Abstract
Natural rubber, one of the widely used renewable resources, is susceptible to degradation upon exposure to sunlight, oxygen, heat and ozone due to the presence of double bonds in cis-polyisoprene. This work describes a versatile means to improve the stability of natural rubber by saturating the double bonds through grafting and hydrogenation reactions as a one-pot method. Graft copolymerization of methyl methacrylate onto natural rubber latex was carried out using emulsion polymerization in the presence of cumene hydroperoxide/tetra-ethylene pentamine mixture as initiator whereas hydrogenation of the natural rubber was performed using the diimide reduction method. The effects of initiator concentration, monomer concentration, reaction temperature, and reaction time on reaction efficiency were investigated. In addition, the thermal, mechanical, ozone ageing, and solvent resistance properties of modified natural rubbers were characterized and found to be superior to the unmodified natural rubber. The decomposition temperature of the grafted-hydrogenated natural rubber is almost 30 °C better than that of the unmodified rubber. The mechanical strength was also improved. In order to test the versatility of this one-pot technique, styrene was also used as a monomer to graft onto the natural rubber. These results show that the one-pot method developed in this work is a simple, versatile means for improving the chemical and physical properties of the natural rubber. This work is also a proof-of-concept for other combinations of reactions or other polymers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural rubber (NR) is one of the renewable resources obtained from the rubber tree Hevea brasiliensis and used for a variety of applications due to its attractiveness in flexibility and fatigue resistance [1]. Despite its excellent properties, NR is susceptible to degradation upon exposure to sunlight, oxygen, heat and ozone. Its susceptibility to deterioration is due to the presence of double bonds in the main chain of cis-polyisoprene, the main polymeric material of the NR [2, 3]. Upon exposure to ambient conditions, several undesirable reactions such as crosslinking or chain scission occur on the unsaturated backbones of polyisoprene [4]. Hence, a means to improve the stability of NR will add value to this renewable resource and can expand its range of applications.
Several chemical-based methods have been used to improve the stability of NR. One of the common methods is based on addition reactions such as hydrogenation [5, 6], chlorination [7, 8], bromination [9, 10], sulfonation [11, 12], and grafting [13, 14]. It has been found that these modified NRs possess enhanced thermal and physical properties and chemical resistance, which are attractive for applications that require strong and durable materials. For example, the thermal, oxidative, and weather resistance of the hydrogenated NR is enhanced compared to the unmodified NR [15–17]. The chlorination and bromination of NR led to reduction of tackiness, frictional resistance, and adhesion, but an increase in the glass transition temperature [7, 9]. The presence of carbon-halogen bonds also increases the hydrophilicity of the rubber surface and therefore improves the oil swelling resistance [18, 19]. Sulfonation leads to the addition of ionic groups to cis-polyisoprene such that it can be potentially used as an ion conductor in many electronic applications, and an ionic exchange membrane for water purification [20, 21]. In previous studies, some examples of monomers that have been grafted onto NR are methyl methacrylate [13, 22, 23], styrene [14, 24, 25], 2-hydroxyethyl methacrylate [26], and maleic anhydride [27]. The grafted NRs are typically used as compatibilizers or modifiers for various blend systems [28, 29]. For example, styrene-grafted NR has been used to increase the tensile and impact strengths and the thermal resistance of acrylonitrile–butadiene–styrene (ABS) blends [30]. These chemical modifications are thus an effective means to enhance the properties of NR as well as to introduce new functionalities that are not usually associated with the pristine NR.
The main objectives of this research were to improve the stability of natural rubber and to demonstrate that natural rubber latex can be consecutively modified by multiple reactions in a single reaction vessel. For this purpose, a one-pot method combining the grafting and hydrogenation reactions to modify the natural rubber was developed. The grafting reaction was carried out using free radical polymerization in the presence of cumene hydroperoxide/tetra-ethylene pentamine as an initiator. Methyl methacrylate was chosen for grafting onto the NR. The grafting efficiency of methyl methacrylate was studied as a function of initiator concentration, monomer concentration, reaction temperature, and reaction time. The hydrogenation reaction was carried out using diimide reduction, and the hydrogenation efficiency was studied as a function of grafting efficiency, hydrazine hydrate and hydrogen peroxide concentration, dropping rate, reaction temperature, and reaction time. The thermal, mechanical, ozone ageing, and solvent resistance properties of the grafted-hydrogenated NR were examined and compared with the unmodified NR. In order to test the versatility of this one-pot method, styrene was also used for grafting with the NR. This knowledge of synthetic strategies could make beneficial contributions to the natural rubber modifications and applications.
