Abstract
Groundwater is an important source for drinking water supply in the hard rock terrain of Ondo town, southwestern Nigeria. Groundwater quality was investigated in Ondo town to determine its suitability for domestic use and human consumption. To achieve this aim, twenty-five groundwater samples were collected and analyzed for physiochemical parameters. Water quality was classified using the Water Quality Index (WQI). Except for phosphate, the results demonstrate that the groundwater characteristics were below the World Health Organization's allowed range. The dominance of main ions was Na +> Ca2+ > K+ > Mg2+ \({Na}^{+}\)> \({Ca}^{2+}\) ˃ \({K}^{+}\)> \({Mg}^{2+}\) for cations and \({{HCO}_{3}}^{-}{{>{Cl}^{-}>NO}_{3}}^{-}\) ˃ \({{PO}_{4}}^{2-}\) ˃ \({{SO}_{4}}^{2-}\) for anions. Within the research region, the WQI varies from 52.69 to 118.07. With the exception of a few isolated regions in the study area, WQI shows that the bulk of the samples are of good quality, implying that the groundwater is safe for consumption and other household applications. Groundwater in places where the quality of the water is declining has to be treated before being used.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The survival of human, food production, well-being, as well as socioeconomic growth all depend on having access to clean and safe water (Adimalla et al. 2020; Boateng et al. 2016; Gugulothu et al. 2022). The variety uses of water are significantly influenced by its quality. Water often derived from two main natural sources: surface water from streams, rivers and groundwater such as well and borehole water (Boateng et al. 2016; Houatmia et al. 2016; Punna et al. 2022). An estimated 1.5 billion people depend on groundwater for residential use, either directly or indirectly (Carrard et al. 2019). Because of a dearth of surface water, need for groundwater resources has soared globally during the last twenty years, particularly for drinking and irrigation (Adimalla 2019; Adimalla et al. 2018a; Awosika et al. 2020; Dasho et al. 2017; Li et al. 2018). Contamination of groundwater has increased rapidly in many geological terrains where there has been massive population growth, rapid urbanization and industrialization, significant agricultural development, excessive fertilizer usage, poor rainfall and large evaporation (Adimalla et al. 2018b; Boateng et al. 2016; Kumar and Sangeetha 2020; Subba Rao 2017). Studies have shown that groundwater contamination poses environment risk and have a huge effect on human health (Adebayo et al. 2021; Adimalla 2019; Akakuru et al. 2021a, b; Akinola et al. 2018; Aromolaran et al. 2019; Mgbenu and Egbueri 2019). Poor water quality causes a variety of health issues, including kidney stones, gout, and water-borne illnesses (Khatri et al. 2020). According to study carried out by the World Health Organization (WHO), 1.7 million children under the age of five (5) die each year as a result of groundwater pollution (WHO 2017). As a result, monitoring groundwater quality is critical to ascertain the suitability of water for drinking and agricultural uses (Eyankware and Akakuru 2023; Khalid 2019). Hydrogeochemical processes that regulate the amounts of main and minor ions in groundwater ranges from, dissolution, weathering to ion exchange (Adimalla et al. 2020; Houatmia et al. 2016). Studies on the suitability of groundwater for irrigation and drinking, as well as the determination of the primary sources of groundwater contamination have been carried out worldwide (Adebayo et al. 2021; Aghaei et al. 2023; Akakuru et al. 2021a, b; Akinola et al. 2018; Nsabimana and Li 2023; Onwe et al. 2023; Sunkari et al. 2023; Tegegne et al. 2023). (Emenike et al. 2018) found that the main processes influencing the groundwater chemistry in Abeokuta, Southwest Nigeria, are the weathering of rocks and the dissolving of minerals from the soil and aquifer. The research conducted by (Egbueri 2019) in Ogbaru farming district in southeastern Nigeria found that both anthropogenic (mostly agricultural) and geogenic factors affect the ion concentrations and quality of drinkable water in the studied area. (Mgbenu and Egbueri 2019) conducted a groundwater quality study in the Umunya district of southeast Nigeria and discovered that human activities and geogenic processes are the primary variables affecting changes in groundwater chemistry and quality.
