Abstract
Statistical index analysis using, contamination factor, metal pollution index, enrichment factor, and ecological risk were successfully applied for the assessment of the impact of heavy metal contamination in groundwater resources within the study area. Fifteen (15) groundwater samples were obtained methodically around the abandoned quarry pit of the Nkalagu area. The samples were analyzed using the America Public Health Association standard (APHA) method. Results indicated that four components were generated from the principal component analysis; strong correlations were observed in the majority of the parameters. The Pearson’s correlation matrix calculated shows weak correlations. Deduction from the Pollution Index of groundwater showed that values were < 1, and were categorized into the following low, moderate, and very high pollution zones. Ecological Risk Assessment results revealed that the ecological risks associated with Cd, Fe, As, Mn, Cu, Co, Ni, Zn, and Pb are generally low. The Contamination Factor of this study reveals that the entire parameters had a low concentration of < 1 in the entire study area except for HCO3 which had a concentration above 1(moderate contamination), and Fe (moderate contamination to considerable contamination). Pollution Load Index values imply that no pollution exists. Metal Pollution Index results in the entire groundwater sample in the study area are below 0.3, which signifies that they are in Class I and are said to be very pure. Hazard quotient order is Cd > Ni > Co > Cu > Mn > Zn > Fe. Hydrogeochemical characterization shows that HCO3¯ + CO3 and Na + K are the dominant ionic species; also, 80% of the groundwater resources in the study area are within the geochemical zone of 3, while 20% fall within the geochemical zone of 4 with a hydrogeochemical trend of HCO3¯ + CO3 > SO4 > Cl+ > Na+ + K+ > Mg+ > Ca+. Based on these results, pre-use treatment before use of the water resources is highly recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the most essential aspects of water research is water quality assessment (Eyankware, Obasi, et al., 2021). According to Arif et al. (2016) and Eyankware, Igwe, et al. (2021), almost 1.1 billion people worldwide lack access to safe drinking water, and water-related diseases claim the lives of millions of people every year. Globally, over 80% of wastewater is discarded without being treated or reused, according to the United Nations (Alvim et al., 2020; Connor, 2015; Mustafa, 2020; Usman et al., 2020). In some nations around the world, this percentage might reach as high as 95% (Lyu et al., 2016). Every year, approximately one billion people, mostly from low-income groups, become ill as a result of contaminated water (Bartram et al., 2005; Gleick, 2002). In 2015, 1.8 million people died all around the world as a result of it (Mayo & Hanai, 2017). According to Eyankware and Ephraim (2021), water may be abundant, but if it is contaminated, it is deemed unsafe for consumption. Hence, it is preferable for water resources to be rarely found and free of contaminants. Ulakpa and Eyankware (2021) were of the view that the major sources of water for domestic and mining purposes around most mining hubs in Ebonyi state are stream, a few manual, and motorized boreholes. Most of the time, when most other water sources (manual and motorized boreholes) have dried up, the residents in the study area turn to these abandoned pits for domestic water (Eyankware et al., 2018).
Lately, groundwater is one of the most water resources faced with various forms of contamination as a result of geogenic and anthropogenic activities. Contamination of groundwater is a global issue that affects all living things and the ecology (Chaudhry and Malik, 2017; Schwarzenbach et al., 2010). Contamination of groundwater within Ebonyi state, southern Nigeria, is mostly attributed to mining/quarrying (Eyankware et al., 2022; Obasi et al., 2021). In the sedimentary basin of southern Nigeria, the southern Benue Trough hosts a variety of ore deposits, such as sulphide ore, lead, or limestone, among others (Fatoye & Gideon, 2013; Eyankware et al., 2018; Ike et al., 2021; Eyankware et al., 202b), with history of quarrying/mining dated over 60 decades as reported by Nnabo (2016). The presence of these mineral deposits has attracted both local and foreign miners. The most frequent quarrying/mining method in the world and the study area is surface mining, open-pit mining accounts for the majority of surface mining in southeastern Nigeria (Eyankware et al., Eyankware, Obasi, et al., 2020, Eyankware, Obasi, et al., 2020, 2022). Marzouk (2018) on the other hand, claims that the methods and equipment employed in solid mineral mining/quarrying are dependent on the purpose for which the mineral is mine/quarried.
The Nkalagu limestone quarry was mostly used for cement manufacture in the 1990s and early 2000s. This quarrying activity has left a lot of abandoned quarry pits in the study area, which is thought to be a threat to water quality within the study. The vast limestone deposit within the study area, was discovered within the Turonian Eze-Aku Formation and looks to be one of the country's largest and most economically viable limestone resource bases, is well-known in the research region (Eyankware, Igwe, et al., 2021; Odukwe, 1980). Because of the presence of this mineral deposit, quarrying activity has been drawn to the area. The Nigerian Cement Corporation (NIGERCEM) is a notable enterprise that utilizes this resource for cement manufacture in Nkalagu, Nigeria. It is Nigeria's first indigenous cement manufacturing company (Fatoye & Gideon, 2013). Mining is the most frequent method of mineral extraction, and it has resulted in the formation of numerous pits, most of which have been abandoned without being adequately closed or reclaimed (Eyankware, Obasi, et al., 2020). Because they are filled with rainwater during the rainy season yet function as water supplies for the local community throughout the dry season (Igwe et al., 2021; Moses & Ruth, 2015), these pits pose significant environmental risks (Igwe et al., 2021; Moses & Ruth, 2015).