Experimental
All chemicals were purchased from Sigma-Aldrich. NR latex (60 % dry rubber content) was purchased from Yang Thong Latex Limited, Thailand. Methyl methacrylate (MMA) and styrene (ST) were purified by passing through silica gel columns.
Preparation of grafted-hydrogenated NR
The NR latex (10 g) was placed into a round-bottom flask. A solution of potassium hydroxide (0.1 g) and sodium lauryl sulfate (0.1 g) as an emulsifier was then added to the flask while stirring at 300 rpm. Subsequently, i-propanol (1 g) as a stabilizer was added to the reaction mixture. The monomer (40–175 phr with respect to dry rubber content) was then added continuously and the reaction mixture was stirred for an additional hour. The mixture was heated to a predetermined temperature (40–90 °C) and cumene hydroperoxide (CHP) (0.5–2.0 phr with respect to dry rubber content) was added. A solution of tetra-ethylene pentamine (TEPA) in distilled water (0.5 wt %) was then added. A ratio of CHP:TEPA of 1:1 was used. The reaction mixture was allowed to react for a specified length of time (0–48 h). Cupric sulfate pentahydrate (20 mg, 0.008 mmol) and hydrazine hydrate (1–7 mol ratio with respect to isoprene units) were then added to the reaction mixture. After that hydrogen peroxide (0.5–4.0 mol ratio with respect to N2H4) was added dropwise (at a rate of 0.03–0.15 mL min−1). The NR latex was then stirred for a specified amount of time (1–36 h). The mixture was then dropped into a 5 wt % formic acid solution and the modified NR precipitates out. The precipitated polymer was then filtered by vacuum filtration, washed with plenty of distilled water, and dried at 60 °C overnight. The polymer was purified by soxhlet extraction in acetone for 24 h to remove any contaminants and dried at 60 °C for 48 h.
The grafting efficiency was determined from 1H NMR spectra using the following equation:
where I 3.6 is the integrated signal area of the methoxy proton of MMA unit and I 5.2 is the integrated signal area of the unsaturated methine proton of cis-polyisoprene.
The hydrogenation efficiency was calculated from 1H NMR spectra using the following equation:
where I 0.8 is the integrated signal area of the saturated–CH3 of cis-polyisoprene and I 5.2 is the integrated signal area of the unsaturated methine proton.
Preparation of rubber vulcanizates
The vulcanized rubber was prepared via conventional vulcanization using a two-roll mixing mill. First, the rubber was masticated for 3 min at 70 °C followed by an addition of stearic acid (2 phr), zinc oxide (1 phr), and N-cyclohexyl-2-benzothiazole sulfonamide (1 phr) and stirred for 3 min. Then, sulfur (1.5 phr) was added and the mixture was stirred for three additional minutes. Prior to the processing step, the adequate curing time (T90) was determined on the basis of the results of curing characteristics from a moving die rheometer (TECHPRO, rheotech MD+) following ASTM D5289 using a temperature of 150 °C. The rubber compound was compression molded into a sheet of 2 mm thickness at 150 °C with a force of 1,800 psi using a hydraulic press and a cure time of T90. The rubber vulcanizates were conditioned for 24 h before testing.
Characterization
1H (400 MHz) NMR spectra were obtained on an AVANCE Bruker NMR Spectrometer using chloroform-d as solvent. The FTIR spectra were obtained using a Perkin Elmer FTIR (Spectrum 2000 model) and NaCl salt windows. The thermogravimetric analysis (TGA) of the polymers was carried out with a Perkin Elmer TGA7 analyzer. The samples were heated at a rate of 10 °C min−1 from 25 to 600 °C under a nitrogen atmosphere.
