Abstract
Scylla paramamosain have been considered as an economic candidate for aquaculture, however, high mortality during early larval stages exhibits a significant bottleneck to their mass seed production. Operational enzymatic variables were investigated for further studies on feeding optimization. Ontogenetic change of the digestive system of S. paramamosain was enzymatically (trypsin, chymotrypsin, pepsin, amylase and alkaline phosphatase) evaluated. Results showed that these enzymes were already presented in the larvae before exogenous feeding. The first detection after hatching was low activity and gradually increased from Z3 except trypsin, and chymotrypsin activity increased from Z5 stage. Alkaline phosphatase activity peaked at Z2 and Z4, followed by a sharp fall in Z5 and megalope. Trypsin and Chymotrypsin activities were also decreased from Z3 to Z5, then sharply increased from Z5; however, Chymotrypsin activity decreased after megalope stage. Pepsin activity was detectable after hatching and regularly increased through the larval development. Amylase activity was low from hatching to Z3 and then suddenly increased. Alkaline phosphatase activity was recorded with the highest activity at Z2 and Z4, then complex variation, particularly at Z3 and megalope stage. The study constitutes physiological information on ontogenic development as well as the digestive abilities of mud crab larvae further, facilitates feeding diet formulation and larviculture of this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mud crab (Scylla paramamosain) is an important commercial species for aquaculture in Southeast Asian countries. Due to the increase in crab farming, the wild crab seed was overexploited but still unmet demand (Lindner 2005). Therefore, there is a requirement to improve the mud crab hatchery technology for sustainable development of crab culture. However, the current technology is facing mass mortality during larval metamorphosis. Many reasons caused this phenomenon, such as vibriosis (Jithendran et al. 2010; Wu et al. 2016), nutrition (Holme 2008; Holme et al. 2009) and water quality (Li et al. 2008; Li et al. 2012). The major impediment is high mortality at Zoea 1 and Megalope stage (Keenan et al. 1998; Serrano and Traifalgar 2012); emphasized the roles of larval ontogenic development and digestive physiological capacity for larviculture technology improvement.
During planktonic stages (from Zoea 1 to Zoea 5), the hepatopancreas of mud crab is not fully functional, leading to the limitation of digestive enzymatic capacities (Kamarudin et al. 1994; Serrano 2012). Hence it might be closely related to the larval feeding strategies and trophic status (Le Vay et al. 2001; Andrés et al. 2010). Furthermore, due to the lack of essential enzymes for the hydrolysis of food particles, live feed become an excellent alternative enzymatic source to enhance the digestive capacity of the larvae (Holme et al. 2009; Res 2013).
Give these hypotheses in feeding preferences among different stages in the life cycle of mud crab, the question arises whether the activity and presence of digestive enzymes were reflected through the feeding preferences? How is the variation in levels of enzyme activities among the planktonic stages? especially at two critical stages as Z1 and Megalope. Comprehensive knowledge of digestive process could contribute to developing an efficient seed production technology for S. paramamosain in forming appropriate feeds and feeding regimes. Ontogenetic changes in the types and concentrations of digestive enzymes were indicative of shifts in the ability of crab larvae to hydrolyse dietary components and consequently highlight possible shifts in diet (Hammer et al. 2000). Thus, this study aims to illustrate digestive enzymes activities during ontogenetic development of mud crab larvae.
Materials and Methods
Larviculture and Sample Collection
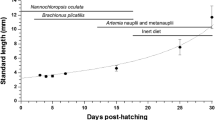
The ovigerous crab was obtained from Ca Mau province, Viet Nam and separately kept in 100 L tanks after disinfecting by 200 ppm formalin for 10 min. During broodstock selection and hatchery husbandry practices, crabs were quarantined and tested by following the standards of testing and assessing aquatic breeds (The circular No: 11/2014/TT-BNNPTNT). The crab was daily fed with blood cockles and exchanged water at 100%. After hatching, the strong photopositive larvae were collected and reared in 500 L tanks at 300 inds/L of stocking density and 30 ppt of salinity. Larvae were fed Artemia (Art) (Vinh Chau strain, Viet Nam) throughout rearing period (Fig. 1). The diameter of decapsulated Artemia cysts was from 200 to 250 μm, and Artemia nauplii ranged from 470 to 550 μm, consisting 56.2% of crude protein, 13.4% of lipid, and 14.6% of carbohydrate.
