Abstract
The pikeperch (Sander lucioperca) is a species with a high potential for aquaculture and a valuable food with high market acceptance. The aim of the study was to evaluate the functional ontogeny of digestive enzyme of pikeperch from hatching to 45 days-post fertilization, 777 degree-day (DPF, dd) under culture condition. The average total length (TL) of larvae measured at hatching was 3.6 ± 0.4 mm (5 DPF; 67 dd) and at the end of experiment (45 DPF, 777 dd) was 27.1 ± 1.1 mm. The survival rate was 80–90% during the experiment period. Inhibition zimography reveals the presence of nine bands with proteolytic activity in the digestive tract of juvenile pikeperch. Zimography results during the ontogeny revealed that in larvae at 8 DPF (108 dd) and 13 DPF (189 dd), three bands were presented. The variations observed in the enzymatic activity reflected a high amount of total protease activity at 10 DPF (133.5 dd). Regarding pepsin, its activity was observed for the first time at 26 DPF (378.9 dd). Lipase activity remained constant from hatching to 26 DPF (378.9 dd). The highest amount of α-amylase activity was detected at 15 DPF (211.5 dd) and 45 DPF (777 dd). The low lipase enzyme activity suggested that live feeds with low lipid were more suitable than diets containing high lipid levels; larvae had also early capability to digest nutrient-dense diet that was high in protein. According to results the pikeperch larvae possess after the exogenous feeding, a functional digestive system with high activities that indicated the gradual development of the digestive system.
Similar content being viewed by others
Introduction
Digestive enzyme study is the first stage of describing and refining the nutritional status during fish ontogeny1. The digestive system of fish larvae in the early life stages is incomplete and consists of a straight intestine and absence of stomach function2. According to Rønnestad et al.3 and Yúfera et al.4, feeding activity and utilization of exogenous enzyme of fish larvae are dependent on digestive system function, in which pancreatic enzymes are responsible of the digestion in the early fish stage2. The nutritional transition between endogenous (yolk sac reabsorbance) and exogenous feeding is a sensitive period in larviculture, which is considered as a key challenge in feeding protocols adaption with digestive capacities during development5,6.
The patterns of digestive enzymes activity are different during fish ontogeny and also among fish larvae and are essential for understanding fish nutritional physiology5,7. This changing in digestive enzyme capacities identify the digest diets which can be used and absorbed during the development3,6,8,9. So for the larvae survival, it is necessary to fed with digestible food after the depletion of endogenous sources7.
The pikeperch Sander lucioperca as a member of percidae family is commercially valuable species and is one of the high demand percid species in market capitalization10. The fast-growing trait of pikeperch compare to other percids and its great potential in aquaculture make it a notable species for mass cultivation for commercial purposes. Hence, the breeding and rearing of pikeperch increases in many European countries such as Denmark, Czech Republic, Poland, Romania, Hungary, Tunisia, Ukraine and Netherlands11. Consequently, the knowledge of the functional digestive system can help to improve the fish diet optimization and feeding strategies during ontogenetic development10. In this respect, in the last decades, some studies have focused on the pikeperch larval digestive system and its functional development. Mani-Ponset et al.12 studied the development of digestive system in pikeperch larvae according to the first diet. Moreover, the changes in digestive tract of pikeperch larvae fed formulated diets was tested by Ostaszewska et al.13. However, the effects of precocious weaning and diets on ontogeny of digestive activities have been documented in this species10. After that, Kamaszewski et al.14 evaluated the effect of feeding on liver and pancreas and activity of some digestive enzymes in pikeperch, and recently the effect of different feeding regimes with rotifers (Brachionus plicatilis) and Artemia salina on gene expression and digestive enzymes in pikeperch larvae highlighted by Imentai et al.15. Hence, no study was carried out on the ontogeny of the digestive enzyme activity of the pikeperch from hatching to juvenile stages. Our work investigated the functional ontogeny of digestive enzyme of pikeperch from hatching through 40th days post hatching (DPH) under culture condition.
Results
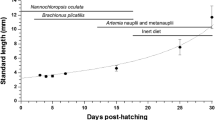
From hatching (5 days post fertilization; 67 degree-day) to the final period of development (45 DPF, 777 dd), the TL of the pikeperch grew continuously and increased from 3.6 ± 0.4 mm to 27.0 ± 1.1 mm, respectively (Fig. 1). The survival rate was 80–90% during the experiment period.
Digestive enzyme activities during pikeperch ontogeny from egg to juvenile stage (45 DPF, 777 dd) is presented in Fig. 2. Pepsin activity was observed for the first time at 26 DPF (378.9 dd; 0.18 ± 0.02 U mg protein−1) then it increased to maximum level at 45 DPF (777 dd; 1.05 ± 0.21 U mg protein−1) (Fig. 2a). According to the results, significant changes were detected in alkaline protease activity during ontogeny (p < 0.05). A high amount of alkaline protease activity was detected at 10 DPF (133.5 dd; 2.71 ± 0.18 U mg protein−1) then sharply increased at 45 DPF (777 dd; 50.19 ± 5.46 U mg protein−1) (Fig. 2b). Differences in lipase activity during ontogeny were significant (p < 0.05). Lipase activity remained constant from hatching to 26 DPF (378.9 dd; 14.89 ± 1.6 mU mg protein−1), then increased to highest level at 45 DPF (777 dd; 125.69 ± 20.32 mU mg protein−1) (Fig. 2c). α-amylase activity was detected from hatching to 45 DPF (777 dd), and it showed significantly differences during larval development (p < 0.05), the highest amount was detected on 15 DPF (211.5 dd; 5.45 ± 0.98 mU mg protein−1) and 45 DPF (777 dd; 20.40 ± 2.41 mU mg protein−1) (Fig. 2d).
Digestive enzymes activity during ontogeny of pikeperch Sander lucioperca. (a) pepsin, (b) total protease, (c) lipase and (d) α-amylase activities. Values are presented as mean ± SEM, n = 4 from egg to 26 DPF and n = 9 for juveniles at 45 DPF. Letters show significant differences between groups (p < 0.05). DPF: day post fertilization.
Inhibition zimography revealed the presence of nine bands with proteolytic activity in the digestive tract of juvenile pikeperch. All of them were serine proteases and independent of Ca2+, as could be seen in lanes 6, 7, and 8. The more significant bands correspond to 31, 24, 23, 19 and 15 KDa. The higher molecular weight bands, 62 and 52 KDa, and the band with 23 KDa showed a weak inhibition by both trypsin and chymotrypsin inhibitors. The band of 34 KDa was inhibited less than 15% by both trypsin and chymotrypsin inhibitors. Moreover, bands with intermediate molecular weight 31 and 24 KDa presented strong inhibition (more than 50%) by the action of TPCK, ZPCK and TLCK. Finally, three bands with low molecular weights, 19, 17 and 15 KDa, were well identified as proteases with chymotrypsin activity (Fig. 3).