The tensile properties of unmodified and modified NR samples were investigated. The specimens were cut into a dumbbell shape using a die C according to ASTM D412. The tensile properties of all vulcanized rubber samples were determined on a universal testing machine (INSTRON 3366). The average of three specimens was used as the representative value. Hardness measurement of samples was investigated according to ASTM D2240 (Shore A) using a hardness tester (WALLACE). Solvent resistance of all vulcanized samples was measured in toluene following ASTM D471. The test specimens were immersed in toluene at 25 °C for 48 h. The test specimens were then removed from the toluene and wiped with tissue paper to remove excess toluene from the surfaces. The percentage change in mass of the specimen after solvent immersion was used to determine the solvent resistance of the samples. Ozone ageing of all vulcanized samples was performed according to ISO 1461-1 (A) using an ozone ageing tester (TOYOSEIKI). The rubber specimens were subjected to a deformation of 20 % under stress using a specimen holder and exposed to an ozone concentration of 50 parts per hundred million (pphm) at 40 °C for 24 h. The results were recorded by photograph with magnification of 9×.
Results and discussion
Synthesis of MMA grafted-hydrogenated NR
In this work, the one-pot synthesis of MMA grafted-hydrogenated NR is described. The grafting reaction was carried out using free radical polymerization in the presence of CHP/TEPA as initiator whereas hydrogenation was carried out using the diimide reduction in the presence of hydrazine hydrate and hydrogen peroxide. Methyl methacrylate was chosen because it is a common monomer for grafting onto NR. Since the NR source comes in the form of latex, emulsion polymerization is a suitable method for modifying cis-polyisoprene as it provides several advantages, for example, low viscosity, good heat transfer, and fast rates of polymerization. A mixture of potassium hydroxide and sodium lauryl sulfate was used as the emulsifier.
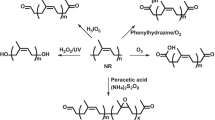
Grafting and hydrogenation reactions could not be performed simultaneously due to the interference of the reagents. Therefore, the grafting and hydrogenation were carried out sequentially by two routes: route A, where grafting was carried out prior to hydrogenation, and route B, where the sequence was reversed (Fig. 1). It was found that only route A successfully yielded the grafted-hydrogenated NR. From route B, the NR was hydrogenated, but not grafted unless very large amounts of CHP and TEPA were added. This is possibly because the presence of hydrazine/H2O2 in the reaction mixture destroyed the CHP and TEPA. Therefore, route A was used for all latter experiments described in this work.
The FTIR spectra of the grafted-hydrogenated NR (GHNR) were obtained and compared with those of the unmodified NR (UNR), the grafted NR (GNR), and the hydrogenated NR (HNR) (Fig. 2). The FTIR spectra of the GHNR and GNR showed C=O stretching and C–O stretching peaks at 1,732 and 1,149 cm−1, respectively. This confirmed the presence of MMA in the polymer chains as these two signals are not present in the spectra of the UNR and HNR. The reduction of signal intensity of C=C stretching peak at 1,664 cm−1 of the HNR and GHNR spectra, compared to the UNR, indicated that the hydrogenation took place at the double bonds of cis-polyisoprene. In addition, the increase in the band at 749 cm−1 attributed to the –CH2– groups confirms that the double bonds in cis-polyisoprene were hydrogenated [15].
The 1H NMR spectra of the UNR, GNR, HNR, and GHNR were obtained (Fig. 3). The 1H NMR spectra of UNR show signals at 1.7, 2.1, and 5.2 ppm which are attributed to –CH3, –CH2–, and olefinic protons, respectively. The 1H NMR of GNR shows signals at 1.7, 2.1, 5.2 and 3.6 ppm which are respectively attributed to –CH3, –CH2–, olefinic proton, and –OCH3. It can be seen that in the NMR spectrum of GHNR, the signal at 5.2 ppm decreases and new signals appear at 0.8 and 1.2 ppm. This is attributed to –CH3, –CH2–, and –CH, confirming that carbon–carbon double bonds in the GNR were hydrogenated to carbon–carbon single bonds.
The grafting and hydrogenation efficiencies of the one-pot synthesis as a function of reaction conditions were studied as follows.