The larval stage of mud crab S. paramamosain consists five zoeal stages (Z), megalope (M), and then molts into crablet (C) (Linh et al. 2017). At every stage from Z 1 until Crab 1, the whole body of crab larvae was poll sampled to 100 mg per sample in triplicate, and was collected before feeding (wet weight) and rinsed in saline water, removed water on tissue, then kept in 1.5 ml tube at −80 °C for enzymatic analyses.
Digestive Enzyme Assays
Pooled samples (the whole larvae was each pooled to 100 mg per each sample) were prepared in a 1.5 ml tube with 500 μl demineralized water by homogenization (Saborowski et al. 2006), followed by centrifugation for 30 min at 15,000 g at 4 °C. The extract was transferred to other tubes and proceed for enzymatic assay in triplicate and repeated three batches of mud crab. Total protein content was determined according to the protocol of Marichamy et al. (2011).
Trypsin activity was assayed using Nα-benzoyl-DL-arginine p-nitroanilide (BAPNA, Sigma B4875) according to Geiger and Fritz (1988). The 2.25 mL reaction volume consisted of 1.25 mL substrate solution, 0.1 mL of purified trypsin solution and buffer. The BAPNA solution was added to start the reaction for 5 min, then added 0.25 mL of 30% acetic acid to stop reaction and the absorbance measured at 405 nm. One unit is defined as the amount of trypsin that cleaves the substrate, yielding 1.0 μmol of p-NA per minute at 25 °C.
Chymotrypsin activity was assayed according to Hummel (1959), reaction mixture comprising1.4 ml Benzoyl-L-tyrosine ethyl ester (BTEE) 1.07 mM in 50% (w/w) methanol, 1.0 ml 80 mM Tris-HCl buffer (pH 7.8) contained 0.1 M CaCl2, and 0.3 ml crude enzyme extract in final volume of 2.7 ml was used for the assay. The reaction was stopped by adding 0.3 ml of 30% acetic acid and absorbed at 256 nm. One unit will hydrolyze 1.0 μmole of BTEE per minute at pH 7.8 at 25 °C.
Pepsin activity was measured using hemoglobin as substrate by modification of Worthington (1982) and Suzer et al. (2007). The assayed enzyme was mixed with the pH 2.0 substrate containing 2% hemoglobin solution in 0.3 N HCl; the incubation was conducted for 10 min at 37 °C. The reaction was stopped by adding 5% Trichloroacetic Acid, before centrifuging at 4000 g for 6 min at 4 °C and absorbed at 280 nm. One unit of pepsin will produce ∆A280 of 0.001 per minute at pH 2.0 and 37 °C measured as trichloroacetic acid soluble products using hemoglobin as the substrate.
α-Amylase activity was assayed using EnzyChromTM a-Amylase Assay Kit (ECAM-100) Quantitative Colorimetric Amylase Determination (BioAssay Systems, USA), absorbed at 585 nm. One unit of enzyme catalyzes the production of 1 μmole of glucose per min under the assay conditions.
Alkaline phosphatase activity was analyzed using QuantiChromTM Alkaline Phosphatase Assay Kit (DALP-250) Colorimetric Kinetic Determination of Serum Alkaline Phosphatase Activity (BioAssay Systems, USA), read at 405 nm. Alkaline phosphatase activity was expressed as μM of ρ-nitrophenol formation per minute per microgram of total proteins.
Statistical analysis was carried out with the computer program Origin 2018 (OriginLab, Northampton, Massachusetts, USA) and SPSS 24.0 (IBM, Chicago, IL, USA). One-way ANOVA was used to test the significant variation between specific activity of enzymes (U/mg protein) at various larval stages. Differences among treatments were considered significant at p < 0.05. LSD was applied to determine multiple comparisons between enzymatic assay activities at different stages.