Cropped image from inhibition zimogram of alkaline protease activity in digestive tract extract from juvenile pikeperch at 45 DPF. All samples were analyzed individually; the figure shows a representative result. Samples by lane and from left to right: MWM: Molecular Weight Marker, H: homogenate, H + TPCK (chymotrypsin inhibitor), H + ZPCK (chymotrypsin inhibitor), H + TLCK (trypsin inhibitor), H + SBTI, H + PMSF and H + EDTA. Figures shows the molecular weight of bands with proteolytic activity. Original gels are presented in the supplementary Fig. S1.
Zimography results during the ontogeny revealed that in the egg stage and in larvae at 10 DPF (133.5 dd) no digestive enzyme activity could be detected by this technique. The difference between zimography results from digestive enzyme activity and those of total proteolytic activities especially in the egg stage and larvae at 10 DPF (133.5 dd), could be due to the presence of other proteases of non-digestive origin. In homogenates from larvae at 8 DPF (108 dd), three bands were observed, with 34, 23 and 15 KDa. These bands were also detected in larvae at 13 DPF (189 dd), but its activity began to diminish as they grew, finding in larvae at 19 DPF (275.5 dd) just the band with the lower molecular weight. At 26 DPF (378.9 dd) most of the bands that were presented at 45 DPF (777 dd) began to be detected (Fig. 4).
Cropped image of model zimogram of the alkaline proteolytic activity during ontogeny from egg to 21 DPH and digestive tract extract of juvenile pikeperch at 40 DPH. All samples were analyzed individually; the figure shows a representative result. Samples by lane and from left to right: MWM (Molecular Weight Marker), Egg, 8, 10, 13, 15, 19, 26 and 45 DPF. Figure shows the molecular weight of bands with proteolytic activity. DPF: day post fertilization. Original gels are presented in the supplementary Fig. S2.
Discussion
The ontogenetic development of the digestive system in fish larvae provides valuable information and understanding of fish digestive physiology for diet optimization in mass marine fish larviculture16,17,18. Among fish species, the digestive enzymes activity varies in species, age and type of diet15,19.
The maturation of the digestive system gradually progresses with larval development. In pikeperch, the functional organs of the newly hatched larvae developed over a relatively short period, less than 15 DPF (211.5 dd) and the inflection points in body proportion changes in pikeperch occurred when the body length was between 9.0 and 12.5 mm. The importance of morphological modifications occurred during the early life periods of pikeperch, showing that these changes coincide with the functional needs throughout the early life period and adaptation to adult life style. In the stage of change from endogenous to exogenous feeding, fish larvae are highly sensitive. Sufficient quantities of food with highest ingestion rate provided enough assimilation to guarantee metabolism and survival during ontogeny20. Success of aquaculture in terms of healthy cultured stock depends on highly nutritious quality diet that is readily accepted and digested. The survival rate of pikeperch larvae was high in this study, however, the use of live feed enrichments due to the added nutritional value to pikeperch larvae feed may help the fast growth rate and survival. So future work is recommended to improve the pikeperch larval growth and survival with the modification of the current feeding scheme.
In fish species the successful food consumption depends on the availability of appropriate food items, a developed digestive system and enzyme activities, and the capacity for locomotion3. In this study, the mouth was not yet open and the jaw was not yet formed in the endogenous feeding stage. The preflexion stage coincided with the first exogenous feeding at 5 DPH (10 DPF, 133.5 dd) and at the initial of this stage, the depletion of the yolk sac and oil globule were observed along with the endo-exogenous feeding period. According to Ostaszewska et al.13 the yolk sac material absorption is accompanied by an intense development of digestive system and the macroscopic yolk sac resorption initiated on the 120 dd (fed with Artemia nauplii and formulated diets). The newly hatched larvae of fish soon after yolk absorption starts exogenous feeding, but they have very small mouth size (less than 0.1 mm), primitive digestive system and low digestibility21. The size of mouth is very important and determines the ability of the larvae to consume different food organisms22. So these larvae require small size, easily digestible, and nutrient riched live feed as their diet. Live feed organisms contain all the nutrients such as essential proteins, lipids, carbohydrates, vitamins, minerals, amino acids and fatty acids21. Cladocerans serve as an important food for small fish, because of their small size and jerky movement which make them more visible to fish larvae. Rotifers, Artemia and copepods are the main live feeds. Rotifers are suitable for the earliest stages of fish growth because of their small size23. In this study, the size of Artemia and Daphnia magna was less than 1 mm which is suitable for capturing and ingestion of pikeperch larvae between 16 DPF (228.5 dd) to 32 DPF (479 dd). Copepods are considered to be superior in terms of nutritional composition as they contain higher levels of highly unsaturated fatty acids21, and they can enhance growth and survival of first-feeding fish larvae.
The water temperature affects the activity of all enzymes. The effects of water temperature on fish digestive enzyme activities seem species-specific, of which it directly affects the digestibility and metabolism of nutrients such as proteins and lipids24,25. There is an optimal temperature for digestive enzymes to better participate in the biological reaction process. According to the kinetics of enzymatic reaction and the protein properties of digestive enzymes, the enzymatic activity increases with the increase in temperature up to a certain level25. In this study, the growth and activity of some digestive enzymes of pikeperch larvae reached the maximum at higher temperatures (777 dd), under light density of 500 lux and fed with live feed organisms. In Lutjanus malabaricus the enzymes activity increased at high temperature (30 °C, as carnivorous fish), which is in accordance with our results26. So it can be stated that metabolism increases with temperature increases. Also the similar results were observed in Catla catla (optimal temperature 18–28 °C, as omnivorous fish), in which low temperature at 10 °C decreased digestive enzyme activities compared to fish held at 25 °C27.