In Ondo town, groundwater is a significant source of water for drinking, residential use, and agriculture purposes. As a result of the recently established and the existing institutions in the study area, the region population has increased significantly, consequently increasing the withdrawals and consumption of groundwater. For a better knowledge of state of groundwater quality and the evolution of hydrogeochemical processes of groundwater systems, related health hazards and suitability for drinking purpose, an evaluation of groundwater quality is essential. The objectives of this study were to determine the physical properties, concentrations of cations, and anions in the drinking water (groundwater) samples in Ondo town and evaluate the quality of groundwater used for drinking and domestic purposes in Ondo City. The findings of this study can serve as a guide for future policies governing the development, operation, and maintenance of groundwater resources in the studied region.
2 Materials and Methods
2.1 Study Area, Climate, Hydrogeology and Geology
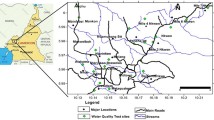
With an estimated 275,917 residents, Ondo metropolis is the second-largest metropolis in Ondo State, Nigeria (NPC 2010). The overall area of the city is 970 km2. The area falls within the humid tropical climatic zone which has prominent dry and wet seasons. A typical wet season extends from April to October, while the dry season extends from November to March. The climate is influenced by rainfall, humidity, temperature and vegetation. Annual rainfall varies between 100 and 1500 mm, with average wet days of about 100 (Iloeje 2001). The almost seven (7) months long wet season ensures aquifer recharge and high humidity. Ondo town is situated inside the Basement Complex area part of Ondo State (Fig. 1). According to Ako and Olorunfemi (1989) and Afolayan et al. (2004), the weathered and fractured basement aquifers are the two main aquifer units that set distinct the Basement Complex region of southwest Nigeria. In contrast to the fractured basement aquifer, which is the outcome of tectonic action, the weathered aquifer unit is created by chemical processes. In the same site, the fractured aquifer and the weathered layer aquifer may coexist.
3 Sample Collection and Analysis
Water samples from twenty-five (25) manually dug wells were collected from different locations (Fig. 2) in February 2020 using double-distilled water pre-cleaned high quality polyethylene plastic bottles, which were also rinsed four times with sampling water. The samples were then analyzed for a variety of hydro-chemical variations of water quality parameters. Total dissolved solids (TDS), pH and electrical conductivity (EC) were determined in-situ.
The in situ measurement was performed using a laboratory calibrated multi-parameter Hanna edge HI 9813–6 pH/EC/TDS/°C probe. Depending on the characteristics of interest, several analytical techniques were utilized for the water samples, and all field and laboratory results were made in accordance with the accepted practices prescribed by American Public Health Association (APHA 2005). Titrimetric techniques were used to determine Cl−, PO43−, and HCO3−. Na+ and K+ were determined using a flame photometric method, while Ca2+ and Mg2+ were determined using an Ethylenediaminetetraacetic acid (EDTA) titrimetric approach (APHA 2005).
4 Statistical Analyses
The SPSS 16.0 version of the statistical software for social sciences was used to conduct descriptive statistical analysis on laboratory data. The R package software cor plot was used to investigate the relationship between the elements while multiple regression analysis was performed to determine the element with the maximum contribution to overall water quality.
5 Multiple Linear Regression
Regression models is one of the effective statistical methods for investigation of relationship between dependent and independent variables (Abyaneh 2014; Akakuru et al. 2022; Omokungbe et al. 2020). MLR is used to model the linear relationship of dependent variables and one or more independent variables (Agbasi and Egbueri 2023; Omokungbe et al. 2020). MLR equation is given in Eq. (1) as:
where \(y\) is the predicted, \({x}_{1}\), \({x}_{2}\), and \({x}_{3}\) are the predictors or descriptors, \({c}_{o}\) is the intercept and \({c}_{1}\), \({c}_{2}\), and \({c}_{3}\) are regression coefficients for predictors. The independent contributions of each independent variable to the dependent variable's prediction are shown by regression coefficients (Akakuru et al. 2022; Akakuru et al. 2023; Omokungbe et al. 2020). The MLR algorithm was employed in this work to simulate and forecast indices and parameters related to water quality. Physicochemical parameters (\({{{HCO}_{3}}^{-}{{, {Cl}^{-}, NO}_{3}}^{-}, {{PO}_{4}}^{2-}, {{SO}_{4}}^{2-}, Na}^{+}\), \({Ca}^{2+}\), \({K}^{+},{Mg}^{2+}\)) analyzed were used as predictors for WQI. Coefficient standardization function in MLR model was used to evaluate the effectiveness of the MLR simulations. MLR was run in R package software.