Quarrying/mining activities across Ebonyi state have been reported to be a major source of heavy metal in the water resources around most active and abandoned quarry/mine sites across southern Nigeria (Eyankware, Obasi, et al., 2020; Eyankware, Nnabo, et al., 2020; Kalu & Ogbonna, 2018; Eyankware et al., Eyankware, Obasi, et al., 2020; Ozoko, 2013; Obasi, & Akudinobi, 2020; Okolo et al., 2018; Obasi et al., 2021; Igwe et al., 2020). They believed that mine tailings and waste from quarrying/mining activities interact with surface water, after which percolates into the ground thereby contaminating groundwater. A study conducted by Nnabo, (2015) revealed the presence of heavy metals in water resources around the mining district of Enyigba Pb–Zn mine showed the presence of heavy metals in order Cd > > > As > > Pb > Ni > Zn > Cu. As earlier pointed out, decline in water resource quality has worsened throughout multiple mines and quarries in Ebonyi State, Nigeria, over the last few decades, and potable drinking water is scarce (Eyankware et al., Eyankware, Obasi, et al., 2020; Obasi et al., 2021). Globally, the variables that contribute to the deterioration of water resource quality differ from one location to the next, depending on the geogenic or anthropogenic activities that take place in the area (Eyankware, Obasi, et al., 2021; Ulakpa & Eyankware, 2021). Infiltrated water, precipitation, surface water, and subsurface geochemical processes, according to Igwe et al. (2020), all affect water quality, particularly groundwater quality. Similarly, water discharge from human activities has an impact on the quality of groundwater (Obasi et al., 2021). Groundwater pollution is a serious concern, since harmful or heavy metals are permanent, and the majority of them have toxic effects on living species when they exceed the permissible limit (Sirajudeen et al., 2014; Chakraborty et al., 2009). According to Eyankware, Nnabo, et al. (2020), 72% of water resources and soil quality are declining across Ebonyi state in southeastern Nigeria, particularly near quarrying/mining activity. Eyankware and Obasi (2021); Eyankware, Nnabo, et al. (2020) further pointed out that mining/quarrying activities introduce heavy metals into water thereby leading to contamination. As a result, there is a pressing need to assess the impact of quarrying activities on the study area's groundwater resource, with a focus on cations, anions, and heavy metals. Three key goals guided this research: (i) compile a list of abandoned limestone quarry discharge sites and collect groundwater samples for analysis; (ii) describe the chemical composition of water within the study area using hydrogeochemical facies; (iii) evaluate water using statistical approach (PCA); (4) evaluate the presence of heavy metal in water using different indices and determine its suitability for domestic use.
The area of research
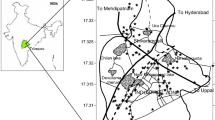
The location of the study is in the Nkalagu district of Ebonyi State, Nigeria's southeastern region. On the Enugu–Abakaliki expressway, approximately 50 km northeast of Enugu. It's between 6° 10′ and 6° 40′ north latitude, and 7° 35′ and 7° 50′ east longitude. A network of major highways and pathways connects the area.
Topography and physiography
According to Igbozuruike (1975) the study location is located within Nigeria's rainforest zone. Orchard bush with small trees and varied densities of prominent elephant grasses characterize the vegetation. There are two distinct seasons in this area: wet and dry. The rainy season starts in March and ends in December, the wet season lasts from November to October, whereas the dry season lasts from November to February. The Dry Harmattan Wind, a North–East Trade Wind from the Sahara Desert, which dominates during the dry season, and the Marine Wind, SW Trade Wind from the Atlantic Ocean, which ushers in the rainy season, are the two prevailing winds that blow across the country at different times of the year. The temperatures in the dry season range from 20 to 38 °C, whereas temperatures in the wet season range from 16 to 28 °C. In January, the monthly rainfall averages 3.1 mm, while in July, it averages 270 mm (Eyankware et al., 2018). The yearly rainfall ranges from 1750 to 2250 mm. The climate in the area surely aids dispersal.
Geology
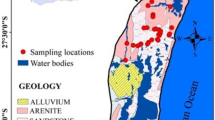
The Benue Trough is a three-part NE–SW trending trough divided into Northern, Central, and Southern, as well as Lower parts (Petters, 1982, 1991). In the Abakaliki Basin of the southern Benue Trough, sedimentation began with the Upper Albian Asu River Group, which disconformably overlies the Precambrian Basement Complex rocks. The Asu River Group is made up of the Abakaliki Shale, which includes volcanoclastics, sandstone, and sandy limestone lenses (Benkheil, 1989). On top of the Asu River Group is the Turonian Eze-Aku Formation (Table 1). This formation (Reyment, 1965) is made up of flaky, grey, or black shales with sandstones and subordinate limestone (Fig. 1). Thick limestone or sandstone strata alternate with calcareous shales in areas, where the Eze-Aku Formation exists (Ikhane et al., 2009). The Nkalagu Formation, described by Petters (1991) and Umeji (2000) in the Nigerian Cement Company (NIGERCEM) quarries (up to 6 m thick), is an example of the aforementioned description.
Hydrogeology
The Nkalagu area's hydrogeology is characterized by poor aquifer conditions (Eyankware et al., 2018). The reason for this is that the area's principal shale units (Aquiclude) are neither porous nor permeable, and hence do not transmit water to surrounding wells. As a result, finding groundwater on a regional scale has become impossible. For example, groundwater could be present in weathered/fractured zones or at sandstone intercalation points.
Materials and methods
Sampling laboratory analysis
Boreholes and hand-dug wells were used to collect water samples around the abandoned quarry site. Groundwater was collected and stored in plastic beakers for analysis once it was collected. Before the field sampling activity, the sample beakers/containers were meticulously cleaned and soaked in distilled water acidified with 1.0 ml HNO3 for 3 days. To gather samples that accurately reflect the water resource, 15 groundwater samples were collected in a methodically manner from various water sources (Fig. 1). Potential surface films in open hand-dug wells and surface water sources were removed before sampling, and samples from boreholes were acquired after 5–10 min of pumping. Each sample was filtered into a sample bottle using disposable filters with a diameter of 0.45 m after washing the bottle with the aliquot to ensure full removal of suspended particles. The samples were acidified in the field with 1.0 ml of conc. HNO3 to prevent heavy metal precipitation, and three drops of HNO3 were added with new syringes to prevent sorption. The samples were stored in hermetically sealed ice-packed beakers at 4 °C. To avoid evaporation, the temperature was maintained during the journey to the analytical laboratory (Sehgal et al., 2012; Singh et al., 2005). Heavy metals like Fe, Ag, Cu, Co, Cd, Mn, Zn, and Pb were measured with a fast sequential (FS) (Varian 240 AA) atomic absorption spectrophotometer (AAS), whereas HCO3¯ and SO42¯ were measured with a Hach DR/2010 Spectrophotometer. The pH values were obtained using a portable pH meter (WGS 84) with temperature electrode accessories, even though the temperature of each sample was taken with a potable thermometer. All data collection and analysis followed the APHA guidelines ().
Analyzing data
Using Microsoft Excel and Surfer 11, the spread of contaminants in the area was visualized. The data were compared to the World Health Organization's (WHO) water quality criteria in terms of the related health risk (Table 2).
Analyses statistics
Principal component analysis (PCA), Pearson correlation analysis, contamination, and metal pollution indices were all used to assess the researched parameters.
In the PCA, component loading entailed reducing a large data set of many variables into a smaller number of linear combinations in the component that accounted for an appropriate fraction of the total data variance and easily associated the variables to the sources or processes via Eq. (1):
when Xi represents the statistical mean, I represents the intervals between variables, and N represents the number of outcomes.