Effects of initiator concentration on grafting efficiency
The effect of initiator concentration on grafting efficiency was investigated. In this study, the concentration of CHP/TEPA mixture was varied from 0.5 to 2.5 phr. The other reaction conditions used were as follows: temperature at 50 °C, [MMA] of 100 phr, and reaction time of 24 h. It was found that as the initiator concentration was increased from 0.5 to 2.0 phr, the grafting efficiency increased from 15 to 20 % (Fig. 4a). However, when the initiator concentration was increased to 2.5 phr, the grafting efficiency decreased from 20 to 9 %. The excessive free polymer radicals react with each other to form free copolymers, rather than grafting to the natural rubber. This result indicates that initiator concentration of 2.0 phr leads to higher grafting efficiency. This result is consistent with previous studies where CHP is used as the initiator [16].
Effects of monomer concentration on grafting efficiency
The effect of MMA concentration on grafting efficiency was studied. The MMA concentration was varied from 40 to 175 phr. The reaction was set at 50 °C using a reaction time of 24 h and [CHP/TEPA] of 1.5 phr. It was found that as the monomer concentration increased from 40 to 175 phr, the grafting efficiency increased from 21 to 31 % (Fig. 4b). This result indicates that a high concentration of monomer is necessary for high grafting efficiency.
Effects of reaction temperature on grafting efficiency
The effect of reaction temperature on grafting efficiency was investigated. In this study, the reaction temperature was varied from 40 to 70 °C. The other reaction conditions used were as follows: [CHP/TEPA] of 1.5 phr, [MMA] of 100 phr, and reaction time of 24 h. It was found that the grafting efficiency increased from 16 to 26 % as the reaction temperature increased from 40 to 60 °C and then plateaued when the temperature was further increased to 90 °C (Fig. 5a). This result is consistent with previous studies where CHP is used as the initiator [31, 32].
Effects of reaction time on grafting efficiency
The effect of reaction time on grafting efficiency was investigated. In this study, the reaction time was varied from 15 min to 48 h and the other reaction conditions used were as follows: temperature at 50 °C, [CHP/TEPA] of 1.5 phr, and [MMA] of 100 phr. It was found that the grafting efficiency was 19 % when the reaction time was 15 min and increased further to 20 % when the reaction time was 2 h (Fig. 5b). After that, the grafting efficiency did not increase further. This result suggests that a reaction time of 15 min is sufficient for the grafting process to complete.
Effects of grafting efficiency on hydrogenation efficiency
From the FTIR and NMR results, it was found that both grafting and hydrogenation reactions exclusively occurred at the double bonds of cis-polyisoprene. Since the grafting reaction was conducted prior to the hydrogenation, the number of remaining double bonds after the grafting reaction would influence the efficiency of hydrogenation reaction. In addition, the presence of grafted MMA on the polyisoprene chains could impose some steric effects on the hydrogenation reaction. Therefore, the effect of grafting efficiency on hydrogenation efficiency was studied. In this study, unmodified NR latex was directly employed for hydrogenation reaction, and is denoted as 0 % grafting efficiency. The other two NR latex samples with different grafting efficiencies were prepared by monomer concentrations at 60 and 120 phr while other grafting conditions were held constant as follows: [CHP/TEPA] of 1.5 phr, reaction temperature of 60 °C, and reaction time of 24 h. From the NMR characterization, these two samples possessed grafting efficiencies of 18 and 28 %. Then all three NR latex samples were used to carry out the hydrogenation reaction using reaction conditions as follows: mole ratio of N2H4: H2O2 of 1:2, ratio of N2H4:isoprene unit of 5:1, reaction temperature of 60 °C, H2O2 dropping rate of 0.08 mL min−1, and stirring time after adding H2O2 of 1 h. It was found that, as expected, the hydrogenation efficiency was inversely proportional to the grafting efficiency (Fig. 6a). The sample with lowest grafting efficiency gave the highest hydrogenation efficiency and vice versa. These results indicate that the grafting efficiency, or the amount of MMA on the cis-polyisoprene, influences the hydrogenation reaction of diimide.