Results
Water Parameters
During rearing period, water was sustained at 26.5–29.5 °C of temperature and 30‰ of salinity. There was no significant fluctuation of the water quality water at daytime and night time. Total ammonium (0.1–0.5 mg/L), nitrite (0.3–0.5 mg/ l) and pH (7.2–7.8) were overall stable and within appreciate ranges (Boyd 1998). During the molting, however, the nitrite level increased to 0.5 mg/l but later was controlled to near zero by water exchange.
Enzyme Assay
The activities of digestive enzymes, trypsin, chymotrypsin, pepsin, and amylase, could be detected, but extremely low at early stages (from Z1 to Z3), while alkaline phosphatase remarkably high (Fig. 2). The pepsin activity was regularly increased and reached a peak (8.32 ± 0.26 U/mg protein) at Crab 1 (Fig. 5). At the Z1 stage, the specific activity of pepsin was low (1.69 ± 0.09 U/mg protein). Thereafter, the activity continuously and significantly increased until the larvae reach to Crab 1.
In contrast, trypsin and chymotrypsin, amylase and alkaline phosphatase strongly fluctuated. The trypsin activity was low in Z1 (0.92 ± 0.05 U/mg protein), significantly increased in Z2 (2.43 ± 0.57 U/mg protein) but rapidly decreased from Z3 to Z5 (1.71 ± 0.42 and 0.99 ± 0.49 U/mg protein, respectively). After megalope metamorphosis, trypsin activity raised again and reached the highest level in Crab 1 (3.94 ± 0.06 U/mg protein) (Fig. 3). The same pattern was expressed for chymotrypsin, however, chymotrypsin was low in crab 1 (0.76 ± 0.05 U/mg protein) (Figs. 4 and 5).
Different to trypsin and chymotrypsin, amylase activity increased after hatching (from 1.09 ± 0.06 U/mg protein in Z1 to 1.86 ± 0.08 U/mg protein in Z2) but dropped abruptly in Z3 stage (0.64 ± 0.08 U/mg protein), then sharply increased to a peak in Z5 stage (9.36 ± 0.08 U/mg protein). However, the activity did not remain in megalope and crab 1, afterward, an abrupt decrease was proceeded up to crab 1 (4.21 ± 0.16 U/mg protein) (Fig. 6).
Despite strong variations, the specific activity of alkaline phosphatase remained at a high level and significant difference among metamorphosis stage during ontogeny. From Z1, the activity increased significantly formed two peaks in Z2 and Z4 (52.42 ± 5.25 and 55.23 ± 10.62 U/mg protein, respectively) (Fig. 7). However, the megalope stage was recorded with a decrease to a valley of alkaline phosphatase activity (6.63 ± 3.27 U/mg protein), and then eventually increased in crab 1 (13.39 ± 0.15 U/mg protein), significantly low compared to zoea stages.
Discussion
Digestive enzyme activity was indicated to be associated with the development of the digestive tract, genetically modulated by specific stage and diet composition (Biesiot and Capuzzo 1990; Serrano and Traifalgar 2012). The result from this study showed that proteolytic enzymes (trypsin, chymotrypsin, pepsin), amylase and alkaline phosphatase could be detected after hatching and varied among different development stages. These enzymes are responsible for the ability of larvae to digest and mineralize protein, carbohydrates. The early detection and fluctuation during larval development of these assayed enzyme activities show that the enzyme synthesis during early ontogenic stages is genetically programmed and at different time (Biesiot and Capuzzo 1990). The comparable pattern has been reported on Penaeid shrimp larvae after hatching (Jones et al. 1997), mud crab Scylla serata larvae (Hong et al. 1995; Serrano and Traifalgar 2012) and spider crab, Maja brachydactyla (Andrés et al. 2010).