The Metal Pollution Index (MPI), which estimated the aggregate impact of individual heavy metals on water quality (Horton, 1965; Mohan et al., 1996), was evaluated using Caeiro et al. (2015), Eq. 2:
where Mn = concentration of the metal.
Microsoft Excel and Surfer 12 software were used to model the concentration configuration and dispersion trend in the area. The findings were matched to WHO guidelines. Pearson's correlation coefficient was calculated using the Statistical Package for Social Science (SPSS) version 23.
Contamination factor (CF)
The CF was calculated using the Hakanson (1980) formula:
where Cn is the metal concentration, Bn is the background/target value (Akakuru et al., 2021b; Yahaya et al., 2021).
Pollution Load Index (PLI)
Hakanson's (1980) formula was used to calculate the PLI:
where CF stands for contamination factor and n stands for element number.
Pollution index of groundwater (PIG)
The PIG is a tool for evaluating the quality of groundwater for drinking purposes (Subba Rao et al., 2018). When using the PIG, there are five steps to consider. Step I: Determine the relative weight (Rw) of each factor in the assessment of water quality as it relates to human health (on a scale of 1–5). (Table 8). Step II is the determination of the weight parameter (Wp) for each of the groundwater quality factors, intending to determine the relative contribution of each to the overall groundwater quality status (Eq. 5). Step III entails calculating the concentration status (Sc), which was calculated by dividing each sample's water variable content (C) by its associated quality standard limit (Ds) (Eq. 6). In this study, the PIG was assessed using the WHO (2011) criteria. Step IV entails calculating the overall quality of groundwater (Ow) by multiplying the weight parameter (Wp) by the concentration status (Sc) (Eq. 7). Step V entails adding up all of the Ow values per sample (Eq. 8):
Health risk assessment
The non-carcinogenic risk associated with drinking contaminated river water was calculated using the criteria of the US Environmental Protection Agency (USEPA, 1989). In Eq. 9, the risk was calculated using the following function for both children and adults:
where CDI denotes Chronic Daily Intake or exposure dose (mg/kg/day), Cw denotes contaminant concentration in water (mg/L), IRW denotes ingestion rate (for adults, IRW is 2 L/day, while for children, IRW is 1 L/day), EF denotes Exposure Frequency (equivalent to 365 days/year), ED denotes exposure duration (for adults and children, ED is 70 and 6 (Bortey-Sam et al., 2015; Duggal et al., 2017; Barzegar et al., 2018). The non-carcinogenic risk posed by various elements in Eq. 10 was evaluated using the hazard quotient (HQ), as defined by Li et al. (2016) and Zhang et al. (2018):
RfD stands for reference dose (mg/kg/day) for a given element. RfD for various elements is similar to 0.7 (Fe), 0.3 (Zn), 0.0001 (Hg), 0.03 (Co), and 0.0035 (Co), according to Duggal et al. (2017) and Barzegar et al. (2018). (Pb). Finally, using Eq. 6, the total HQ values for the elements is computed to determine the hazard index (HI) of the water samples:
HI and HQ values larger than 1 indicate that the given element's noncarcinogenic risk exceeds the limit of acceptance (HI = 1), while values less than 1 show that the noncarcinogenic risk is within acceptable limits (USEPA, 1989; Su et al., 2017). As reported by Bortey-Sam et al. (2015), Barzegar et al. (2018), Table 3 illustrates the USEPA (1989) classification of non-carcinogenic risk.
Results and discussion
Principal component analysis (PCA)
PCA is a pattern identification technique that aims to explain the variation of a large number of interrelated variables (2021b; Akakuru et al., 2021a). It shows how variables are related, lowering the data set's complexity. From the covariance matrix of original data, PCA extracts eigen values and eigenvectors. The uncorrelated (orthogonal) variables obtained by multiplying the original correlated variables with the eigenvectors are known as principal components (PCs) (loadings).
The PCs' associated variance is measured by their eigen values, the original variables' participation in the PCs is determined by the loadings, and the transformed observations are referred to as scores (Helena et al., 2000; Wunderlin et al., 2001; Singh et al., 2005). From Table 4 it shows four components, PC1 shows loadings in EC, TDS, pH, Mg, Ca, Cl, Mn, Zn, Cu, Co, and Ni; PC2 shows loadings in temperature, Na, Ca, SO4, As, Pb, K, and HCO3; PC3 indicates loadings in temperature, Mg, Ca, CO3, and Cd; PC4 shows loadings in EC, CO3, Fe, As, Ni and HCO3. Strong correlations observed in the majority of the parameters imply that the groundwater mixed with saline water could be a result of saltwater intrusion. This further means that saltwater was the major recharge source for the groundwater in the review region (2021b; Akakuru et al., 2021a; Yahaya et al., 2021).
Pearson’s correlation matrix
In the examination of groundwater quality, Pearson's Correlation matrix analysis is a valid technique for determining the relationship and origin of hydrogeochemical indicators (Akakuru et al., 2021a). Correlation coefficients more than 0.7 indicate a strong link between the two limits; correlation coefficients between 0.5 and 0.7 indicate a shaky link, implying a moderate correlation (Akakuru et al., 2021b; Shyu et al., 2011; Qian et al., 2016) Table 5 shows a relationship between pH and EC, TDS and EC; Mg and Temp; Na and pH, Temp; Cl and Temp., Ca; SO4 and Ca; CO3 and Ca, Cl; Cd and Mg, CO3; Fe and Ca, Cl; As and Na; Pb and Temp, Ca, SO4; Mn and Mg, SO4; Zn and EC,TDS, pH; Cu and Temp., SO4, Mn. Because the majority of the data have weak relationships, it is likely that the groundwater did not interact with saline water, and hence there was no saltwater intrusion. This also implies that freshwater was the primary source of groundwater recharge in the study area (Egbueri et al., 2019; Yahaya et al., 2021; Akakuru et al., 2022)
Pollution Indices
To analyze heavy metal contamination, this research used pollution indices grouped into three types as described by Yang et al. (2011): Pollution Index of Groundwater (PIG), Contamination Factor (CF), Pollution Load Index (PLI), Metal Pollution Index (MPI), and Ecological Risk Index (ERI).