Effects of reaction temperature on hydrogenation efficiency
In this study, the effect of reaction temperature on hydrogenation efficiency was investigated. From the previous experiment, it was known that the grafting efficiency affected the hydrogenation efficiency. Therefore, to study the effects of reaction conditions on hydrogenation efficiency, the grafting condition was fixed as follows: [CHP/TEPA] of 1.5 phr, [MMA] of 60 phr, reaction temperature of 60 °C, and reaction time of 24 h. The average grafting efficiency for all samples using this grafting condition was found to be 16 ± 2 %. The hydrogenation reaction temperature was varied from 40 to 70 °C and the other reaction conditions were set as follows: mole ratio of N2H4:isoprene unit of 5:1, mole ratio of N2H4:H2O2 of 1:2, H2O2 dropping rate 0.08 of mL min−1, and stirring time after adding H2O2 of 1 h. It was found that, as the reaction temperature increased from 40 to 70 °C, the hydrogenation efficiency increased from 9 to 19 % (Fig. 6b). However, as the temperature was increased further, the hydrogenation efficiency decreased. This was probably because the diimide molecules tend to self-react at higher temperature (reaction scheme 1).
This result is consistent with previous studies where the hydrogenation reaction was carried out using hydrazine hydrate/hydrogen peroxide [15].
Effects of hydrazine hydrate concentration on hydrogenation efficiency
Since the diimide is generated from the reaction between hydrazine and hydrogen peroxide, the amount of hydrazine hydrate could affect the hydrogenation efficiency. In this study, the effect of hydrazine hydrate concentration on hydrogenation efficiency was examined. The amount of N2H4 was varied from 1:1 to 7:1 mol ratio with respect to the isoprene units in cis-polyisoprene. The number of moles of isoprene units in cis-polyisoprene was calculated from the ratio between weight of dry rubber in grams and the molecular weight of isoprene. Other reaction conditions used were as follows: mole ratio of N2H4:H2O2 of 1:2, reaction temperature of 60 °C, H2O2 dropping rate of 0.08 mL min−1, and stirring time after adding H2O2 of 1 h. It was found that the hydrogenation efficiency increased with increasing N2H4/H2O2 concentration (Fig. 7a). This result can be simply explained using the hydrogenation mechanism.
The diimide hydrogenation reaction consists of two steps: the reaction between hydrazine hydrate and hydrogen peroxide to form the diimide (reaction scheme 2) and the reaction between the diimide and carbon–carbon double bonds (reaction scheme 3). Hence, increasing the N2H4/H2O2 concentration leads to a higher amount of diimide, and consequently higher hydrogenation efficiency.
Effects of hydrogen peroxide concentration on hydrogenation efficiency
Both hydrazine hydrate and hydrogen peroxide are required for diimide formation. In this study, the effect of hydrazine hydrate on hydrogenation efficiency was investigated. The relative mole ratios between H2O2:N2H4 were 0.5:1–4:1. Other reaction conditions used were as follows: mole ratio of N2H4:isoprene unit of 5:1, reaction temperature of 60 °C, H2O2 dropping rate of 0.08 mL min−1, and stirring time after adding H2O2 1 h. It was found that the hydrogenation efficiency increased with increasing hydrogen peroxide, indicating that a large amount of hydrogen peroxide was necessary for efficient hydrogenation (Fig. 7b). It is well known that hydrogen peroxide is thermally unstable, so it was necessary to have an excess of hydrogen peroxide in the reaction.
Effects of hydrogen peroxide dropping rate on hydrogenation efficiency
Upon adding the hydrogen peroxide into the reaction mixture, it was observed that the hydrogenation reaction was vigorous. Therefore, it was predicted that the dropping rate of hydrogen peroxide solution would affect the yield of diimide and consequently the hydrogenation efficiency. In this study, the hydrogen peroxide dropping rate was varied from 0.03 to 0.15 mL min−1. The other reaction conditions were set as follows: a mole ratio of N2H4:isoprene unit of 5:1, mole ratio of N2H4:H2O2 of 1:2, reaction temperature of 60 °C, and stirring time after adding H2O2 of 1 h. It was found that as the H2O2 dropping rate was increased, the hydrogenation efficiency decreased (Fig. 8a). This is possibly due to the fact that a fast rate of H2O2 addition resulted in the rapid formation of a large amount of diimide, leading to a side reaction (Eq. 1). Hence, a slow formation of the diimide from a slow dropping rate leads to a more effective hydrogenation as the diimide must diffuse into the NR latex particles to react with the polymer’s double bonds.