6 Principal components analysis (PCA)
PCA can be used in data reduction to examine similarities/clustering in data and to identify the sources of variance across variables (Akakuru et al. 2022; Boateng et al. 2016). Principal component analysis (PCA) with Varimax with Kaiser Normalization was applied to the data in order to determine the sources of the physiochemical parameters in the samples (Adebayo et al. 2022; Eyankware et al. 2022; Ogundele et al. 2020), which reduced the amount of variables with an elevated loading on each component, making PCA results easier to comprehend.
6.1 Water Quality Index Method (WQI)
The WQI is a useful tool for determining the whole quality of groundwater and its appropriateness for drinking (Adimalla et al. 2018a; Agidi et al. 2022; Khan and Jhariya 2017). It basically simplifies a lot of groundwater data into a single value and makes information about the quality of the water easier to understand. Weight (wi) was assigned to physiochemical parameters according to their importance for the overall quality of drinking water in order to calculate WQI.
The WHO standards, relative weight \({(W}_{i})\), and assigned weight \({(w}_{i})\), for each parameter are shown in Table 1. A maximum weight of 5 was assigned to variables including nitrate, total dissolved solids, chloride, and sulfate due to their importance in assessing water quality (Khan and Jhariya 2017). Because bicarbonate and phosphate play minimal roles in water quality assessment, they were assigned a minimum weight of 1. Other characteristics, such as calcium and magnesium, were assigned weights ranging from 1 to 5 based on their importance in the overall quality of drinking water (Boateng et al. 2016).
The relative weight is determined using Eq. (2)
\(n\) represents the number of parameters, \({w}_{i}\) represents the weight of each parameter, and \({W}_{i}\) represents the relative weight.
The quality rating scale of each parameter was calculated according to WHO (2011) using Eq. (3)
where \({S}_{i}\) represents the WHO (2011) guideline for each parameter, \({C}_{i}\) represents the concentration (mg/L) of each chemical parameter, and qi represent the quality rating. For each chemical parameter, sub-index of \(ith\) parameter (\({SI}_{i})\) was calculated using Eq. (4). \({SI}_{i}\) was then utilized to compute the WQI using Eq. (5)
7 Result and Discussion
Table 2 showed the physicochemical properties of groundwater samples and WHO guideline values for drinking water.
As noticed, the average pH value of 5.23 indicates that the groundwater is mildly acidic, with all samples falling below the permissible range of 6.5–8.5. This low pH value may be related to the oxidation and hydrolysis of pyrite found in groundwater sediment and the weathering of complex subsurface rocks in southwest Nigeria (Egbueri 2019). According to reports, acidic water may cause salts in the aquifer rocks to dissolve and raise the metal and TDS levels in the groundwater (Shakerkhatibi et al. 2019). The results of this study are similar to several other studies conducted in other parts of the country (Egbueri 2019; Emenike et al. 2018). Except for S3, the TDS values for the groundwater at the study sites were within the WHO (2011) permissible limits. High value of TDS in S3 sample may be as a result of rich geogenic deposits of minerals. (Freeze and Cherry 1979) classification showed that all groundwater samples of the study site are classify as freshwater (TDS < 1000 mgL−1). The groundwater EC values at the study sites were below WHO's acceptable limit. The decomposition of minerals in groundwater due to water rock interaction often cause higher EC concentration (Baig et al. 2010). High EC values in groundwater have a huge effect is the physiological drought condition because it prevent plants from competing with the ions in the soil solution (Naseem et al. 2010). The concentrations of all the cations were discovered to be below WHO's acceptable limits (WHO 2017) and (NSDWQ, 2007). The average concentration of the cations in the samples decreased in the following trend \({Na}^{+}\) (28.50) > \({Ca}^{2+}\) (16.28) ˃ \({K}^{+}\) (6.13) > \({Mg}^{2+}\)+(5.59). The dissolution of the limestone and