Pollution Index of groundwater (PIG)
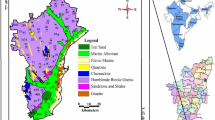
PIG is a useful instrument for measuring the quality of water (surface and groundwater), and it has been used by many researchers (Subba Rao, 2012; Subba Rao and Chaudhary, 2019). Table 6 lists the components required to calculate the PIG, whereas Table 6 lists the PIG results for 15 groundwater samples collected around the study area, as well as a PIG distribution map (Fig. 0.3). PIG values vary from 0.395 to 2.511(Table 7). According to PIG's classification (Table 8), 60% of collected groundwater samples are unpolluted, while 13.3%, 20%, and 6.7 percent of the samples are in low, moderate, and extremely high pollution zones, respectively. The regional distribution of PIG in the research area is depicted in Fig. 2.
Ecological risk assessment (ERI)
Water quantity risk (physical risk), water quality risk (chemical risk), and aqua-ecosystem risk (biological risk) are the three main components of watershed ecological risk, according to Kun et al. (2017) and Akakuru et al. (2015). The overall amount and speciation of heavy metals in water sediments are linked to their potential environmental harm (Jain et al., 2005; Nayek et al., 2013). ERI values for Cd range from 0.075 to 2.325, 0.85–3.975 for Fe, 0–0.02 for As, 0–0.002 for Pb, 0.00008–0.00063 for Mn, 0.00005–0.00128 for Zn, 0.005–0.023 for Cu, 0.009–0.03 for Co, 0.001–0.0082 for Ni. Table 9 shows that the ecological hazards associated with Cd, Fe, As, Mn, Cu, Co, Ni, Zn, and Pb are generally low (less than 110) based on the ERI results. Table 10 shows the ERI categorization.
Contamination factor
In groundwater studies, the CF has been used to calculate the concentration ratio of heavy metals to background values.
The values of the contamination factor are described using the following criteria: CF < 1 Low contamination 1 ≤ CF ≥ 3 (CF < 1), moderate contamination 3 ≤ CF ≥ 6, significant contamination CF > 6, and very high contamination. (Akakuru et al., 2017a, 2017b; Bhutian et al., 2017). The CF of this investigation, as shown in Table 11, shows that all parameters had low values of < 1 across the study area, except for HCO3, which showed a concentration above 1 (moderate contamination), and Fe, which ranged from moderate to severe contamination. This finding indicates that geogenic processes are the primary cause of pollution. The findings of Bhutian et al. (2017) in India and Nigeria are similar (Yayaha et al., ; Akakuru et al., 2021a).
Pollution Load Index (PLI)
PLI is a useful tool for determining heavy metal toxicity in representative samples (Akakuru et al., 2021b; Yang et al., 2011). No pollution (PLI1), moderate pollution (PLI > 2), heavy pollution (2PLI > 3), or extremely heavy pollution (3 > PLI) are the most common PLI classifications. The study area's groundwater had a concentration value of less than one, according to the results (Table 11). This implies that no pollution exists. This work contradicts the study undertaken in India (Bhutian et al., 2017; Gopinath et al., 2019), but it is consistent with the work done in Nigeria by Yahaya et al. (2021).
Metal pollution index (MPI)
MPI has proven to be a valuable tool in the classification of groundwater. Water with an MPI of 0.3 or less is classified as Very Pure, whereas water with an MPI of 0.3–1.0 is classified as Pure. Those with an MPI of 1.0–2.0 are classified as minimally afflicted in Class III, while those in Class IV are classified as severely affected. With an MPI of 2.0–4.0, moderately affected. Water samples with MPI between 4.0 and 6.0 are classified as significantly affected in Class V, while those with MPI > 6.0 are classified as seriously affected in Class VI (Table 12). According to the MPI results in Table 13, groundwater sample in the research region has a value of less than 0.3, indicating that it is in Class I and thus very pure (Akakuru and Akudinobi, 2018; Eyankware et al., 2016; Eyankware et al., 2017).
Health risk assessment
There are numerous health concerns associated with drinking heavy metal-contaminated water. Because most metals have a high contamination factor, residents are more susceptible to health concerns from their consumption. Heavy metals in water can create an unpleasant taste in beverages, stains on products and laundry, sticky coatings, and depositions in water distribution pipes, in addition to drinking (WHO, 2011; Mgbenu & Egbueri, 2019). Despite the fact that there is no health-based temperature guideline, it has been found that high water temperature promotes the growth of bacteria that may be hazardous to human systems, as well as problems with taste, odor, color, and corrosion (WHO, 2011; Mgbenu & Egbueri, 2019). Furthermore, the hazard quotients (HQ) (for heavy metals) reported in Table 14 were used to determine the non-carcinogenic risk posed by drinking water containing trace metals for both children and adults in the research area. Cd > Ni > Co > Cu > Mn > Zn > Fe is the HQ order for both children and adults, indicating that Cd and Ni are the most polluting elements in water supplies. The findings suggest that injecting heavy metal poses a substantial health risk to both adults and children. Mgbenu and Egbueri (2019) found similar results somewhere in Nigeria.
Hydrogeochemical facies
The hydrochemical evolution, grouping, and areal distribution of groundwater's major dissolved constituents (major cations and major anions) can be seen graphically (Akakuru et al., 2015, Akakuru et al., 2017a, b; Eyankware, Igwe, et al., 2021). Piper trilinear diagrams, Scholler plots, and Durov plots were used to assess hydrochemical facies variation in this work.
Piper trilinear and Durov plots
The Piper Trilinear plot (Piper, 1944) is one of the most effective graphical representations in groundwater quality studies; it aids in understanding the geochemistry of shallow groundwater and brings out chemical interactions in more precise terms than other plotting approaches (Akakuru et al., 2013; Eyankware, Nnabo, et al., 2020; Eyankware, Obasi, et al., 2020). The Piper trilinear diagram for the study region shows that the anion area's main ionic species are HCO3 + CO3, whereas the cation area's leading ionic species are Na + K. The graphic also shows that 80% of the research area's groundwater resources are in the geochemical zone 3 (weak acid exceeds strong acid), whereas 20% are in the geochemical zone 4. (strong acid exceed weak acid). Cation exchange has long been blamed for the formation of sodium–bicarbonate groundwater in aquifers.
Microbial oxidation of organic carbon produces carbonic acid, which dissolves calcite and yields dissolved calcium and bicarbonate, according to this concept. Calcium is exchanged for sodium adsorbed on clays, and sodium–bicarbonate groundwater is the consequence (Saha et al., 2019). This result is similar to Akakuru et al. (2021b), in Nigeria and Xu et al. (2018) in China, but not to Umar et al. (2019) in Nigeria. Figure 3 depicts a Piper diagram of the samples in the research area, whereas Fig. 4 depicts a Durov plot of the samples in the region.