Effects of stirring time after adding hydrogen peroxide on hydrogenation efficiency
In this experiment, the effect of stirring time after adding hydrogen peroxide on hydrogenation efficiency was studied. The stirring time after adding H2O2 was varied from 1 to 38 h. The other reaction conditions used were as follows: mole ratio of N2H4:isoprene unit of 5:1, mole ratio of N2H4:H2O2 of 1:2, reaction temperature of 60 °C, and dropping rate of 0.08 mL min−1. It was found that the stirring time after adding H2O2 did not significantly affect the hydrogenation efficiency (Fig. 8b). This result suggests that most of the hydrogenation reaction occurred during the addition of H2O2 and therefore prolonged stirring time did not further increase the hydrogenation efficiency.
Mechanical properties
The mechanical properties of the vulcanized rubber samples were measured (Table 1). It was found that the tensile strength of the polymers can be ranked as follows: UNR > GNR > HNR > GHNR. These results show that both grafting and hydrogenation decreased the tensile strength of the polymers, possibly due to a decrease in crystallinity [16]. The modulus at 100 % and hardness of the unmodified and modified NR samples agree well and can be ranked as follows: GNR > GHNR > UNR > HNR. MMA grafting increased the stiffness of the rubber, possibly due to the increased polar interaction in the polymer chains overcoming the negative effect from the reduced crystallinity. In contrast, hydrogenation decreased the modulus and hardness of the NR compared to the unmodified NR, as the saturation of the polymer backbones reduces the crystallinity of the polymer.
Reverse of the modulus and hardness values, the elongation at break of the polymers can be ranked as follows: HNR > UNR > GHNR > GNR. Hydrogenation increased the elasticity of the rubber whereas the presence of MMA did the opposite. This result is consistent with the modulus and hardness results as a sample with high elasticity usually possesses low modulus and hardness.
Solvent resistance
Solvent resistance of the vulcanized rubber samples was investigated. Toluene was used as the solvent and the mass change percentages of the samples were recorded and shown in Table 2. High mass change percentage value equals poor resistance against solvent. It was found that the solvent resistance of the polymers can be ranked as follows: GNR > GHNR > UNR > HNR. The solvent resistance of GNR is the highest, possibly because of the presence of mildly hydrophilic MMA moiety in the rubber. HNR showed the lowest solvent resistance, possibly because the saturation of the double bonds increases the hydrophobicity of the polymer. GHNR is similar to UNR in terms of solvent swelling resistance due to the cancelling effect between the grafted MMA and hydrogenated backbones.
Ozone ageing
As mentioned above, cis-polyisoprene has unsaturated backbones that can easily degrade when exposed to ozone. In this study, the ozone ageing of the vulcanized rubber samples was investigated. The surface topography images of the UNR, GNR, HNR, and GHNR after ozone treatment are shown (Fig. 9). It was found that the UNR, GNR, and GHNR samples developed a large number of small cracks, while the HNR sample developed cracks, but in smaller numbers. It can be clearly seen that the cracks in the GHNR sample are not as deep as those in the other samples. This result indicates that both grafting and hydrogenation processes lead to an improved ozone resistance.
Thermal properties of modified NRs
Thermal properties of unmodified and modified NR samples were investigated using thermogravimetric analysis (TGA). From the thermograms (Fig. 10), the decomposition temperatures of the polymers, which were determined from the intersection of two tangents at the onset of the decomposition temperature, were obtained. It was found that the decomposition temperatures of the polymers can be ranked as follows: GHNR > HNR > GNR > UNR. This suggests that the saturation of the cis-polyisoprene backbone by both grafting and hydrogenation reactions improves the thermal stability of the material.
Synthesis of styrene-grafted, hydrogenated NR
To test the versatility of this one-pot method, styrene was chosen for grafting with the NR. The reaction conditions for grafting were set as follows: potassium hydroxide 0.1 g, sodium lauryl sulfate 0.106 g, i-propanol 0.6 g, [styrene] of 100 phr, [CHP/TEPA] of 2.0 phr, reaction temperature of 50 °C, reaction time of 24 h. The reaction conditions for hydrogenation were: CuSO4 0.002 g, mole ratio of N2H4:isoprene unit of 5:1, mole ratio of N2H4:H2O2 of 1:2, reaction temperature of 60 °C, dropping rate of 0.08 mL min−1, and stirring time after adding H2O2 of 1 h.