shale are the possible sources of calcium and magnesium that may be present in the water samples from the study area (Mgbenu and Egbueri 2019). Similarly, potassium is considered to come via agricultural waste leaching and silicate rock weathering (Egbueri 2019; Ezugwu et al. 2019). The order of anion enrichment in the groundwater decreased in the following trend \({{HCO}_{3}}^{-}(42.79){{>{Cl}^{-}(21.67)>NO}_{3}}^{-}\) (18.01) ˃ \({{PO}_{4}}^{2-} (9.95)\) ˃ \({{SO}_{4}}^{2-}\) (8.95). Except for phosphate, none of the anions have concentrations beyond their respective maximum allowed limits for drinking water, which is similar to the results of the cations concentration in the groundwater samples. Ondo is known for intensive agricultural activities, majority of nitrate pollution may be attributed to percolating of inorganic and organic fertilizers from agricultural land via irrigation water and infiltration of rain, biological fixation and atmospheric precipitation weathering (Egbueri 2019; Ezugwu et al. 2019). High phosphate level could be as a result of sewage materials rich in phosphate from the communities (Boateng et al. 2016). It could also be attributed to drain rich in detergents. According to Collins et al.(2020), the presence of Nitrate in water lowers the pH consequently forming weak acids through process of hydration and oxidation. The formation of bicarbonates in groundwater is mainly from the \({CO}_{2}\) dissolution in soil, the environment and organic matter (Egbueri et al. 2019). Chloride is thought to be derived from the onsite septic contamination, animal wastes and leachates from organic agricultural waste dumps (Egbueri 2019). The occurrence of sulphate and phosphate in the groundwater sample maybe related to fertilizer applications in the vicinity of the study region and wastewaters from agricultural operations.
The reported results (Table 3) for \({Ca}^{2+}\) and \({Cl}^{-}\) in this study were shown to be lower than the previously reported data from Akoko, Abeokuta, Abakaliki and Umunya (Agidi et al. 2022; Eyankware 2022; Afuye et al. 2015; Emenike et al. 2018). Except for pH, the concentrations of major ions (Akakuru et al. 2021a; Akakuru et al. 2021b) were lower than in the present study.
The reported concentrations of \({Ca}^{2+}\), \({{NO}_{3}}^{-}\) and \({K}^{+}\) from Benue were lower than the present reported concentrations, while \({Cl}^{-}\), \({{HCO}_{3}}^{-}\), and \({K}^{+}\) concentrations were reported higher than the present reported concentrations (Agidi et al. 2022). Comparatively, Abeokuta, Abakaliki, Umunya and Benue groundwater were found to contain higher concentrations of \({{Ca}^{2+}, Cl}^{-}\), and \({{HCO}_{3}}^{-}\), respectively.
7.1 Water Quality Index Result
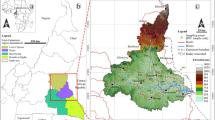
The spatial distribution of groundwater quality status of studied groundwater samples is presented in Fig. 3. According to Adimalla and Qian (2019), there are five types of groundwater quality rating based on WQI values. The rating are excellent water quality \((WQI<50)\), good quality type \((WQI: 50-100)\), poor quality type \((WQI: 100- 200)\), very poor quality type \((WQI: 200-300)\) and water not suitable for drinking type \((WQI>300)\). As can be seen from Fig. 3, high water quality index was found at Q2—Q4 indicating that the groundwater greatly is impaired. Low water quality index was at the vicinities well location Q10. Q13 and Q25, According to the WQI classification, \(22 (88\%)\) of the total groundwater samples had good water quality for domestic purposes, approximately three (3) samples, or 12% of all groundwater samples, had low-quality water. Conclusively, except for a few sampling places (Q2—Q4), the WQI results revealed that the groundwater in the examined area is of good drinking quality.
To ascertain the parameters which had the greatest influence on the WQI, multiple regression analysis where coefficient standardization was utilized. The result showed that Phosphate, Calcium, \(TDS\), and Magnesium have the greatest impact due to the higher standardized beta coefficients. As a result, magnesium, calcium, and \(TDS\) are critical markers in the determination of groundwater quality Table 4.