Metabolism of carbohydrate was determined to be necessary for decapod larvae and irrespectively of their feeding habits (Andrés et al. 2010), in which α-amylases have a central physiological role in the primary steps of starch hydrolysis and glycogen storage (Date et al. 2015; Asaro et al. 2018). Previous studies have presented on various crustacean larvae, either in penaeid shrimps (Carrillo-Farnés et al. 2007), crabs (Saborowski et al. 2006; Asaro et al. 2017; Asaro et al. 2018) or lobsters (Johnston 2003) and carideans (Kamarudin et al. 1994; Romero et al. 2017), ranging from herbivorous, omnivorous and carnivorous, respectively. In this study, Amylase with low level was observed from Z1 to Z3 indicated the carnivorous behavior of crab larvae at early stages. However, a sharp increase of amylase level from Z4 to megalope was recorded, reflected the changing into omnivorous feeding behavior. The slightly decreasing of ability to digest carbohydrates in megalope metamorphosis to the first crab, agreed to demonstrated patterns in Panaeus juponicus and Macrobrachium rosenbergii larvae (Kamarudin et al. 1994), Scylla serata (Serrano and Traifalgar 2012).
Phosphatase enzymes play an essential role in chitin synthesis, to convert Dolichol pyrophosphate to Dolichol phosphate or Dolichol Phosphate to Dolichol (Urich 1994; Salaenoi et al. 2012). Dolichol phosphate joined as a carrier in the pyrophosphate-linked oligosaccharides assembly (Monin and Rangneker 1974), and an acceptor in the Dolichyl phosphate glucose synthesis (Morris and Greenaway 1992; Urich 1994). Alkaline phosphatase also associates precipitation of calcium phosphate complexes within the vesicles in calcification (Sandhu and Jande 1982), joins in the hydroxyapatite crystals formation which maintains phosphate, calcium homeostasis and supports for hydrolyzation of phosphate compounds to supply phosphate for calcification process (Mornet et al. 2001; Marcin et al. 2003). The most considerable change was remarked in Alkaline phosphatase activity with different larval stages in this study. However, the operation was maintained at high levels compared to other enzymes. At Z3 and Z5 metamorphosis to megalope stage, Alkaline phosphatase activity drastically declined, and mass mortality of larvae was also confirmed in these periods because of incomplete molt, which have reported on Scylla serata (Hamasaki et al. 2002; Suprayudi et al. 2012), on blue swimming crab Portunus pelagicus (Fujaya et al. 2013). Therefore, it’s reasonable to hypothesize on the lack of alkaline phosphatase in critical stages (Z1 to Z3, Z5 to megalope) closely related to the mortality of larvae. The molting cycle of decapod was devised in early post-molt, post-molt, inter-molt, and late inter-molt (Salaenoi et al. 2012; Chamchuen et al. 2014). The alkaline phosphatase activities in different phases were highly significant, presenting high range at inter-molt, pre-molt stages extended to late pre-molt and low range at early post-molt due to the process of deterioration from premolt (Saborowski et al. 2006; Salaenoi et al. 2012). Crab integument contained a high level of alkaline phosphatase to transfer phosphate and phosphoryl group from seawater or among cells into the tissues supplied for phosphate metabolism and bio-mineralization (Salaenoi et al. 2012). The performance and rearrangement of the new cuticle during molting required a supposed increase of alkaline phosphatase, however, the interrupt of alkaline phosphatase occurred at Z3 and megalope could lead to incomplete molt syndrome related to mass mortality during larviculture.