Scholler Plot
Schoeller Semi-logarithm plot that makes use of logarithmic scales in representing water quality status. The chart proposed by (Schoeller, 1967) portrays a gathering of investigations on equidistant verticals, the quantity of which relies upon the number of constituents being communicated. This diagram is particularly valuable for looking at waters of low focus and waters that don't vary incredibly in fixation (Sakram et al., 2013; Saha et al., 2019). The Schoeller diagram (Fig. 5) of the groundwater samples also reveals a hydrogeochemical trend of HCO3¯ + CO3 > SO4 > Cl + > Na+ + K+ > Mg+ > Ca+. The Piper plot is confirmed by the Schoeller semi-logarithmic plots, which reveal a dissimilarity in ionic mobility, showing that the water is not from the same source (Akakuru et al., 2017a&b; Eyankware, Akakuru, et al., 2021).
Conclusions
Heavy metal contamination in the research region was successfully assessed utilizing statistical index analysis employing contamination factor, metal pollution index, enrichment factor, and ecological risk. These results were gotten: Four components were generated from the PCA analysis, Strong correlations observed in the majority of the parameters imply that the groundwater mixed with saline water could be a result of saltwater intrusion. The Pearson's correlation matrix reveals Weak correlations in the majority of the parameters which implies that the groundwater did not mix with saline water, hence there was no saltwater intrusion. PIG ranges from 0.395 to 2.511 points. 60% of collected groundwater samples are polluted insignificantly, while 13.3%, 20%, and 6.7 percent of the samples are polluted in low, moderate, and very high pollution zones, respectively. The Ecological Risk Index values range from are generally modest, according to the Ecological Risk Index results (less than 110). The CF of this study reveals that the entire parameters had a low concentration < 1 in the entire study area except for HCO3 which had a concentration above 1(moderate contamination), and Fe which ranged from moderate contamination to considerable contamination. PLI values of the study area had a concentration value of less than one, according to the results. This implies that no pollution exists. MPI results in the entire groundwater sample in the study area is below 0.3, which signifies that they are in Class I and are said to be very pure. Mean values show that the Hazard Quotient order confirms that Cd and Ni have the highest pollution factor in the water resources. Piper trilinear diagram for the study area shows that the anion area HCO3 + CO3 are the dominant ionic species, while Na + K is the dominant ionic species for the cation area. The diagram further reveals that 80% of the groundwater resources in the study area are within the geochemical zone of 3 (weak acid exceeds strong acid), while 20% fall within the geochemical zone of 4 (strong acid exceeds weak acid). The Schoeller diagram of the groundwater samples, shows a hydrogeochemical trend of HCO3− + CO3 > SO4 > Cl+ > Na+ + K+ > Mg+ > Ca+. Based on these results, pre-use treatment of the water resources before use is highly recommended.
References
Akakuru, O.C., Akudinobi, B.E.B., & Aniwetalu, E.U. (2015). Qualitative evaluation and hydrogeochemical attributes of groundwater in Owerri Capital Territory, Southeastern Nigeria. IOSR Journal of Applied Geology and Geophysics, 3(2), 12–18.
Akakuru, O., Akudinobi, B., Okoroafor, P., & Maduka, E. (2017a). Application of geographic information system in the hydrochemical evaluation of groundwater in parts of Eastern Niger Delta Nigeria. American Journal of Environmental Policy and Management, 3(6), 39–45.
Akakuru, O.C., and Akudinobi, B.E.B (2018). Determination of water quality index and irrigation suitability of groundwater sources in parts of coastal aquifers of Eastern Niger Delta, Nigeria. International Journal of Applied and Natural Sciences, 7 (1), 1–6.
Akakuru, O. C., Akudinobi, B. E., Nwankwoala, H. O., Akakuru, O. U., & Onyekuru, S. O. (2021b). Compendious evaluation of groundwater in parts of Asaba Nigeria for agricultural sustainability. Geosciences Journal. https://doi.org/10.1007/s12303-021-0010-x.
Akakuru, O. C., Akudinobi, B., Opara, A. I., Onyekuru, S. O., & Akakuru, O. U. (2021a). Hydrogeochemical facies and pollution status of groundwater resources of Owerri and environs. Southeastern Nigeria. Environmental Monitoring and Assessment, 193, 623. https://doi.org/10.1007/s10661-021-09364-9.
Akakuru, O. C., Akudinobi, B. E. B., & Usman, A. O. (2017b). Organic and heavy metal assessment of groundwater sources around Nigeria National Petroleum Corperation oil depot Aba, South-eastern Nigeria. Journal of Natural Sciences Research, 7(24), 48–58.
Akakuru, O. C., Eze, C. U., Okeke, O. C., Opara, A. I., Usman, A. O., Iheme, O. K., Ibeneme, S. I., & Iwuoha, P. O. (2022). Hydrogeochemical evolution, water quality indices, irrigation suitability and pollution index of groundwater (PIG) around Eastern Niger Delta. Nigeria. International Journal of Energy and Water Resources. https://doi.org/10.1007/s42108-021-00162-0.
Akakuru, O. C., Maduka, E. C., & Akakuru, O. U. (2013). Hydrogeochemical characterization of surface water sources in Owerri Capital Territory, Southeastern Nigeria. IOSR Journal of Applied Geology and Geophysics, 1(2), 32–38.
Alvim, C. B., Mendoza-Roca, J., & Bes-Piá, A. (2020). Wastewater treatment plant as microplastics release source quantification and identifcation techniques. Journal of Environmental Management, 255, 109739.
American Public Health Association (APHA). (1995). Standard methods for the examination for water and wastewater (19th ed.). Byrd Prepress.
American Public Health Association (APHA). (2012). Standards methods for the examination of water and wastewater (22nd ed., p. 1360). American Public Health Association (APHA), American Water Works Association (AWWA) and Water Environment Federation (WEF).
Arif, S., Khan, U., Turab, A., Hussain, I., Habib, A., Mansoor, T., Mallick, D., Suhag, Z., Bhatti, Z., & Ahmed, I. (2016). Evaluation of solar disinfection of water intervention delivered through Lady Health Workers in reduction of diarrheal episodes in under five children. International Journal Pedestrian Child Care, 1, 1.
Bartram, J., Lewis, K., Lenton, R., & Wright, A. (2005). Focusing on improved water and sanitation for health. The Lancet, 365(9461), 810–812.
Barzegar, R., Moghaddam, A. A., Nazemi, A. H., & Adamowski, J. (2018). Evidence for the occurrence of hydrogeo-chemical processes in the groundwater of Khoy plain, northwestern Iran, using ionic ratios and geochemical modeling. Environment and Earth Science, 77, 597. https://doi.org/10.1007/s12665-018-7782-y.