7.2 Correlation Between Physiochemical Parameters
Figure 4 depicts the Cor plot of the parameters. The plot showed that the bulk of the parameters have a statistically significant correlation with one another, showing a close relationship between these parameters. Except for \({{NO}_{3}}^{-}, {{SO}_{4}}^{2-}\), \({{PO}_{4}}^{2-}\) and \({{HCO}_{3}}^{-}\) significant positive correlations were observed between essential ion, TDS and EC, with correlation coefficient \((r)\) ranging from (\(0.54-0.95\)) suggesting that ions are affected by ion exchange, mineral dissolution and solubility, topographical features and anthropogenic activities. Significant positive correlation was also observed between \({Mg}^{2+}\) and \({Ca}^{2+}\) (r = 0.80), an indication of water hardness, Na+ strongly correlated with \(TDS\) (\(r = 0.95\)) while \(pH\) has no strong link with \(TDS\) or the principal ions studied, indicating that pH does not play a vital role in determining groundwater chemistry. The most significant positive associations of bicarbonate and calcium (\(r = 0.77\)), bicarbonate and magnesium (\(r = 0.77\)) are related to the breakdown of carbonates rocks (dolomite) found in sedimentary rocks (Houatmia et al. 2016).
7.3 Sources Identification
The result of factor analysis is shown in Table 5. The components PCA analysis yielded three factors. \(TDS\), \(EC\), \({Ca}^{2+}\), \({Cl}^{-}, {Mg}^{2+}\), and \({Na}^{+}\) show strong positive factor loadings in \(PC1\), which explains \(43.84 \%\) of the variance, according to the extracted factor data. The factor may be caused by host rock weathering and leaching. \(PC2\) which describes \(27.94 \%\) of the total variance was positively loaded with \({{HCO}_{3}}^{-}\), \({{NO}_{3}}^{-}\) and \({{PO}_{4}}^{2-}\). Factor 2, can thus be linked to the influence of human-induced activities from the communities. In \(PC3\), \(pH\) and \({Na}^{+}\) have elevated positive loading coefficient and it has \(9.37 \%\) as variance percentage explained. Chloride is assumed to be from the dissolution of top layers of the soil that collect wastewater and sewage from residential activities (Boateng et al. 2016).
7.4 Limitations and Uncertainties of the Study
In this study, the metal content of the groundwater was analyzed and compared to permitted limit values, the water quality index was calculated, and the suitability of the groundwater for human consumption was examined. The limitation of this study was that naturally occurring radionuclides materials (NORMs) and heavy metals are not observed. This may underestimate the overall water quality level in the study area.
8 Conclusion
Unmanaged groundwater extraction due to a surge in population may cause a serious water imbalance problem in Ondo City, Ondo State. This study, therefore, was conducted to clarify the groundwater quality conditions and its suitability for residential use. The mean concentration values of \({Ca}^{2+}\), \({Mg}^{2+}, {K}^{+}\), \({Na}^{+}\), \({Cl}^{-}, {{HCO}_{3}}^{-}, {{NO}_{3}}^{-},\) and \({{SO}_{4}}^{2-}\) in the sampled groundwater were within their respective permissible limits. The sequence of ions in the groundwater was \({Na}^{+}\)> \({Ca}^{2+}\) ˃ \({K}^{+}\)> \({Mg}^{2+}\) for cations and \({{HCO}_{3}}^{-}{{>{Cl}^{-}>NO}_{3}}^{-}\) ˃ \({{PO}_{4}}^{2-}\) ˃ \({{SO}_{4}}^{2-}\) for anions. Groundwater quality is mostly influenced by anthropogenic activities, weathering of the rocks, and leaching of surface soil, according to the PCA results. The calculated WQI values of the ions vary from 52.69 —118.07 with a mean of 86.44, implying that the groundwater is safe for consumption and other household applications. Therefore, it can be concluded that the sampled groundwater is unpolluted and safe for domestic consumption.
Data Availability
No datasets were generated or analysed during the current study.