The assayed proteases (trypsin, chymotrypsin, and pepsin) are typical enzymes in aquatic organisms. Particularly, proteinases present in midgut and stomach at high level to promote the breaking of alimentary proteins (Saborowski et al. 2006). They have been documented in several decapod species such as Macrobrachium rosenbergii (Deru and Wales 1990; Kamarudin et al. 1994), lobster Homams americanus (Biesiot and Capuzzo 1990), Scylla serata (Hong et al. 1995; Serrano and Traifalgar 2012), spiny lobster, Jasus edwardsii (Johnston 2003), blue swimming crab Portunus pelagicus (Chamchuen et al. 2014). After hatching, proteinases of mud crab were detected at certain level, appeared in all larval stages and reached to significantly high activity in crab 1, comparable to Scylla serata (Hong et al. 1995; Serrano and Traifalgar 2012) and Portunus pelagicus (Chamchuen et al. 2014). It also confirmed the ability to digest protein at beginning by endogenous proteolytic enzymes. Moreover, Jantrarotai et al. (2005), investigated the digestive system of Scylla olivacea by histological method, have reported that anterior midgut and posterior midgut gland did not performed at Z1 and Z2. These structures were progressively increased in Z3 to Z5, could result to the fluctuation of trypsin and chymotrypsin. The expand of hepatopancreas was observed on both sides of the digestive tract from Z3 (Jantrarotai et al. 2005), augured the completion of the development of the digestive system. Additionally, the appearance of the sixth abdominal segment, the highly functional gastric organ, and hepatopancreas at Z4, mandibular palp at Z5, eventually performed of feeding apparatus at Megalope confirmed the completed development of digestive system (Kumlu and Jones 1995; Li and Li 1998; Holme et al. 2009). When reached to crab 1, the high activities of proteinases and amylase were evident for an effective digestion. However, there was not homogenous among composition enzymes. Pepsin was recorded with a linear increase while trypsin and chymotrypsin showed a strong fluctuation from Z3 to Z5, these results support the histological findings and describe the functional complexity.
In conclusion, this study presented the lack of endopeptidases during larval development of mud crab (Scylla paramamosain). The larvae could digest external food at Z1, and the enzymatic activity was significantly increased and archived at crab 1. However, the activity of examined enzymes was low at early stages, and impracticable to stimulate of enzyme production, suggest that additional enzyme sources (live feed/ digestive enzymes) should be integrated into diets for mud crab larviculture.
References
Andrés M, Gisbert E, Díaz M, Moyano FJ, Estévez A, Rotllant G (2010) Ontogenetic changes in digestive enzymatic capacities of the spider crab, Maja brachydactyla (Decapoda: Majidae). J Exp Mar Biol Ecol 389(1–2):75–84
Asaro A, Paggi RA, De Castro RE, López Mañanes AA (2017) Amylase in the hepatopancreas of a euryhaline burrowing crab: characteristics and modulation. Turk J Zool 41(3):443–453
Asaro A, Alejandro R, Cristina J, Antonia A, Mañanes L (2018) Glucose homeostasis in the euryhaline crab Cytograpsus angulatus : effects of the salinity in the amylase , maltase and sucrase activities in the hepatopancreas and in the carbohydrate reserves in different tissues. Comp Biochem Physiol B 216(November 2017):39–47
Biesiot PM, Capuzzo JM (1990) Changes in digestive enzyme activities during early development of the American lobster Homams americanus Milne Edwards. J Exp Mar Biol Ecol 136:107–122
Boyd CE (1998) Water quality for pond aquaculture. Research and development series No. 43. Alabama, international Center for Aquaculture and Aquatic Environments, Alabama Agricultural Experiment,Station, Auburn University, Auburn, pp 37