The digestive potential of fish is highly variable, changing with species, water temperature, food and feeding history and development stage. According to our results, the alkaline protease activity of pikeperch larvae showed high levels during the ontogeny from hatching to exogenous feeding, and decreased at the end of larval and juvenile stages. During the larval stages, proteins are digested mainly by alkaline proteases such as trypsin and chymotrypsin19. Thus, this study showed that pikeperch larvae have trypsin and chymotrypsin since 8 DPF (108 dd). Before the absorption of the yolk sac, trypsin has the highest level of activity in white sea bass (Atractoscion nobilis) larvae cultured at 18 °C28. This result is in agreement with the observation in turbot (Scophhthamus maximus) cultured under fed and fasting condition, which increased the activity of trypsin, chymotrypsin and alkaline phosphatase enzymes before first feeding29. In Siberian sturgeon (Acipenser baerii) the activity of chymotrypsin increased after the consumption of exogenous food30. An increase in alkaline phosphatase activity was also observed in marine fish, sea bream (Sparus aurata), during feeding31. In wolf cichlid (Parachromis dovii) larvae cultured at 25–29 °C (fed with Artemia nauplii and formulated feed), trypsin activity reached the highest level during 19 DPF (275.5 dd)32. Generally, in this study trypsin activity was observed during 8 DPF (108 dd) to 19 DPF (275.5 dd), then tended to decrease until 45 DPF (777 dd), although a high activity was observed at the 10 DPF (133.5 dd), which is the day of the start of active (exogenous) feeding. The chymotrypsin activity increased similar to that of trypsin during the first 8 (108 dd) to 19 (275.5 dd) DPF in pikeperch larvae. It may be due to the use of a high protein diet, such as zooplanktons, during exogenous feeding. Vijverberg and Frank33 determined 71.2% protein, 19.3% lipids and 9.5% carbohydrates in zooplanktons such as cladocerans. In this study, pikeperch larvae were fed with small zooplanktons until 15 DPF (211.5 dd), followed by Artemia nauplii and Daphnia magna and other natural live feed until 45 DPF (777 dd). Ahmadi et al.34 reported that Artemia nauplii has a high amount of C18:3n3 fatty acid and in fresh hatched Artemia nauplii proximate composition were calculated as protein 37–71%, lipid 12–13%, carbohydrate 11–23% and ash 4–21%. The contents of protein 47.7%, lipid 6.1%, carbohydrate 20% and ash 15.6% were calculated in Daphnia magna35. So it can be concluded that the proximate composition of food items influence the digestive enzymes of pikeperch. The pepsin activity was observed at 26 DPF (378.9 dd) and showed the maximum level in the juvenile stage. During the early life stage, pancreas enzymes play a key role in digestion due to the poor developed digestive system36. Daries et al.37 stated that the complete maturation of gastric glands takes place when these are able to secret pepsin and hydrochloric acid. The appearance of pepsin activity in the transition from larval to juvenile stage in fish species such as pikeperch and common pandora (Pagellus erythrinus)10,38, red snout cichlid (Petenia splendida)39, red porgy (Pagrus pagrus)37 and spotted rose snapper (Lutjanus guttatus)40 were reported. According to the results of this study, live feeds were considered as a food item during the growth of pikeperch larvae. Live feed organisms facilitate the process of digestion and assimilation by autolysis and by providing their digestive enzymes to fish larvae41.
It seems that fish larvae adapt their functional metabolism to nutrients in their environment through enzyme secretion37. Therefore, the increase in pepsin activity is associated with a sudden decrease in trypsin and chymotrypsin activity after the yolk sac absorption and the start of exogenous feeding. In other words, the transfer of enzyme activity from alkaline proteases (trypsin and chymotrypsin) to acidic proteases (pepsin) was observed during the growth and development of pikeperch larvae, which can be related to physiological changes that occur during larval development, such as increased protein ingestion, the appearance of hormones or other enzymes, although a type of genetic programming can not be discarded19,42.
The α-amylase activity in pikeperch decreased during the larval stages and it increased at 15 DPF (211.5 dd) and in the juvenile stage, which can be related to pikeperch prey during the first stages of exogenous feeding and in larvae-juvenile transition. Castro-Ruiz et al.6 suggested that α-amylase activity decreased during larval period in tiger shovelnose catfish (Pseudoplatystoma punctifer) larvae cultured at 27.8 ± 0.7 °C (fed with Artemia nauplii and formulated feed). In agreement with Pradhan et al.5 on butter catfish (Ompok bimaculatus) larvae reared at 27.0 ± 1.1 °C (fed with Artemia nauplii and zooplankton), and Frías-Quintana et al.43 on tropical gar (Atractosteus tropicus) larvae cultured at 29.0 ± 1.0 °C (fed with Artemia nauplii and trout feed), α-amylase showed low activity at hatching, and it increased gently after exogenous feeding. One of the reasons for digestive enzyme changes during fish ontogeny can be due to larval feeding behaviour and chemical composition of food items44. The enzyme activity in fish was closely related to the diet45,46 and diet affects the enzyme activity especially at the last stage of larval development when the larvae adapted well to the diet47. Hence, it was suggested that different patterns of α-amylase activity during the ontogenesis between species, could be linked to their digestive physiology differences or feeding habits6,48. In current study, pikeperch larvae received no food until absorbing the yolk sac, so the presence of amylase at this stage indicates that it is synthesized during the early stages of larval development even in the absence of food. In general, this feature is a genetic programming in fish larvae to digest carbohydrates after the early larval stages49.
The study of lipase activity during larvae ontogeny allows to have a clue about the utilization of dietary lipids6,50. Based on the current study, an early lipase activity was detected after hatching and the levels of this enzyme in juveniles were significantly higher than those found in larvae. The lipase activity after hatching refers to yolk lipid catabolism as an energy source for larval development before exogenous feeding51. The high amount of lipase activity at juvenile stage of pikeperch can be related to the amount of lipid contents in diets, or changes in the nutritional requirements, which are reflected in the growth rate, or the acquisition of full digestive capacities of this species6. According to Oozeki and Bailey51, the lipase activity divided to two parts: one part is related to yolk sac absorption and the second is related to digestion of exogenous lipids. Since pikeperch larvae received no food until absorbing the yolk sac, so the presence of lipase at this stage can be categorized as the first type. The digestive organs generally increase in both volume and surface area as the larvae grew. Similar to other fish larvae, it seems that the pikeperch larvae would preferably rely on dietary lipids to meet their energy requirements. The increase in volumetric capacity of digestive tract during development and lipase activity increases has been shown for some fishes such as Soldatov's catfish (Silurus soldatovi)52, striped catfish (Pangasianodon hypophthalmus)53 and butter catfish5.