References
Abyaneh HZ (2014) Evaluation of multivariate linear regression and artificial neural networks in prediction of water quality parameters. J Environ Health Sci Eng 12:1–8
Adebayo AS, Akinola BS, Adeyemi AF (2021) Groundwater contamination and human health risk assessment in Ikire community, Osun State. Nigeria Sustain Water Resources Management 7(5):1–10
Adebayo AS, Olufemi AP, Ogundele LT, Okunnuwa OQ, Toyeje AB, Olowookere CJ (2022) Ecological and human health risk assessments of metals in soil and tailing from Ife-Ijesha gold mining area. Southwest Nigeria Environ Earth Sci 81(18):462
Adimalla N (2019) Groundwater quality for drinking and irrigation purposes and potential health risks assessment: a case study from semi-arid region of South India. Exposure and Health 11(2):109–123
Adimalla N, Qian H (2019) Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicol Environ Saf 176:153–161
Adimalla N, Li P, Venkatayogi S (2018a) Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environmental Processes 5:363–383
Adimalla N, Vasa SK, Li P (2018b) Evaluation of groundwater quality, Peddavagu in Central Telangana (PCT), South India: an insight of controlling factors of fluoride enrichment. Modeling Earth Systems Environ 4:841–852
Adimalla N, Dhakate R, Kasarla A, Taloor AK (2020) Appraisal of groundwater quality for drinking and irrigation purposes in Central Telangana. India Groundwater Sustain Development 10:100334
Afolayan JF, Olorunfemi MO, Afolabi O (2004) Geolelectrical/Electromagnetic VLF survey for groundwater development in a Basement terrain case study. Ife J Sci 6(1):74–78
Afuye GG, Oloruntade AJ, Mogaji KO (2015) Groundwater quality assessment in Akoko south east area of Ondo State, Nigeria. Int J Sci Tech 5(9)
Agbasi JC, Egbueri JC (2023) Intelligent soft computational models integrated for the prediction of potentially toxic elements and groundwater quality indicators: a case study. J Sedimentary Environ 8(1):57–79
Aghaei Y, Nazari-Sharabian M, Afzalimehr H, Karakouzian M (2023) Hydrogeochemical Assessment of Groundwater Quality and Suitability for Drinking and Agricultural Use The Case Study of Fars Province. Iran Eng Technol App Sci Res 13(3):10797–10807
Agidi BM, Akakuru OC, Aigbadon GO, Schoeneich K, Isreal H, Ofoh I, ... & Esomonu I (2022) Water quality index, hydrogeochemical facies and pollution index of groundwater around Middle Benue Trough, Nigeria. Int J Energy Water Resources. 8(1):35–54
Akakuru OC, Akudinobi BE, Nwankwoala HO, Akakuru OU, Onyekuru SO (2021a) Compendious evaluation of groundwater in parts of Asaba, Nigeria for agricultural sustainability. Geosci J 25(6):915–927
Akakuru OC, Akudinobi B, Opara AI, Onyekuru SO, Akakuru OU (2021b) Hydrogeochemical facies and pollution status of groundwater resources of Owerri and environs, Southeastern Nigeria. Environ Monit Assess 193:1–26
Akakuru OC, Eze CU, Okeke OC, Opara AI, Usman AO, Iheme O, ... & Iwuoha PO (2022). Hydrogeochemical evolution, water quality indices, irrigation suitability and pollution index of groundwater (PIG) around Eastern Niger Delta, Nigeria. International Journal of Energy and Water Resources, 1–23.
Akakuru OC, Akaolisa CCZ, Aigbadon GO, Eyankware MO, Opara, AI, Obasi PN., ... & Akudinobi BEB (2023) Integrating machine learning and multi-linear regression modeling approaches in groundwater quality assessment around Obosi, SE Nigeria. Environment, Development and Sustainability. 25(12):P14567–14606.