Benkheil, J. (1989). The origin and evolution of the Cretaceous Benue Trough, Nigeria. Journal of Africa Earth Sciences, 8, 251–282.
Bhutian, R., Dipali-Bhaskar, K., & Khanna, D. R. (2017). A Gautam (2017) Geochemical distribution and environmental risk assessment of heavy metals in groundwater of an industrial area and its surroundings, Haridwar, India. Energy, Ecology and Environment., 2(2), 155–167. https://doi.org/10.1007/s40974-016-0019-6.
Bortey-Sam, N., Nakayama, S. M., Ikenaka, Y., Akoto, O., Baidoo, E., Mizukawa, H., & Ishizuka, M. (2015). Health risk assessment of heavy metals and metalloid in drinking water from communities near gold mines in Tarkwa Ghana. Environmental Monitoring Assessment, 187(7), 397.
Caeiro, S., Costa, M. H., Ramos, T. B., Fernandes, F., Silveira, N., Coimbra, A., Medeiros, G., & Painho, M. (2015). Assessing heavy metal contamination in Sado estuary sediment: An index nnuivsis approach. Ecological Indicators, 5, 151–169.
Chakravorty, U., Hubert, M. H., & Nøstbakken, L. (2009). Fuel versus food. Annual Review of Resource Economics., 1(1), 645–663.
Chaudhry, F., & Malik, M. (2017). Factors affecting water pollution: A review. Journal of Ecosystem & Ecography, 7(225), 2.
Connor, R. (2015). The United Nations world water development report 2015: water for a sustainable world (Vol. 1). UNESCO Publishing.
Duggal, V., Sharma, S., Saini, K. et al. (2017) Assessment of carcinogenic and non-carcinogenic risk from exposure to uranium in groundwater from western Haryana, India. J Geol Soc India 89, 663–668 . https://doi.org/10.1007/s12594-017-0675-y.
Egbueri JC. (2019). Evaluation and characterization of the groundwater quality and hydrogeochemistry of Ogbaru farming district in southeastern Nigeria. SN Applied Sciences, 1:851. https://doi.org/10.1007/s42452-019-0853-1.
Eyankware, M. O. (2017). Hydrogeochemical evaluation of groundwater for irrigation purposes in mining areas of Umuoghara near, Abakaliki, SE Nigeria. Journal of Science and Technology, 3(19), 1–19.
Eyankware, M. O., Akakuru, C. O., & Eyankware, E. O. (2022). Interpretation of hydrochemical data using various geochemical models: a case study of Enyigba mining district of Abakaliki Ebonyi state SE Nigeria. Sustainable Water Resources Management. https://doi.org/10.1007/s40899-022-00613-4.
Eyankware, M. O., Akakuru, C. O., Ulakpa, R. O. E., & Eyankware, E. O. (2021). Sustainable management and characterization of groundwater resource in coastal aquifer of Niger delta basin Nigeria. Sustainable Water Resources Management. https://doi.org/10.1007/s40899-021-00537-5.
Eyankware, M. O., & Ephraim, B. E. (2021). A comprehensive review of water quality monitoring and assessment in Delta State Southern Part of Nigeria. Journal of Environmental and Earth Sciences., 3(1), 16–28. https://doi.org/10.30564/jees.v3i1.2900.
Eyankware, M. O., Eyankware, O. E., & Ulkapa, R. O. E. (2016). Assessment of impact of leachate on soil physicochemical parameters in the vicinity of Eliozu Dumpsite, Port Harcourt, Nigeria. Basic Research of Journal of Soil and Environ Science., 4(2), 15–25.
Eyankware, M. O., Igwe, E. O., & Onwe, M. O. (2021). Geochemical study of groundwater using modeling approach in Ojekwe region of southern Benue Trough Nigeria. International Journal of Energy and Water Resources. https://doi.org/10.1007/s42108-021-00163-z.
Eyankware, M. O., Nnabo, P. N., & Ogwah, C. (2020). Impact of past mining activity on water resources around active and abandoned mines in Ebonyi State, South-Eastern Nigeria—A mini review. Hydro Science and Marine Engineering, 2(2), 29–35.
Eyankware, M. O., Nnajieze, V. S., & Aleke, C. G. (2018). Geochemical assessment of water quality for irrigation purpose, in abandoned limestone quarry pit at Nkalagu area Southern Benue Trough Nigeria. Environmental Earth Science. https://doi.org/10.1007/s12665-018-7232-x.
Eyankware, M. O., & Obasi, P. N. (2021). A holistic review of heavy metals in water and soil in Ebonyi SE, Nigeria; with emphasis on its effects on human, aquatic organisms and plants. World News of Natural Science, 38, 1–19.
Eyankware, M. O., Obasi, P. N., & Ogwah, C. (2021). A comparative study of groundwater between geological groups of southern Benue Trough, Nigeria Using Modelling Approach. Journal of Environmental & Earth Sciences, 3(2), 72–86. https://doi.org/10.30564/jees.v3i2.3694.
Eyankware, M. O., Obasi, P. N., Omo-Irabor, O. O., & Akakuru, O. C. (2020). Hydrochemical characterization of abandoned quarry and mine water for domestic and irrigation uses in Abakaliki, southeast Nigeria. Modeling Earth Systems and Environment. https://doi.org/10.1007/s40808-020-00827-5
Fatoye, F. B., & Gideon, B. Y. (2013). Geology and occurrence of Limestone and Marble in Nigeria. Journal of Natural Science Research, 3(11), 60–65.
Gleick, P. H. (2002). Dirty-water: estimated deaths from water-related diseases 2000–2020: Citeseer.
Gopinath, S. K., Srinivasamoorthy, K., Saravanan, R., Prakash, D., & Karunanidhi, D. (2019). Characterizing groundwater quality and seawater intrusion in coastal aquifers of Nagapattinam and Karaikal, South India using hydrogeochemistry and modeling techniques. Human and Ecological Risk Assessment: an International Journal., 25, 1–22.
Hakanson L (1980) An ecological risk index for aquatic pollution control. Sedimentol Approach Water Res 14(8):975–1001.
Helena, B., Pardo, R., Vega, M., Barrado, E., Fernandez, J. M., & Fernandez, L. (2000). Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga River, Spain) by principal component analysis. Water research, 34(3), 807–816.
Horton, R. K. (1965). An index-number system for rating water quality. Journal of Water Pollution Control Federation, 37, 300–306.
Igbozurike, M. U. (1975). Vegetation type. In G. E. K. Oformata (Ed.), Niger in Maps, Eastern States (p. 286). Ethiope publishing House.