To our knowledge, this is the first study that has done a characterization of pikeperch trypsin and chymotrypsin-like activities during the ontogeny. In this sense, the results showed the presence of three proteases of 34, 23 and 15 KDa whose activity was reduced during the early development, being only detected the 15 KDa protease at 19 DPF (275.5 dd). This suggests that a change in digestive protease activity was taking part during this developmental stage, as at 26 DPF (378.9 dd) most of the proteases found in juveniles at 45 DPF (777 dd) were found. Accordingly, a different proteases band pattern was found in seabass fingerlings reared at 25 ± 0.5 °C (fed with high starch or lipid diets) compared with juveniles54,55. Moreover, in juveniles 3 proteases with low molecular weights (19, 17 and 15 KDa) had been identified as chymotrypsin-like activities as was found in other species, like seabream reared at 22 °C (fed with high dietary carbohydrate inclusion by both protein and lipid replacement) and in sea bass fingerlings reared at 21–22 °C, where the dietary protein sparing effect by lipids and carbohydrates were studied54,56. Unlike in those species, no trypsin-like activities were clearly identified since 31 and 24 KDa proteases presented strong inhibition by both trypsin and chymotrypsin inhibitors, whereas for 62, 52 and 23 KDa presented weak inhibition. This dual inhibition could be related with the concentration of each inhibitor. In this sense, further studies are needed to characterize trypsin and chymotrypsin activities, since an imbalance in the trypsin/chymotrypsin rate can lead to an impairment in the availability of amino acids and therefore in nutrient absorption affecting fish growth57,58.
Conclusion
In conclusion, our study shows that temperature is likely one of the important physical environmental factors affecting the growth of pikeperch and may improve the ontogeny and maturation of the digestive enzymes. The pikeperch larvae reared under higher temperature presented a better growth at the juvenile stage, which might be due to higher digestion rates and better food efficiency. Pikeperch larvae showed the low lipase enzyme activity compared to other enzymes, so it can be suggested that live feeds with low lipid levels are more suitable than the diets containing high lipid level. Based on our results, pikeperch larvae have early capability to digest nutrient-dense diet that is high in protein. So, differences in the enzymatic activity are related to type of feeding habits of the studied fish and also high activities of digestive enzymes after the exogenous feeding reflected the gradual maturation of the digestive system.
Materials and methods
Larval and juvenile rearing
Pikeperch larvae were obtained by spontaneous spawning of pikeperch broodstock held at controlled conditions under temperature of 13.9 ± 0.4 °C. The wild broodstock were captured from the Aras dam reservoir in northwest Iran and transported to the Dr. Yousefpour Marine Fishes Restocking and Genetic Conservation Center (Siahkal, Guilan, Iran). The caught female and male fish were 4–5 years old and the body weight was 1.1 ± 0.1 kg.
Before the spawning, 44 females and 48 males were separately acclimated in twelve concrete ponds (1.8 × 1.8 × 0.5 m) for 5 to 7 days under identical conditions and without feeding. For hormonal induction, fourteen females and sixteen males were held in each rectangular concrete tank (13.0 × 3.1 × 1.1 m) with an artificial spawning nest (50 × 50 cm)59 for each pair. The type of spawning nest was artificial turf. The female spawning was induced by injection of 200 IU human chorionic gonadotropin kg−1 body weight, whereas male fish received no injections because they were ready to spawn. The pikeperch pairs spawned on artificial spawning nests (for females spawning occured at 80–85 h after injection).
Larval rearing conditions
The spawned adhesive eggs attached to the nests were then transferred to the circular concrete tanks. The size of the tank was 180 × 50 cm; 1270 L water volume. Hatching occurred at 3nd–5th DPF (day-post fertilization). After hatching the eggs, artificial spawning nests were taken away from the circular concrete tank to supply enough space for the growth and movement of larvae. The mean larval density in each circular concrete tank was 70 ind. l−1. The light intensity was 500 lux60, and larvae were exposed in natural photoperiod of 12L:12D. The air blowers provided enough oxygen for larvae. The water flow was maintained at 0.3–0.5 L min−1. Water exchange by daily siphoning was carried out to remove any waste matter from the bottom of the tanks.
The water quality characteristics were recorded daily in all raring tanks at the same time from 11:00 to 12:00 A.M. During larval culture period, the water physico-chemical characters were: temperature 18.7 ± 0.3 °C, dissolved oxygen 7.2 ± 0.6 mg l−1, ammonia nitrogen 0.03 mg l−1 and pH 7.8 ± 0.2. Rotifers, cyclopoid copepods, copepod nauplii, or some small cladocerans were mainly used as first prey for the 10 DPF larvae61,62. It could be noted that after absorption of yolk sac the pikeperch larvae are very sensitive to starvation due to high metabolic and anabolic demands11. The larvae feed were composed of Artemia nauplii and then with Daphnia magna at the 16 DPF, of which density was kept at about 30 ind. ml−1, three times per day (at 8.00; 13.00; and 18.00). The small zooplanktons were obtained from a stock produced in an earthen pond (using caw manure as an organic fertilizer for microalgae growth on which the zooplankton feed)63. The Artemia nauplii was hatched based on the standard procedure64. After 16–24 h, about 70% of the cysts at 29 °C were hatched and fed to the larvae. From 20 DPF to the juvenile stage, larvae were reared in three earthen ponds under identical conditions including water exchange (5–20% every day), feeding (natural zooplankton such as rotifers, caldocerans and copepods)65, water temperature (21.5 ± 2.5 °C), water ammonia nitrogen (controlled under 0.03 mg l−1) and natural photoperiod. Pond size was 4 ha and larval density was 400 × 103 larva ha−1 in the earthen pond.
Sampling procedure
During the experiment, random samples of eggs before hatching (3nd-5th DPF (day-post fertilization) and daily samples of larvae were collected randomly from each tank (from 6 to 20 DPF) and pond (from 20 DPF to the juvenile stage) at the same time of day. Larvae were killed with an overdose of clove powder extract and snap frozen in liquid nitrogen. All samples were stored at − 80 °C until further analyses.
Pikeperch survival rate during developmental stages was calculated by the following formula66.
Biochemical analyses
Sample preparation
Sample homogenization, pepsin, total alkaline protease activities, trypsin and chymotrypsin characterization were performed according to García Meilán et al.56,67. Briefly, eggs, whole larvae from day 8 to 15, larvae at 19 and 26 DPF without head and tail, and individually digestive tract without gallbladder in juveniles at 45 DPF were weighed and buffer solution (Tris–HCl, 50 mM, pH 7.5) was added to a final concentration of 200 mg ml−1. Samples were then homogenized using rapid vibration (6500 rpm; 3 × 20 s with three breaks of 20 s; 4 °C) in a Precellys Evolution® Homogenaizer combined with Cryolys® as a cooling system (Bertin Technologies, France). Next, homogenates were centrifuged for 15 min (2400 rpm; 4 °C; Eppendorf, 5418R) and supernatants were stored at − 80 °C for future analysis. The 4 different homogenates were used from larvae at the different stages (DPF) and 9 individual samples for juveniles.
