(12):1810–1819
Wang Z, Luo W, Li S, Lin F, Liao S, Li L, Hua L, He C (2014) Rheological behavior of raw natural rubber coagulated by microorganisms. Polimeros 24(2):143–148
Tarachiwin L, Sakdapipanich JT, Tanaka Y (2003) Gel formation in natural rubber latex: 1. Effect of (NH4)(2)HPO4 and TMTD/ZnO additives. Rubber Chem Technol 76(5):1177–1184
Nimpaiboon Adun, Sriring Manus, Sakdapipanich Jitladda T (2016) Molecular structure and storage hardening of natural rubber: insight into the reactions between hydroxylamine and phospholipids linked to natural rubber molecule. J Appl Polymer Sci 133(31):1–9
Fuller KNG, Fulton WS (1990) The influence of molecular weight distribution and branching on the relaxation behaviour of uncrosslinked natural rubber. Polymer 31(4):609–615
Kim C, Beuve JS, Guilbert S, Bonfilsb F (2009) Study of chain branching in natural rubber using size-exclusion chromatography coupled with a multi-angle light scattering detector (SEC-MALS). Eur Polymer J 45(8):2249–2259
Luo MC, Jian Z, Xuan F, Huang G, Wu J (2016) Toughening diene elastomers by strong hydrogen bond interactions. Polymer 106:21–28
Tarachiwin L, Sakdapipanich J, Ute K, Kitayama T, Bamba T (2005) Structural characterization of alpha-terminal group of natural rubber. 1. Decomposition of branch-points by lipase and phosphatase treatments. Biomacromolecules 6(4):1858–1863
Tanaka Y (1989) Structure and biosynthesis mechanism of natural polyisoprene. Prog Polymer Sci 14(3):339–371
Tanaka Y, Tarachiwin L (2015) Recent advances in structural characterization of natural rubber. Rubber Chem Technol 82(3):283–314
Zhang H, Zhang L, Chen X, Wang Y, Zhao F, Luo M, Liao S (2020) The role of ron-rubber components on molecular network of natural rubber during accelerated storage. Polymers 12(12):2880
Tanaka Y, Aik-Hwee E, Ohya N, Nishiyama N, Tangpakdee J, Kawahara S, Wititsuwannakul R (1996) Initiation of rubber biosynthesis in Hevea brasiliensis : characterization of initiating species by structural analysis. Phytochemistry 41(6):1501–1505
Mekkriengkrai D, Sakdapipanich JT, Tanaka Y (2006) Structural characterization of terminal groups in natural rubber: origin of nitrogenous groups. Rubber Chem Technol 79(2):366–379
Song L, Zhu T, Yuan L, Zhou J, Zhang Y, Wang Z, Tang C (2019) Ultra-strong long-chain polyamide elastomers with programmable supramolecular interactions and oriented crystalline microstructures. Nat Commun 10(1):1315
Gan SN, Ting KF (1993) Effect of treating latex with some metal ions on storage hardening of natural rubber. Polymer 34(10):2142–2147
Tarachiwin L, Sakdapipanich J, Ute K, Kitayama T, Tanaka Y (2005) Structural characterization of alpha-terminal group of natural rubber. 2. Decomposition of branch-points by phospholipase and chemical treatments. Biomacromolecules 6:1858–1863
Amnuaypornsri S, Sakdapipanich J, Toki S, Hsiao B, Tanaka Y (2008) Strain-induced crystallization of natural rubber: effect of proteins and phospholipids. Rubber Chem Technol 81:753–766
Acknowledgements
The research was funded by the Strategic Priority Research Program of the Chinese Academy of Sciences (no. XDC06010100), Key-Area Research and Development Program of Guangdong Province (no. 2020B020217003), Major Science and Technology Plan Projects of Hainan Province (no. ZDKJ2016020) and Hainan Province Postgraduate Innovation Research Project (no. Hys2019-121).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, X., Zhang, HF., Zhang, L. et al. Insight on natural rubber’s relationship with coagulation methods and some of its properties during storage. J Rubber Res 24, 555–562 (2021). https://doi.org/10.1007/s42464-021-00139-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42464-021-00139-y