Igwe, E. O., Ede, C. O., & Eyankware, M. O. (2021). Heavy metals concentration and distribution in soils around Oshiri and Ishiagu lead–zinc mining areas, southeastern Nigeria. World Scientific News, 158, 22–58.
Igwe, E. O., Ede, C. O., Nnabo, P. N., & Ukpai, S. N. (2020). Impact of heavy metals dispersion on water supplies around Oshiri and Ishiagu mine districts of Southern Benue Trough Nigeria. Modeling Earth Systems and Environment. https://doi.org/10.1007/s40808-020-00950-3.
Ike, J. C., Ezeh, H. N., Eyankware, M. O., & Haruna, A. I. (2021). Mineralogical and geochemical assessment of clay properties of Edda Afkpo Sub Basin Nigeria for possible use in the ceramics industry. Journal of Geological Research. https://doi.org/10.30564/jgr.v3i2.2964.
Ikhane, P. R., Folorunso, A. F., Nton, M. E., & Oluwalaanu, J. A. (2009). Evaluations of turonian limestone formation exposed at NIGERCEM Quarry, Nkalagu, Southeastern Nigeria: A geochemical approach. Pacific Journal of Science and Technology, 10(2), 763–771.
Jain, C. K., Singhal, D. C., & Sharma, M. K. (2005). Metal pollution assessment of sediment and water in the river Hindon, India. Environmental Monitoring Assessment, 105, 193–207.
Kalu, I. E., & Ogbonna, N. J. (2018). Investigation of environmental effect of stone quarrying activities on soil and water in Akpoha and Ishiagu communities of Ebonyi state Nigeria. International Journal of Construction Management. https://doi.org/10.1080/15623599.2019.1604115.
Kun, B., Jing-ling, L., Xiao-guang, Y., Xuan, S., & Bo, M. (2017). A new comprehensive ecological risk index for risk assessment on Luanhe River China. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-017-9978-6
Li P, Wu J, Qian H (2016). Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci. 69:2211–2225.
Lyu, S., Chen, W., Zhang, W., Fan, Y., & Jiao, W. (2016). Wastewater reclamation and reuse in China: Opportunities and challenges. Journal of Environmental Science, 39, 86–96.
Marzouk, S. H. (2018). Influences of limestone stone quarries on groundwater quality. International Journal of Human Capital in Urban Management, 3(4), 315–324.
Mayo, A. W., & Hanai, E. E. (2017). Modeling phytoremediation of nitrogen polluted water using water hyacinth (Eichhorniacrassipes). Physics Chemistry of the Earth Parts, a/b/c, 100, 170–180.
Mgbenu, C. N., & Egbueri, J. C. (2019). The hydrogeochemical signatures, quality indices and health risk assessment of water resources in Umunya district, southeast Nigeria. Applied Water Science. https://doi.org/10.1007/s13201-019-0900-5.
Mohan, S.V., Nithila, P. and Reddy, S.J. (1996) Estimation of Heavy Metal in Drinking Water and Development of Heavy Metal Pollution Index. Journal of Environmental Science and Health, A-31, 283–289. https://doi.org/10.1080/10934529609376357.
Moses, O. E., & Ruth, O. E. (2015). Environmental degradation on land in Enyigba with reference to Artisan Lead-Zinc miner in south eastern Nigeria. Journal of Multidisciplinary Scientific Research, 3(3), 32–34.
Mustafa, M. (2020). Removal of micropollutants from wastewater: evaluation of effect of upgrading ozonation to electro-peroxone. Umeå University.
Nayek, S., Gupta, S., & Saha, R. N. (2013). Heavy metal distribution and chemical fractionation in water, suspended solids and bed sediments of industrial discharge channel: An implication to ecological risk. Research Journal of Chemistry and Environment, 17(6), 26–33.
Nnabo, P. N. (2015). Assessment of Contamination of Underground Water Sources in Enyigba Pb–Zn District, South Eastern Nigeria using Metal Enrichment and Pollution Indices. International Journal of Science, 4(9), 47–56.
Nnabo, P. N. (2016). Surface water contamination by heavy metals from Enyigba Pb-Zn Mine District, Southeastern Nigeria Using Metal Enrichment and Pollution Indices. International Journal of Science and Technology, 5(1), 8–16.
Obasi, A. I., Ogwah, C., Selemo, A. O. I., Afiukwa, J. N., & Chukwu, C. G. (2020). In situ measurement of radionuclide concentrations (238U, 40K, 232Th) in middle Cretaceous rocks in Abakaliki-Ishiagu areas southeastern Nigeria. Arabian Journal of Geosciences, 13, 374. https://doi.org/10.1007/s12517-020-05360-4.
Obasi, P. N., & Akudinobi, B. E. B. (2020). Potential health risk and levels of heavy metals in water resources of lead–zinc mining communities of Abakaliki southeast Nigeria. Applied Water Science, 10, 184. https://doi.org/10.1007/s13201-020-01233-z.
Obasi, P. N., Eyankware, M. O., & Akudinobi, B. E. B. (2021). Characterization and evaluation of the effects of mine discharges on surface water resources for irrigation: a case study of the Enyigba Mining District Southeast Nigeria. Applied Water Science. https://doi.org/10.1007/s13201-021-01400-w.
Odukwe, G. C. (1980). Industrial minerals of Nigeria. In: Proceedings 4th Industrial Minerals International Congress Atlanta, London, pp 103–108.
Okolo, C. C., Oyedotun, T. D. T., & Akamigbo, F. O. R. (2018). Open cast mining: threat to water quality in rural community of Enyigba in south-eastern Nigeria. Applied Water Science, 8, 204. https://doi.org/10.1007/s13201-018-0849-9.
Ozoko, D. C. (2013). Heavy metal pollution of natural waters in Abakaliki, Ebonyi State, Nigeria. International Journal of Science and Research, 4(6), 482–486.
Petters, S. W. (1982). Central West African cretaceous—tertiary benthic foramnifera and Stratigraphy. Paleogeographica, 179, 1–104.
Petters, S. W. (1991). Regional Geology of Africa. Lecture Notes in Earth Sciences (p. 722). Springer-Verlag.
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water analysis. Transactions of the American Geophysical Union, 25, 914–923.
Qian, C., Xiong, W., Wen-Ping, M., Rui-Zhi, F., Ge Zhu, Z. W., & Dan-dan, W. (2016). Hydrogeochemical characterization and suitability assessment of groundwater in an agro-pastoral area, Ordos Basin. NW China. Environmental Earth Science, 75, 1356. https://doi.org/10.1007/s12665-016-6123-2.