The 1H-NMR spectrum of styrene-grafted, hydrogenated NR showed signals at 0.8, 1.2, and 6.5–7.5 ppm which are attributed to –CH3, –CH2–, and –aryl protons, respectively (Fig. 11). This result confirms that NR can be grafted with styrene and hydrogenated in a one-pot fashion. From the NMR characterization, the styrene-grafted, hydrogenated NR possessed grafting and hydrogenation efficiencies of 13 and 24 %, respectively. This result demonstrates the versatility of the one-pot method developed in this work.
Conclusions
In this work, a one-pot method for the synthesis of grafted-hydrogenated NR was developed. It was found that both grafting and hydrogenation efficiencies depend on the reaction conditions and one reaction affects the efficiency of the other. Under optimized conditions, the grafting and hydrogenation efficiencies exceeded 30 and 20 %, respectively. The one-pot strategy is an attractive strategy for modifying the polymers because it affords a synthetic method with a reduction of synthetic steps and reaction work-up time without the purification step of the intermediate product. The reduced amount of reagents used and toxic wastes produced make this a greener chemical method. This simple means to improving the stability and adding new functionalities using an environmentally friendly approach will add value to NR.
References
Graves DF (2007) Rubber. In: Kent JA (ed) Handbook of industrial chemistry and biotechnology. Springer, US, pp 689–718
Gamlin C, Markovic MG, Dutta NK, Choudhury NR, Matisons JG (2000) Structural effects on the decomposition kinetics of EPDM elastomers by high-resolution TGA and modulated TGA. J Therm Anal Calorim 59:319–336
dos Santos KAM, Suarez PAZ, Rubim JC (2005) Photo-degradation of synthetic and natural polyisoprenes at specific UV radiations. Polym Degrad Stab 90:34–43
Chaikumpollert O, Sae-Heng K, Wakisaka O, Mase A, Yamamoto Y, Kawahara S (2011) Low temperature degradation and characterization of natural rubber. Polym Degrad Stab 96:1989–1995
Hinchiranan N, Charmondusit K, Prasassarakich P, Rempel GL (2006) Hydrogenation of synthetic cis-1,4-polyisoprene and natural rubber catalyzed by [Ir(COD)py(PCy3)]PF6. J Appl Polym Sci 100:4219–4233
Kongparakul S, Prasassarakich P, Rempel GL (2008) Effect of grafted methyl methacrylate on the catalytic hydrogenation of natural rubber. Eur Polym J 44:1915–1920
Radabutra S, Thanawan S, Amornsakchai T (2009) Chlorination and characterization of natural rubber and its adhesion to nitrile rubber. Eur Polym J 45:2017–2022
Zhong JP, Li SD, Wei YC, Peng Z, Yu HP (1999) Study on preparation of chlorinated natural rubber from latex and its thermal stability. J Appl Polym Sci 73:2863–2867
Xue X, Wu Y, Wang F, Ling J, Fu X (2010) Preparation and thermal stability of brominated natural rubber from latex. J Appl Polym Sci 118:25–29
Lewis C, Bunyung S, Kiatkamjornwong S (2003) Rheological properties and compatibility of NR/EPDM and NR/brominated EPDM blends. J Appl Polym Sci 89:837–847
Kado N, Patjaree S, Akabori K, Yamamoto Y, Kawahara S (2012) A novel proton conductive polymer electrolyte prepared from natural rubber. Kobunshi Ronbunshu 69:228–234
Xavier T, Samuel J, Manjooran KB, Kurian T (2002) New ionic polymer: synthesis and properties of “zinc sulfonated natural rubber”. J Elastomers Plast 34:91–101
Satraphan P, Intasiri A, Tangpasuthadol V, Kiatkamjornwong S (2009) Effects of methyl methacrylate grafting and in situ silica particle formation on the morphology and mechanical properties of natural rubber composite films. Polym Adv Technol 20:473–486
Suksawad P, Yamamoto Y, Kawahara S (2011) Preparation of thermoplastic elastomer from natural rubber grafted with polystyrene. Eur Polym J 47:330–337