Carrillo-Farnés O, Forrellat-Barrios A, Guerrero-Galvan S, Vega-Villasante F (2007) A review of digestive enzyme activity in penaeid shrimps. Crustaceana 80(3):257–275
Chamchuen P, Pratoomchat B, Engkakul A, Kovitvadhi U, Rungruangsak-Torrissen K (2014) Development of enzymes and in vitro digestibility during metamorphosis and molting of blue swimming crab (Portunus pelagicus). J Mar Biotechnol 2014:1–12
Date K, Satoh A, Iida K, Ogawa H (2015) Pancreatic α-amylase controls glucose assimilation by duodenal retrieval through N-glycan-specific binding, endocytosis, and degradation. J Biol Chem 290(28):17439–17450
Deru J, Wales (1990) Studies on the development and nutrition of the caridean prawn Macrobrachium rosenbergii (de Man) (Crustacea: Decapoda). University College of North Wales, UK
Fujaya Y, Trijuno DD, Nikhlani A, Cahyono I, Hasnidar H (2013) The use of mulberry (Morus alba) extract in the mass production of blue swimming crab (Portunus pelagicus L.) larvae to overcome the mortality rate due to molting syndrome. Aquatic Science and Technology 2(1):1
Geiger R, Fritz H (1988) Trypsin. In: Bergmeyer J, Grab M (eds) Methods of enzymatic analysis, vol. V. Chemie Verlag, Weinheim, p 119–129
Hamasaki K, Suprayudi MA, Takeuchi T (2002) Mass mortality during metamorphosis to megalops in the seed production of mud crab Scylla serrata ( Crustacea , Decapoda , Portunidae ). Fish Sci 68:1226–1232
Hammer HS, Bishop CD, Watts SA (2000) Activities of three digestive enzymes during development in the crayfish Procambarus clarkii (Decapoda). J Crustac Biol 20:614–620
Holme M-H (2008) Towards development of a formulated diet for mud crab (Scylla serrata) larvae, with emphasis on lipid nutrition. James Cook University. James Cook University
Holme MH, Zeng C, Southgate PC (2009) A review of recent progress toward development of a formulated microbound diet for mud crab, Scylla serrata, larvae and their nutritional requirements. Aquaculture 286(3–4):164–175
Hong T, Li S, Guizhong WLQ (1995) The experimental studies on the digestive enzyme activities in the larvae of the mud crab Scylla serrata (Forskål). J Xiamen Univ (Nat Sci) 34(1):88–93
Hummel B (1959) A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol 37(2):1393–1399
Jantrarotai P, Srakaew N, Sawanyatiputi A (2005) Histological study on the development of digestive system in zoeal stages of mud crab ( Scylla olivacea ). Kasetsart J 671(39):666–671
Jithendran KP, Poornima M, Balasubramanian CP, Kulasekarapandian S (2010) Diseases of mud crabs (Scylla spp.): an overview. Indian J Fish 57(3):55–63
Johnston DJ (2003) Ontogenetic changes in digestive enzyme activity of the spiny lobster, Jasus edwardsii (Decapoda; Palinuridae). Mar Biol 143(6):1071–1082
Jones DA, Kumlu M, Le Vay L, Fletcher DJ (1997) The digestive physiology of herbivorous, omnivorous and carnivorous crustacean larvae: a review. Aquaculture 155(1–4):285–295
Kamarudin MS, Jones DA, Le Vay L, Abidin AZ (1994) Ontogenetic change in digestive enzyme activity during larval development of Macrobrachium rosenbergii. Aquaculture 123(3–4):323–333
Keenan CP, Davie PJF, Mann DL (1998) A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bull Zool 46:217–245
Kumlu M, Jones DA (1995) Feeding and digestion in the caridean shrimp larva of Palaemon elegans Rathke and Macrobrachium rosenbergii (De Man) (Crustacea: Palaemonidae) on live and artificial diets. Aquac Nutr 1(1):3–12
Le Vay L, Jones DA, Puello-Cruz AC, Sangha RS, Ngamphongsai C (2001) Digestion in relation to feeding strategies exhibited by crustacean larvae. Comp Biochem Physiol A 128(3):623–630
Li FH, Li SJ (1998) Studies on the hepatopancreas of larval Scylla serrata. Oceanologia et limnologia sinica 1:34–40 (In Chinese with English abstract).