Digestive enzyme activity
For determination of acid protease activity, homogenized samples were reacted with 50 mM glycine–HCl buffer containing 1% bovine hemoglobin, pH 2 at 25 °C, whereas for total alkaline protease activity (TPA), samples were reacted with 50 mM Tris–HCl buffer containing 1% casein, pH 9.0 at 25 °C. After 60 min, trichloroacetic acid 10% was used for the reaction stop. The samples from both analyses were kept at 4 °C for an hour and then centrifuged (7500 rpm, 5 min, 4 °C). Individual blanks were established for each sample. Supernatant absorbance was measured at 280 nm (Infinite 200 PRO, Grödig, Tecan, Austria). Pepsin from porcine gastric mucosa (Sigma Aldrich, Madrid, Spain, 3440 U/mg solid) was used as standard and acid protease activity was measured as BAEE units. Bovine trypsin (Sigma Aldrich, Madrid, Spain, 12,100 BAEE U/mg protein, NC-IUB, 1979) was used as the standard.
The protein concentration in homogenate samples was measured according to Bradford68 method using bovine serum albumin as a standard.
Lipase and α-amylase enzymes activity was determined according to the kit manufacturer’s recommendation (Spinreact, Sant Esteve d’en Bas, Girona, Spain). For lipase, methylresorufin formation was measured at 580 nm. For α-amylase, the rate of 2-cloro-4-nitrophenol formation at 405 nm was determined. Activities of both enzymes were reported as mU per mg of protein. Both tests were conducted at 25 ± 0.5 °C using a microplate scanning spectrophotometer (Tecan Infinite 200 PRO, Grödig, Tecan, Austria).
For digestive activities, 4 pools of samples from egg to 26 DPF stage and 9 individual samples for juveniles (45 DPF) were analysed.
Zimogram
Trypsin and chymotrypsin-like activities were characterized by zimography according to Santigosa et al.69. Proteolytic characterization was done by the combination of the homogenate with water or the corresponding inhibition solution for 45 min at a ratio of 1:1. Inhibition solutions selected were: tosyllysyl chloromethyl ketone (TLCK; 10 mM in HCl 1 mM) as trypsin-like activity inhibitor, tosyl phenylalanyl chloromethyl ketone (TPCK; 10 mM in methanol) and carbo benzoxyphenylalanyl chloromethyl ketone (ZPCK; 10 mM in dioxane) as chymotrypsin-like activity modifiers, SBTI (250 µM in H2Od) and PMSF (100 mM in 2-isopropanol) as total serine protease activity inhibitor and EDTA (0.5 in H2Od) to determine protease dependence on divalent cations, according to Alarcon et al.70.
Samples were loaded in 12% polyacrylamide gels (10 × 10.5 × 0.1 cm), pure trypsin and albumin were used as controls and a commercial weight marker RPN 800 (Amersham, GE Healthcare, UK, 12,000–225,000 Da) was used to determine the molecular weight of protease active fractions from the homogenized samples. Electrophoresis was performed at a constant current of 15 mA per gel for 100 min (Biorad 4 °C). The gels were incubated under agitation with TrisHCl buffer containing 2% casein, pH 8.2 for 30 min at 4 °C, and then it was shaken for 90 min at room temperature. Gels were washed and stained in a methanol:acetic acid:water solution (40:40:10) with 0.1% BBC R-250 (Coomassie Brilliant Blue R-250) for 15–20 min. After that, the same solution without colorant was used for destaining for 5–10 min and agitation was applied during both procedures.
Trypsin and chymotrypsin-like activities were characterized by 3 inhibition zimograms for juvenile stage (Supplementary Fig. S1). Protease activity during pikeperch ontogeny was detected by running 4 zimography gels, in which every gels contains one homogenate from egg to juvenile stages (Suplemmentary Fig. S2).
Signifcance statement
This work contains important studies on pancreatic and proteolitic enzymes for the evaluation of functional ontogeny of digestive enzyme of pikeperch from hatching to 45 days post fertilization (DPF) under culture condition. Inhibition zimography reveals the proteolytic activity in the digestive tract of juvenile pikeperch. All digestive enzymes were detected at egg, except pepsin. Also, larvae had the ability to digest diet that was high in protein. Despite this, they showed low lipase activity compared to other enzymes. In summary, larvae possess a functional digestive system with high enzyme activities after the exogenous feeding that indicated the gradual development of the digestive system.
Statistical analyses
Data were analyzed using IBM SPSS Statistics v.25 (Armonk, USA) and were presented as means ± standard error of the means (SEM). The normal distribution was analyzed using the Shapiro–Wilk test and homogeneity of the variances (homoscedasticity) was assessed with Levene’s test. If normal distribution and/or homoscedasticity were not found, data were transformed logarithmically. Significant differences were tested by One-way analysis of variance (ANOVA) and the post-hoc Tukey HSD. If necessary, the nonparametric Kruskal Wallis test and the post-hoc Games-Howell were used. Statistical differences were considered significant when p < 0.05. Zimogram inhibition results were analyzed using Quantity One 1-D Analysis Software 4.6.6 (Bio-Rad Laboratories, Inc, Hercules, California, USA).
Ethics approval
All the experimental animal procedures involved in this study were approved by the animal care and welfare of University of Guilan and followed the experimental basic principles by the ARRIVE guidelines.
All the reporting in the manuscript follows the recommendations in the ARRIVE guidelines for Reporting Animal Research. No distress or suffering was produced by the procedures used to perform this study.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Pérez-Sirkin, D. I. et al. Digestive enzyme activities during pejerrey (Odontesthes bonariensis) ontogeny. Aquaculture 524, 735151. https://doi.org/10.1016/j.aquaculture.2020.735151 (2020).
Srichanun, M., Tantikitti, C., Vatanakul, V. & Musikarune, P. Digestive enzyme activity during ontogenetic development and effect of live feed in green catfish larvae (Mystus nemurus Cuv. & Val.). Songklanakarin J. Sci. Technol. 34, 247–254 (2012).
Rønnestad, I. et al. Feeding behaviour and digestive physiology in larval fish: Current knowledge, and gaps and bottlenecks in research. Rev. Aquac. 5, 59–98. https://doi.org/10.1111/raq.12010 (2013).