Akinola BS, Awoyemi MO, Matthew OJ, Adebayo AS (2018) Geophysical and hydro-chemical investigation of contamination plume in a Basement Complex formation around Sunmoye dumpsite in Ikire, Southwestern Nigeria. Modeling Earth Systems Environ 4:753–764
Ako BD, Olorunfemi MO (1989) Geoelectric Survey for Groundwater in the Newer Basalts of Vom, Plateau State. J Min Geol 25:247–250
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American public health association American water works association/water environment federation, Washington DC
Aromolaran O, Fagade OE, Aromolaran OK, Faleye ET, Faerber H (2019) Assessment of groundwater pollution near Aba-Eku municipal solid waste dumpsite. Environ Monit Assess 191:1–25
Awosika DD, Ariyibi EA, Adebayo AS, Dasho OA, Shode OH, Adenika CI, Olagunju E (2020) Evaluation of groundwater potential zones using integrated geophysical approach in Obun-Ewi, Ondo East local government, Southwestern Nigeria. Sustainable Water Res Management 6:1–12
Baig JA, Kazi TG, Shah AQ, Kandhro GA, Afridi HI, Arain MB, Jamali MK, Jalbani N (2010) Speciation and evaluation of Arsenic in surface water and groundwater samples: A multivariate case study. Ecotoxicol Environ Saf 73(5):914–923
Boateng TK, Opoku F, Acquaah SO, Akoto O (2016) Groundwater quality assessment using statistical approach and water quality index in Ejisu-Juaben Municipality, Ghana. Environmental Earth Sciences 75:1–14
Carrard N, Foster T, Willetts J (2019) Groundwater as a source of drinking water in southeast Asia and the Pacific: A multi-country review of current reliance and resource concerns. Water 11(8):1605
Collins SL, Loveless SE, Muddu S, Buvaneshwari S, Palamakumbura RN, Krabbendam M, Lapworth DJ, Jackson CR, Gooddy DC, Nara SNV (2020) Groundwater connectivity of a sheared gneiss aquifer in the Cauvery River basin, India. Hydrogeol J 28:1371–1388
Dasho OA, Ariyibi EA, Akinluyi FO, Awoyemi MO, Adebayo AS (2017) Application of satellite remote sensing to groundwater potential modeling in Ejigbo area, Southwestern Nigeria. Modeling Earth Systems Environment 3:615–633
Egbueri JC (2019) Evaluation and characterization of the groundwater quality and hydrogeochemistry of Ogbaru farming district in southeastern Nigeria. SN Applied Sciences 1(8):851
Egbueri JC, Mgbenu CN, Chukwu CN (2019) Investigating the hydrogeochemical processes and quality of water resources in Ojoto and environs using integrated classical methods. Modeling Earth Systems Environ 5(4):1443–1461
Emenike PC, Nnaji CC, Tenebe IT (2018) Assessment of geospatial and hydrochemical interactions of groundwater quality, southwestern Nigeria. Environ Monit Assess 190:1–17
Eyankware MO, Akakuru OC (2023) Appraisal of groundwater to risk contamination near an abandoned limestone quarry pit in Nkalagu, Nigeria, using enrichment factor and statistical approaches. Int J Energy Water Res 7(4):603–621
Eyankware MO, Akakuru OC, Eyankware EO (2022) Interpretation of hydrochemical data using various geochemical models: a case study of Enyigba mining district of Abakaliki, Ebonyi State. SE Nigeria Sustain Water Res Management 8(1):33
Ezugwu CK, Onwuka OS, Egbueri JC, Unigwe CO, Ayejoto DA (2019) Multi-criteria approach to water quality and health risk assessments in a rural agricultural province, southeast Nigeria. HydroResearch 2:40–48
Freeze RA, Cherry JA (1979) Groundwater. Prentice-Hall Inc., Eaglewood Cliffs, NJ, pp 383-456
Gugulothu S, Subbarao N, Das R, Dhakate R (2022) Geochemical evaluation of groundwater and suitability of groundwater quality for irrigation purpose in an agricultural region of South India. Appl Water Sci 12(6):142
Houatmia F, Azouzi R, Charef A, Bédir M (2016) Assessment of groundwater quality for irrigation and drinking purposes and identification of hydrogeochemical mechanisms evolution in Northeastern. Tunisia Environmental Earth Sciences 75(9):746
Iloeje NP (2001) A new geography of Nigeria (New Revised Edition). Longman Group, London, UK, pp 32–45
Khalid S (2019) An assessment of groundwater quality for irrigation and drinking purposes around brick kilns in three districts of Balochistan province, Pakistan, through water quality index and multivariate statistical approaches. J Geochem Explor 197:14–26
Khan R, Jhariya D (2017) Groundwater quality assessment for drinking purpose in Raipur city, Chhattisgarh using water quality index and geographic information system. J Geol Soc India 90:69–76