Li YY, Xia XA, Wu QY, Liu WH, Lin YS (2008) Infection with Hematodinium sp. in mud crabs Scylla serrata cultured in low salinity water in southern China. Dis Aquat Org 82(2):145–150
Li S, Zhang Z, Li C, Zhou L, Liu W, Li Y, Wen X (2012) Molecular cloning and expression profiles of nitric oxide synthase (NOS) in mud crab Scylla paramamosain. Fish and Shellfish Immunology 32(4):503–512
Lindner B (2005) Impacts of mud crab hatchery technology in Vietnam ACIAR projects FIS / 1992 / 017 and FIS / 1999 / 076. ACIAR projects FIS/1992/017 and FIS/1999/076
Linh NK, N T, Khoa D, Catherine S, Musa N, Musa N, Shaharom-Harrison F (2017) Development of mud crab crablet , the identification of ciliates and the bioefficacy of leaf extract of Rhizophora Apiculata as anti- protozoal agent. J Sustain Sci Manag 12(2):52–62
Marcin B, Eva H, Le Z, Pi S, Gérard A, Jacqueline R, B J, B R (2003) The roles of annexins and alkaline phosphatase in mineralization process. Acta Biochim Pol 50(4):1019–1038
Marichamy G, Shanker S, Saradha A, Nazar AR, BadhulHaq MA (2011) Proximate composition and bioaccumulation of metals in some finfishes and shellfishes of Vellar Estuary (South east coast of India ). European Journal of Experimental Biology 1(2):47–55
Monin MA, Rangneker PV (1974) Histochemical localization of acid and alkaline phosphatases and glucose-6-phosphatase of the hepatopancreas of the crab, Scylla serrata (Forskal). J Exp Mar Biol Ecol 14(1):1–16
Mornet E, Stura E, Lia-Baldini A-S, Stigbrand T, Ménez A, Le Du M-H (2001) Structural evidence for a functional role of human tissue nonspecific alkaline phosphatase in bone mineralization. J Biol Chem 276(33):31171–31178
Morris MA, Greenaway P (1992) High affinity, Ca2+ specific atpase and Na+K+-ATPase in the gills of a supralittoral crab Leptograpsus variegatus. Comp Biochem Physiol A Physiol 102(1):15–18
Res EJZ (2013) Trypsin and leucine aminopeptidase activity contribution of live food to the developing mucrab ( Scylla serrata ) larvae Augusto E . Serrano , Jr . Institute of Aquaculture , College of Fisheries and Ocean Sciences. University of the Philippines Visayas 2(2):10–14
Romero DSR, García-Guerrero M, Vega-Villasante F, Cortés-Jacinto E, Nolasco-Soria H (2017) Effect of photoperiod and temperature on growth and activity of digestive enzymes in juveniles of the long-arm river shrimp Macrobrachium tenellum (Smith, 1871) (Caridea: Palaemonidae). J Crustac Biol 37(4):445–452
Saborowski R, Thatje S, Calcagno JA, Lovrich GA, Anger K (2006) Digestive enzymes in the ontogenetic stages of the southern king crab, Lithodes santolla. Mar Biol 149(4):865–873
Salaenoi J, Thongpan A, Mingmuang M (2012) Activities of alkaline phosphatase and Ca2+ ATPase over the molting cycle of mud crab ( Scylla serrata ). International Journal of Animal and Veterinary Sciences 6(9):98–103
Sandhu HS, Jande SS (1982) A biochemical and morphological investigation of alkaline phosphatase and Ca+2‐ATPase during initial mineralization in chick embryonic tibia. J. Exp. Zool 221(3):395–398
Serrano AE (2012) Ontogeny of endogenous and exogenous amylase and total protease activities in mud crab, Scylla serrata larvae fed live food. European Journal of Experimental Biology 2(5):1578–1584
Serrano AE, Traifalgar RF (2012) Ontogeny and induction of digestive enzymes in Scylla serrata larvae fed live or artificial feeds or their combination. AACL Bioflux 5(3):101–111
Suprayudi MA, Takeuchi T, Hamasaki K (2012) Cholesterol effect on survival and development of larval mud crab Scylla serrata. Hayati J Biosci 19(1):1–5
Suzer C, Kamaci HO, Coban D, Saka S, Firat K, Ozkara B, Ozkara A (2007) Digestive enzyme activity of the red porgy (Pagrus pagrus, L.) during larval development under culture conditions. Aquac Res 38(16):1778–1785
Urich K (1994) Ester hydrolases, ATPases and Carboanhydrases. In: Comparative animal biochemistry. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 657–684
Worthington TM (1982) Pepsin. In: Division BP (ed) Enzymes and related biochemicals. Worthington diagnostic system Inc. Freehold, New Jersey
Wu Q, Wang S, You C, Li Y (2016) Immune response of mud crab, Scylla Paramamosain, to bacterial lipopolysaccharide. J World Aquacult Soc 47(6):843–853
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khoa, T.N.D., Mai, N.T., Linh, N.K. et al. Ontogenic Development of Digestive Enzymes of Mud Crab (Scylla paramamosain) During Larval Stages. Thalassas 35, 655–661 (2019). https://doi.org/10.1007/s41208-019-00143-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-019-00143-5