Mahittikul A, Prasassarakich P, Rempel GL (2007) Diimide hydrogenation of natural rubber latex. J Appl Polym Sci 105:1188–1199
Arayapranee W, Rempel GL (2009) Synthesis and mechanical properties of diimide-hydrogenated natural rubber vulcanizates. J Appl Polym Sci 114:4066–4075
Simma K, Rempel GL, Prasassarakich P (2009) Improving thermal and ozone stability of skim natural rubber by diimide reduction. Polym Degrad Stab 94:1914–1923
Radabutra S, Thanawan S, Amornsakchai T (2009) Chlorination and characterization of natural rubber and its adhesion to nitrile rubber. Eur Polym J 45:2017–2022
H-p Yu, S-d Li, Zhong JP, Xu K (2004) Studies of thermooxidative degradation process of chlorinated natural rubber from latex. Thermochim Acta 410:119–124
Suksawad P, Kosugi K, Yamamoto Y, Akabori K, Kuroda H, Kawahara S (2011) Polymer electrolyte membrane with nanomatrix channel prepared by sulfonation of natural rubber grafted with polystyrene. J Appl Polym Sci 122:2403–2414
El Sayed AM (2007) Evaluation of new conducting polymeric membrane based on sulfonation of compatibilized NR/EPDM blend. J Appl Polym Sci 104:3804–3812
Kochthongrasamee T, Prasassarakich P, Kiatkamjornwong S (2006) Effects of redox initiator on graft copolymerization of methyl methacrylate onto natural rubber. J Appl Polym Sci 101:2587–2601
Zhang S, Cao L, Shao F, Chen L, Jiao J, Gao W (2008) Grafting of methyl methacrylate onto natural rubber in supercritical carbon dioxide. Polym Adv Technol 19:54–59
Arayapranee W, Rempel GL (2008) Morphology and mechanical properties of natural rubber and styrene-grafted natural rubber latex compounds. J Appl Polym Sci 109:1395–1402
Pukkate N, Kitai T, Yamamoto Y, Kawazura T, Sakdapipanich J, Kawahara S (2007) Nano-matrix structure formed by graft-copolymerization of styrene onto natural rubber. Eur Polym J 43:3208–3214
Amnuaypanich S, Ratpolsan P (2009) Pervaporation membranes from natural rubber latex grafted with poly(2-hydroxyethyl methacrylate) (NR-g-PHEMA) for the separation of water-acetone mixtures. J Appl Polym Sci 113:3313–3321
Nakason C, Kaesaman A, Supasanthitikul P (2004) The grafting of maleic anhydride onto natural rubber. Polym Test 23:35–41
Arayapranee W, Prasassarakich P, Rempel GL (2004) Blends of poly(vinyl chloride) PVQ/natural rubber-g-(styrene-co-methyl methacrylate) for improved impact resistance of PVC. J Appl Polym Sci 93:1666–1672
Afifi H, El-Wakil AA (2008) Study of the effect of natural rubber-graft-maleic anhydride (NR-g-MA) on the compatibility of NR-NBR blends using the ultrasonic technique. Polym-Plast Technol Eng 47:1032–1039
Pisuttisap A, Hinchiranan N, Rempel GL, Prasassarakich P (2013) ABS modified with hydrogenated polystyrene-grafted-natural rubber. J Appl Polym Sci 129:94–104
Kangwansupamonkon W, Gilbert RG, Kiatkamjornwong S (2005) Modification of natural rubber by grafting with hydrophilic vinyl monomers. Macromol Chem Phys 206:2450–2460
Juntuek P, Ruksakulpiwat C, Chumsamrong P, Ruksakulpiwat Y (2011) Glycidyl methacrylate grafted natural rubber: synthesis, characterization, and mechanical property. J Appl Polym Sci 122:3152–3159
Acknowledgments
This work is funded by the 2013 Science & Technology Research Grant by the Thailand Toray Science Foundation. The authors acknowledge the Department of Chemistry, Faculty of Science and Technology, Thammasat University. In addition, the authors would like to thank the reviewers and journal editors for useful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kookarinrat, C., Paoprasert, P. Versatile one-pot synthesis of grafted-hydrogenated natural rubber. Iran Polym J 24, 123–133 (2015). https://doi.org/10.1007/s13726-014-0306-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-014-0306-z