Reyment, R. A. (1965). Aspects of geology of Nigeria (p. 145). Ibadan Univ. Press.
Saha S, Reza A. H. M. S and Roy MK (2019) Hydrochemical evaluation of groundwater quality of the Tista floodplain, Rangpur, Bangladesh. Applied Water Science 9:198. https://doi.org/10.1007/s13201-019-1085-7.
Sakram, G., Sundaraiah, R., & Vishnu Bhoopathi, P. R. S. (2013). The impact of agricultural activity on the chemical quality of groundwater, Karanja Vagu Watershed, Medak District, Andhra Pradesh. Int J Adv Sci Tech Res, 6(3), 769–786.
Schoeller, H. (1967). Geochemistry of Groundwater Studies. An International Guide for Research and Practice, UNESCO, Paris, 1–18.
Schwarzenbach, R. P., Egli, T., Hofstetter, T. B., Von Gunten, U., & Wehrli, B. (2010). Global water pollution and human health. Annual Revised Environmental Resources, 35, 109–136.
Sehgal, M., Garg, A., Suresh, R., & Dagar, P. (2012). Heavy metal contamination in the Delhi segment of Yamuna basin. Environmental Monitoring Assessment. https://doi.org/10.1007/s10661-011-2031-9.
Shyu, G. S., Cheng, B. Y., Chiang, C. T., Yao, P. H., & Chang, T. K. (2011). Applying factor analysis combined with kriging and information entropy theory for mapping and evaluating the stability of groundwater quality variation in Taiwan. International journal of environmental research and public health, 8(4), 1084–1109.
Singh, V. K., Singh, K. P., & Mohan, D. (2005). Status of heavy metals in water and bed sediments of River Gomti—a tributary of the Ganga River, India. Environmental Monitoring Assessment, 105, 43–67.
Sirajudeen, J., Arulmanikandan, S., & Manivel, V. (2014). Heavy metal pollution index of groundwater of Fathima Nagar area near Uyyakondan Channel, Tiruchirappalli District, Tamilnadu, India. World Journal of Pharmacy and Pharmaceutical Sciences, 4(1), 967–975.
Su, X., Lu, S., Gao, R., Su, D., Yuan, W., Dai, Z., & Papavasilopoulos, E. N. (2017). Groundwater flow path determination during riverbank filtration affected by groundwater exploitation: A case study of Liao River Northeast China. Hydrological Sciences Journal, 62(14), 2331–2347.
Subba Rao, N. (2012). PIG: A numerical index for dissemination of groundwater contamination zones. Hydrology Process, 26(22), 3344–3350.
Subba Rao, N., & Chaudhary, M. (2019). Hydrogeochemical processes regulating the spatial distribution of groundwater contamination, using pollution index of groundwater (PIG) and hierarchical cluster analysis (HCA): a case study. Groundwater for Sustainable Development, 9, 100238.
Subba Rao, N., Sunitha, B., Rambabu, R., Nageswara Rao, P. V., Surya Rao, P., Spandana, B. D., Sravanthi, M., & Marghade, D. (2018). Quality and degree of pollution of groundwater, using PIG from a rural part of Telangana State India. Applied Water Science., 8, 227. https://doi.org/10.1007/s13201-018-3950864-x.
Ulakpa, R. O. E., & Eyankware, M. O. (2021). Contamination assessment of water resources around waste dumpsites in Abakaliki, Nigeria; A Mini Review. Journal Clean WAS, 5(1), 13–16. https://doi.org/10.26480/jcleanwas.01.2021.13.16.
Umar ND, Igwe O(2019). Geo‑electric method applied to groundwater protection of a granular sandstone aquifer. Applied Water Science 9:112. https://doi.org/10.1007/s13201-019-0980-2.
Umeji, A. C. (2000). Evolution of the Abakaliki and the Anambra sedimentary basins, Southeastern Nigeria. A report submitted to the Shell Petroleum Development Company Nigeria, Limited, p 155.
US Environmental Protection Agency (USEPA). (1989). Risk assessment guidance for superfund, vol 1, human health evaluation manual (Part A). Office of Emergency and Remedial Response, Washington, DC.
Usman, M., Farooq, M., & Hanna, K. (2020). Existence of SARS-CoV-2 in wastewater: implications for its environmental transmission in developing communities. ACS Publications.
WHO (World Health Organization). (2011). Guideline for drinking water quality Recommendations vol 1 (4th ed., pp. 219–230). WHO.
Wunderlin, D.A., et al. (2001) Pattern Recognition Techniques for the Evaluation of Spatial and Temporal Variations in Water Quality. A Case Study: Suquia River Basin (Cordoba-Artgentina). Water Research, 35, 2881–2894. https://doi.org/10.1016/S0043-1354(00)00592-3
Xu, Y. S., Shen, J. S., Zhou, A. N., & Arulrajah, A. (2018). Geological and hydrogeological environment with geohazards during underground construction in Hangzhou: A review. Arabian Journal of Geosciences, 11(18), 1–18.
Yahaya, S. M., Fatima, A., & Nafiu, A. (2021). Ecological risk assessment of heavy metal contaminated soils of selected villages in Zamfara State. Nigeria. SN Applied Sciences, 3, 168. https://doi.org/10.1007/s42452-021-04175-6.
Yang, Q., Lun, W., & Fang, Y. (2011). Numerical modeling of three dimension groundwater flow in Tongliao (China). Procedia Engineering, 24, 638–642.
Zhang, Y., Wu, J., & Xu, B. (2018). Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environment and Earth Science, 77(7), 273. https://doi.org/10.1007/s12665-018-7456-9.
Acknowledgments
The authors wish to thank all who assisted in conducting this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
As per the literature review, this is neither a repetition of any work nor copied key data from other’s work. The methodology, findings, and conclusions made here belong to original research work as per our knowledge and belief.
Compliance with ethical standards
This research work is carried out in compliance with transparency, moral values, honesty, and hard work. No human participation or animals are involved in this research work.
Informed consent
Every step of processing for publication informed to all co-authors of this paper at the earliest, and everything is carried out with collective decision and consent.
Rights and permissions
About this article
Cite this article
Eyankware, M.O., Akakuru, O.C. Appraisal of groundwater to risk contamination near an abandoned limestone quarry pit in Nkalagu, Nigeria, using enrichment factor and statistical approaches. Int J Energ Water Res 7, 603–621 (2023). https://doi.org/10.1007/s42108-022-00186-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42108-022-00186-0