Yufera, M., Moyano, F. J. & Martínez-Rodríguez, G. The digestive function in developing fish larvae and fry. From molecular gene expression to enzymatic activity. In Emerging Issues in Fish Larvae Research (ed. Yúfera, M.) 51–86 (Springer International Publishing AG, 2018). https://doi.org/10.1007/978-3-319-73244-2_3.
Pradhan, P. K., Jenaa, J., Mitra, G., Sood, N. & Gisbert, E. Ontogeny of the digestive enzymes in butter catfish Ompok bimaculatus (Bloch) larvae. Aquaculture 372, 62–69. https://doi.org/10.1016/j.aquaculture.2012.10.024 (2013).
Castro-Ruiz, D. et al. Ontogeny of the digestive enzyme activity of the Amazonian pimelodid catfish Pseudoplatystoma punctifer (Castelnau, 1855). Aquaculture 504, 210–218. https://doi.org/10.1016/j.aquaculture.2019.01.059 (2019).
Kim, B. G., Divakaran, S., Brown, C. L. & Ostrowski, A. Comparative digestive enzyme ontogeny in two marine larval fishes: Pacific thread fin (Polydactylus sexfilis) and blue fin trevally (Caranx melampygus). J. Fish Physiol. Biochem. 24, 225–241. https://doi.org/10.1023/A:1014054431627 (2001).
Gisbert, E., Morais, S. & Moyano, F. J. Feeding and digestion. In Larval Fish Aquaculture (ed. Quin, J. G.) 73–123 (Nova Science Publishers, 2013).
Hamre, K. et al. Fish larval nutrition and feed formulation: Knowledge gaps and bottlenecks for advances in larval rearing. Rev. Aquacult. 5, 26–58. https://doi.org/10.1111/j.1753-5131.2012.01086.x (2013).
Hamza, N., Mhetli, M. & Kestemont, P. Effects of weaning age and diets on ontogeny of digestive activities and structures of pikeperch (Sander lucioperca) larvae. Fish Physiol. Biochem. 33, 121–133. https://doi.org/10.1007/s10695-006-9123-4 (2007).
Rahmdel, K. J. & Falahatkar, B. Adaptation of pikeperch (Sander lucioperca) to formulated diets: A review. Fish. Aquat. Life. 29, 1–12. https://doi.org/10.2478/aopf-2021-0001 (2021).
Mani-Ponset, L. et al. Development of yolk complex, liver and anterior intestine in pikeperch larvae, Stizostedion lucioperca. (Percidae), according to the first diet during rearing. Aquat. Living Resour. 7, 191–202 (1994).
Ostaszewska, T., Dabrowski, K., Czuminska, K., Olech, W. & Olejniczak, M. Rearing of pikeperch larvaeusing formulated diets-first success with starter feeds. Aquac. Res. 36, 1167–1176. https://doi.org/10.1111/j.1365-2109.2005.01332.x (2005).
Kamaszewski, M. & Ostaszewska, T. Effect of feeding on digestive enzyme activity and morphological changes in the liver and pancreas of pike-perch (Sander lucioperca). Israeli J. Aquac-Bamidge. 62, 225–236 (2010).
Imentai, A. et al. Effect of first feeding regime on gene expression and enzyme activity in pikeperch (Sander lucioperca) larvae. Front. Mar. Sci. 9, 8864536. https://doi.org/10.3389/fmars.2022.864536 (2022).
Diaz, M., Moyano, F., Alarcon, F., Garcıa-Carreno, F. & Navarrete del Toro, M. Characterization of fish acid proteases bysubstrate-gel electrophoresis. Comp. Biochem. Physiol. 121B, 369–377. https://doi.org/10.1016/s0305-0491(98)10123-2 (1998).
Cahu, C. & Zambonino Infante, J. L. Is the digestive capacity of marine fish larvae sufficient for a compound diet feeding?. Aquac. Int. 5, 151–160 (1997).
Asgari, R. et al. Ontogeny of the digestive enzyme activities in hatchery produced beluga (Huso huso). Aquaculture 416, 33–40. https://doi.org/10.1016/j.aquaculture.2013.08.014 (2013).
Zambonino Infante, J. L. & Cahu, C. L. Ontogeny of the gastrointestinal tract of marine fish larvae. Comp. Biochem. Physiol. 130C, 477–487. https://doi.org/10.1016/s1532-0456(01)00274-5 (2001).
Powell, A. B. & Chester, A. J. Morphometric indices of nutritional condition and sensitivity to starvation of spot larvae. Trans. Am. Fish. Soc. 114, 338–347 (1985).
Dhont, J., Dierckens, K., Støttrup, J. G., Stappen, G., Wille, M. & Sorgeloos, P. Rotifers, Artemia and copepods as live feeds for fish larvae in aquaculture. In Advances in Aquaculture Hatchery Technology, 157–202. https://doi.org/10.1533/9780857097460.1.157 (2013).
Huet, M. Text-book of fish culture; Breeding and Cultivation of Fish. 2nd edn (Fishing News Books, 1986).
Fielder, D. S., Purser, G. J. & Battaglene, S. C. Effect of rapid changes in temperature and salinity on availability of the rotifers Brachionus rotundiformis and Brachionus plicatilis. Aquaculture 189, 85–99. https://doi.org/10.1016/S0044-8486(00)00369-0 (2000).
Fang, J., Tian, X. & Dong, S. The influence of water temperature and ration on the growth, body composition and energy budget of tongue sole (Cynoglossus semilaevis). Aquaculture 299, 106–114. https://doi.org/10.1016/j.aquaculture.2009.11.026 (2010).
Volkoff, H. & Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 7, 307–320. https://doi.org/10.1080/23328940.2020.1765950 (2020).
Mazumder, S. K., Das, S. K., Rahim, S. M. & Mazlan, A. G. Temperature and diet effect on the pepsin enzyme activities, digestive somatic index and relative gut length of Malabar blood snapper (Lutjanus malabaricus Bloch & Schneider, 1801). Aquac. Rep. 9, 1–9. https://doi.org/10.1016/j.aqrep.2017.11.003 (2018).
Sharma, J., Singh, S. P. & Chakrabarti, R. Effect of temperature on digestive physiology, immune- modulatory parameters, and expression level of Hsp and LDH genes in Catla catla (Hamilton, 1822). Aquaculture 479, 134–141. https://doi.org/10.1016/j.aquaculture.2017.05.031 (2017).
Galaviz, M., García-Gasca, A., Drawbridge, M., Álvarez-González, C. A. & López, L. Ontogeny of the digestive tract and enzymatic activity in white seabass, Atractoscion nobilis, larvae. Aquaculture 318, 162–168. https://doi.org/10.1016/j.aquaculture.2011.05.014 (2011).