Khatri N, Tyagi S, Rawtani D, Tharmavaram M, Kamboj R (2020) Analysis and assessment of ground water quality in Satlasana Taluka, Mehsana district, Gujarat, India through application of water quality indices. Groundw Sustain Dev 10:100321
Kumar S, Sangeetha B (2020) Assessment of ground water quality in Madurai city by using geospatial techniques. Groundw Sustain Dev 10:100297
Li P, He S, Yang N, Xiang G (2018) Groundwater quality assessment for domestic and agricultural purposes in Yan’an City, northwest China: implications to sustainable groundwater quality management on the Loess Plateau. Environmental Earth Sciences 77:1–16
Mgbenu CN, Egbueri JC (2019) The hydrogeochemical signatures, quality indices and health risk assessment of water resources in Umunya district, southeast Nigeria. Appl Water Sci 9(1):22
Naseem S, Hamza S, Bashir E (2010) Groundwater geochemistry of Winder agricultural farms, Balochistan, Pakistan and assessment for irrigation water quality. European Water 31:21–32
National Population Commission (NPC) (2010) Federal Republic of Nigeria, (2006). Population and Housing Census, Priority Table Volume IV, Population Distribution by Age and Sex. Abuja, Nigeria: National Population Commission (NPC)
Nsabimana A, Li P (2023) Hydrogeochemical characterization and appraisal of groundwater quality for industrial purpose using a novel industrial water quality index (IndWQI) in the Guanzhong Basin. China Geochemistry 83(1):125922
NSDWQ U (2007) Nigerian standard for drinking water quality. Nigerian Industrial Standard, NIS, 554: 13-14
Ogundele LT, Ayeku PO, Adebayo AS, Olufemi AP, Adejoro IA (2020) Pollution indices and potential ecological risks of heavy metals in the soil: a case study of municipal wastes site in Ondo State, Southwestern, Nigeria. Polytechnica 3:78–86
Omokungbe OR, Fawole OG, Owoade OK, Popoola OA, Jones RL, Olise FS, Abiye OE (2020) Analysis of the variability of airborne particulate matter with prevailing meteorological conditions across a semi-urban environment using a network of low-cost air quality sensors. Heliyon 6(6)
Onwe I, Eyankware M, Obasi P, Ifeanyichukwu K (2023) Hydrochemical and statistical approaches in the evaluation of groundwater quality for drinking and irrigation uses around around Ezzangbo-Ngbo area, Southeastern Nigeria. Modeling Earth Systems and Environment 9(1):413–429
Punna BK, Duvva LK, Ramu M, Dhakate R, Prabhakar G (2022) Evaluation of groundwater quality index for suitability for drinking and irrigation purposes: A case study in Bukleru Watershed region. Telangana J App Geochem 24(4):216–228
Shakerkhatibi M, Mosaferi M, Pourakbar M, Ahmadnejad M, Safavi N, Banitorab F (2019) Comprehensive investigation of groundwater quality in the north-west of Iran: Physicochemical and heavy metal analysis. Groundw Sustain Dev 8:156–168
Subba Rao N (2017) Controlling factors of fluoride in groundwater in a part of South India. Arab J Geosci 10:1–15
Sunkari ED, Abangba T, Ewusi A, Tetteh SEK, Ofosu E (2023) Hydrogeochemical evolution and assessment of groundwater quality for drinking and irrigation purposes in the Gushegu Municipality and some parts of East Mamprusi District. Ghana Environ Monitoring Ass 195(1):165
Tegegne AM, Lohani TK, Eshete AA (2023) Evaluation of groundwater quality for drinking and irrigation purposes using proxy indices in the Gunabay watershed, Upper Blue Nile Basin, Ethiopia. Heliyon 9(4)
WHO (2011) Hardness in Drinking-water: background document for development of WHO guidelines for drinking-water quality. World Health Organization, Geneva
WHO (2017) Guidelines for drinking water quality, 3rd edn. World Health Organization, Geneva
Funding
There was no specific grant for this research from public, private, or nonprofit funding organizations.
Author information
Authors and Affiliations
Contributions
Ayodele P. Olufemi: conceptualized, carried out the experiment; and wrote the original manuscript. Adebiyi S. Adebayo, Opeyemi R. Omokungbe: designed and conducted the experiments; data analysis and interpretation; revised the manuscript. Oghenenyovwe Ovie, Adekunle B. Toyeje, Oladimeji A. Babatunde, Juliet O. Ogede Conducted the experiments; site selection, data analysis and interpretation; revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Olufemi, A.P., Adebayo, A.S., Omokungbe, O.R. et al. Groundwater Quality Assessment in Ondo City, Southwestern Nigeria. Polytechnica 7, 6 (2024). https://doi.org/10.1007/s41050-024-00048-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41050-024-00048-0