Cousin, J. C. B., Baudin-Laurencin, F. & Gabaudan, J. Ontogeny of enzymatic activities in fed and fasting turbot Scophthalmus maximus L. J. Fish Biol. 30, 15–33. https://doi.org/10.1111/J.1095-8649.1987.TB05728.X (1987).
Gisbert, E., Sarasquete, M. C., Williot, P. & Castello-Orvay, F. Histochemistry of the development of the digestive system of Siberian sturgeon (Acipenser baeri, Brandt) during early ontogeny. J. Fish Biol. 55, 596–616 (1999).
Sarasquete, M. C., Polo, A. & Gonzales De Canales, M. L. A histochemical and immunochemical study of digestive enzymes and hormones during larval development of the sea bream, Sparus aurata L. Histochem. J. 25, 430–437. https://doi.org/10.1007/BF00157807 (1993).
Frías-Quintana, C. A. et al. Changes in digestive enzymes activities during the initial ontogeny of wolf cichlid, Parachromis dovii (Perciformes: Cichlidae). Neotrop. Ichthyol. 17, e180161. https://doi.org/10.1590/1982-0224-20180161 (2019).
Vijverberg, J. & Frank, H. T. H. The chemical composition and energy contents of copepods and cladocerans in relation to their size. Freshw. Biol. 6, 333–345. https://doi.org/10.1111/j.1365-2427.1976.tb01618.x (1976).
Ahmadi, M. R., Leibovitz, H. & Simpson, K. L. Nutrient composition of the Iranian brine shrimp (Artemia uromiana). Comp. Biochem. Physiol. 95B, 225–228. https://doi.org/10.1016/0305-0491(90)90069-6 (1990).
El-feky, M. & Abo-Taleb, H. Effect of feeding with different types of nutrients on intensive culture of the water flea, Daphnia magna Straus, 1820. Egypt. J. Aquat. Biol. Fish. 24, 655–666. https://doi.org/10.21608/EJABF.2020.76554 (2020).
Gisbert, E., Giménez, G., Monzón, I. F., Kotzamanis, Y. & Estevez, A. Development of digestive enzymes in common dentex Dentex dentex during early ontogeny. Aquaculture 287, 381–387. https://doi.org/10.1016/j.aquaculture.2008.10.039 (2009).
Darias, M., Ortiz-Delgado, J., Sarasquete, C., Martínez-Rodríguez, G. & Yufera, M. Larval organogenesis of Pagrus pagrus L., 1758 with special attention to the digestive system development. Histol. Histopathol. 22, 753–768. https://doi.org/10.14670/HH-22.753 (2007).
Suzer, C., Firat, K. & Saka, S. Ontogenic development of the digestive enzymes in common pandora (Pagellus erythrinus L.) larvae. Aquac. Res. 37, 1565–1571. https://doi.org/10.1111/j.1365-2109.2006.01598.x (2006).
Trevino, L. et al. A histological study of the organogenesis of the digestive system in bay snook Petenia splendida Gunther, 1862 from hatching to the juvenile stage. J. Appl. Ichthyol. 27, 73–82. https://doi.org/10.1111/j.1439-0426.2010.01608.x (2011).
Peña, E., Hernández, C., Ibarra-Castro, L. & Álvarez-González, C. A. In vitro protein digestibility of different grow-out stages of spotted rose snapper (Lutjanus guttatus, Steindachner, 1869). Aquac. Nutr. 23, 1204–1215. https://doi.org/10.1111/anu.12489 (2017).
Kolkovski, S. Digestive enzymes in fish larvae and juveniles—Implications and applications to formulated diets. Aquaculture 200, 181–201. https://doi.org/10.1016/S0044-8486(01)00700-1 (2001).
Lazo, J. P., Mendoza, R., Holt, G. J., Aguilera, C. & Arnold, C. R. Characterization of digestive enzymes during larval development of red drum (Sciaenops ocellatus). Aquaculture 265, 194–205. https://doi.org/10.1016/j.aquaculture.2007.01.043 (2007).
Frías-Quintana, C. et al. Development of digestive tract and enzyme activities during the early ontogeny of the tropical gar Atractosteus tropicus. Fish Physiol. Biochem. 41, 1–17. https://doi.org/10.1007/s10695-015-0070-9 (2015).
Kuz’mina, V. V. Influence of age on digestive enzyme activity in some freshwater teleosts. Aquaculture 148, 25–37. https://doi.org/10.1016/S0044-8486(96)01370-1 (1996).
Hardewig, I. & Van Dijk, P. L. M. Is digestive capacity limiting growth at low temperatures in roach?. J. Fish Biol. 62, 358–374. https://doi.org/10.1046/j.1095-8649.2003.00027.x (2003).
Liu, H. et al. The gut microbiome and degradation enzyme activity of wild freshwater fishes influenced by their trophic levels. Sci. Rep. 6, 24340. https://doi.org/10.1038/srep24340 (2016).
Jones, D. A., Kumlu, M., Le Vay, L. & Fletcher, D. J. The digestive physiology of herbivorous, omnivorous and carnivorous crustacean larvae: A review. Aquaculture 155, 289–299. https://doi.org/10.1016/S0044-8486(97)00129-4 (1997).
Solovyev, M. M., Kashinskaya, E. N., Izvekova, G. I., Gisbert, E. & Glupov, V. V. Feeding habits and ontogenic changes in digestive enzyme patterns in five freshwater teleosts. J. Fish Biol. 85, 1395–1412. https://doi.org/10.1111/jfb.12489 (2014).
Zambonino Infante, J. L. & Cahu, C. L. Dietary modulation of some digestive enzymes and metabolic processes in developing marine fish: Applications to diet formulation. Aquaculture 268, 98–105. https://doi.org/10.1016/j.aquaculture.2007.04.032 (2007).
Zambonino Infante, J. L., Gisbert, E., Sarasquete, C., Navarro, I., Gutierrez, J. & Cahu C. L. Ontogeny and physiology of the digestive system of marine fish larvae. In Feeding and Digestive Functions (eds Cyrino, J. E. P. et al.). 281–348. https://archimer.ifremer.fr/doc/00086/19684/ (2008).
Oozeki, Y. & Bailey, K. M. Ontogenetic development of digestive enzymes activities in larval walleye pollock, Theragra chalcograma. Mar. Biol. 122, 177–186. https://doi.org/10.1007/BF00348930 (1995).
Liu, W., Zhang, X. M. & Wang, L. B. Digestive enzyme and alkaline phosphatase activities during the early stages of Silurus soldatovi development. Zool. Res. 31, 627–632. https://doi.org/10.3724/SP.J.1141.2010.06627 (2010).
Rangsin, W., Areechon, N. & Yoonpundh, R. Digestive enzyme activities during larval development of striped catfish, Pangasianodon hypophthalmus (Sauvage, 727 1878). Kasetsart. J. Nat. Sci. 46, 217–228 (2012).
García-Meilán, I. et al. Effects of dietary protein-to-lipid ratio on digestive and absorptive processes in sea bass fingerlings. Aquaculture 463, 163–173. https://doi.org/10.1016/j.aquaculture.2016.05.039 (2016).
García-Meilán, I., Herrera-Muñoz, J. I., Ordóñez-Grande, B., Fontanillas, R. & Gallardo, M. Á. Growth performance, digestive enzyme activities, and oxidative stress markers in the proximal intestine of European sea bass (Dicentrarchus labrax) fed high starch or lipid diets. Fishes 8, 223. https://doi.org/10.3390/fishes8050223 (2023).
García-Meilán, I., Ordóñez-Grande, B., María Valentín, J., Fontanillas, R. & Gallardo, M. A. High dietary carbohydrate inclusion by both protein and lipid replacement in gilthead sea bream. Changes in digestive and absorptive processes. Aquaculture 520, 734977. https://doi.org/10.1016/j.aquaculture.2020.734977 (2020).
García-Meilán, I., Valentín, J. M., Fontanillas, R. & Gallardo, M. A. Different protein to energy ratio diets for gilthead sea bream (Sparus aurata): Effects on digestive and absorptive processes. Aquaculture 412–413, 1–7. https://doi.org/10.1016/j.aquaculture.2013.06.031 (2013).
Guillaume, J. & Choubert, G. Digestive physiology and nutrient digestibility in fishes. In Nutrition and Feeding Fish and Crustaceans (eds Guillaume, J. et al.) 27–57 (Springer-Praxis Publishing, 2001).
Tielmann, M., Schulz, C. & Meyer, S. The effect of light intensity on performance of larval pike-perch (Sander lucioperca). Aquac. Eng. 77, 1–24. https://doi.org/10.1016/j.aquaeng.2017.03.001 (2017).
Horvath, L., Tamas, G. & Tolg, I. Special Methods in Pond Fish Husbandry (Akademia Kiado, 1984).
Kestemont, P. & Mélard, C. Aquaculture. In Percid Fishes Systematics, Ecology and Exploitation (ed. Craig, J. F.) 191–224 (Blackwell Science, 2000).
Briland, R. D., Doyle, C. M. & Culver, D. A. Large-scale production of yellow perch, walleye, and hybrid walleye in ponds. In Biology and Culture of Percid Fishes (eds Kestemont, P. et al.) 469–498 (Springer, 2015). https://doi.org/10.1007/978-94-017-7227-3_18.
Paray, B. L. & Al-Sadoon, M. K. Utilization of organic manure for culture of cladocerans, Daphnia carinata, Ceriodaphnia carnuta and copepod, Thermocyclops decipiens under laboratory conditions. Indian. J. Mar. Sci. 45, 399–404 (2016).
Sorgeloos, P., Bossuyt, E., Lavina, E., Baeza-Mesa, M. & Persoone, G. Decapsulation of Artemia cysts: A simple technique for the improvement of the use of brine shrimp in aquaculture. Aquaculture 12, 311–315. https://doi.org/10.1016/0044-8486(77)90209-5 (1977).
Falahatkar, B., Efatpanah, I. & Kestemont, P. Pikeperch Sander lucioperca production in the south part of the Caspian Sea: Technical notes. Aquac. Int. 26, 391–401. https://doi.org/10.1007/s10499-017-0222-2 (2018).
Biswas, G., Thirunavukkarasu, A. R., Sundaray, J. K. & Kailasam, M. Culture of Asian seabass Lates calcarifer (Bloch) in brackishwater tide-fed ponds: Growth and condition factor based on length and weight under two feeding systems. Indian J. Fish. 58, 53–57 (2011).
García-Meilán, I., Ordóñez-Grande, B. & Gallardo, M. A. Meal timing affects protein-sparing effect by carbohydrates in sea bream: Effects on digestive and absorptive processes. Aquaculture 434, 121–128. https://doi.org/10.1016/j.aquaculture.2014.08.005 (2014).
Bradford, M. M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing princiole off protein-dye binding. Anal. Biochem. 72, 248–254. https://doi.org/10.1006/abio.1976.9999 (1976).
Santigosa, E. et al. Modifications of intestinal nutrient absorption in response to dietary fish meal replacement by plant protein sources in sea bream (Sparus aurata) and rainbow trout (Onchorynchus mykiss). Aquaculture 317, 146–154. https://doi.org/10.1016/j.aquaculture.2011.04.026 (2011).
Alarcon, F. J., Diaz, M., Moyano, F. J. & Abellan, E. Characterization and functional properties of digestive proteases in two sparids: Gilthead seabream (Sparus aurata) and common dentex (Dentex dentex). Fish Physiol. Biochem. 19, 257–267. https://doi.org/10.1023/A:1007717708491 (1998).
Acknowledgements
This study was financially supported by the Iran National Science Foundation (project number 98010579), University of Guilan, Guilan Fisheries Directory and University de Barcelona. We would like to thank to the staff of Dr. Yousefpour Marine Fishes Restocking and Genetic Conservation Center for their help during the sampling. The authors are also thankful to the colleagues in University de Barcelona, for providing lab facilities and analyses.
Funding
This research was supported by the Iran National Science Foundation (INSF), Grant/Award Number: 98010579, also by the R + D + i project: RTI2018-100757-B-I00 to J.G. and J.B., funded by the Spanish “Ministerio de Ciencia e Innovación”.
Author information
Authors and Affiliations
Contributions
All the authors read and approved the manuscript. F.L.: larval rearing, sampling, biometry, laboratory work, data analysis, writing original draft. B.F.: funding acquisition, supervision, conceptualized the study, draft-editing. I.G.-M. laboratory work, data analysis, draft-editing. M.P.-A.: help in laboratory work. I.E.: preparing all equipment for larval and juvenile rearing. J.G.: funding acquisition, supervision, conceptualized the study, draft-editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lavajoo, F., Falahatkar, B., García-Meilán, I. et al. Ontogeny of the digestive enzyme activity of the pikeperch (Sander lucioperca) under culture condition. Sci Rep 13, 19739 (2023). https://doi.org/10.1038/s41598-023-43845-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43845-w
- Springer Nature Limited