Abstract
Medicago minima (L.) Bartal. is an important rangeland forage and weed, native to Eurasia and Africa but widely distributed elsewhere. Like dozens of its nearest annual Medicago species, it is known to be highly uniform due to extreme inbreeding, and no valid infra-specific taxa meriting recognition have yet been proposed. In this study, 67 individuals from 13 natural populations of M. minima were collected and subjected to genetic diversity evaluation using 6 simple sequence repeat (SSR). Of the genetic variation parameters, expected heterozygosity (He) showed a significant variation from 0.0 in the Meshkinshahr population to 0.337 in the Andimeshk population. The analysis of molecular variance (AMOVA) revealed considerable partitioning of genetic diversity among populations (58%), while intrapopulation diversity accounted for 42%. Moderate to high population genetic differentiation was observed among particular populations, ranging from 0.060 between Marivan and Paveh to 0.562 between Meshkinshahr and Kohdasht populations. A weak total gene flow (0.115) was accompanied by a relatively high PhiPT value (0.617). Structure analysis, principal coordinate analysis, and neighbour-joining analysis, all divided the 13 populations into two genetic groups with significant genetic homogeneity. This discovery of two molecularly distinctive groups of M. minima in Iran, with a limited hybrid zone between them, is remarkable and suggests early divergent speciation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Medicago minima (L.) Bartal. (Fabaceae), a native of Eurasia and Africa has been introduced to much of the world as a minor weed (Small 2011). More importantly, this annual legume is very hardy and highly nutritious and is greatly valued as a rangeland forage (Farag et al. 2007; Judd et al. 2008; Beech et al. 2017). Annual medics (Medicago spp.) have significant advantages over perennials, including high growth rate, higher total crude protein, considerable resistance to pests, vigorous seedlings, and extensive seed banks that enables them to survive harsh environmental conditions and make them essential forage sources (Walsh et al. 2001; Parsamanesh and Sadeghi 2019). Like other legumes, medics are especially friendly to soil due to the nitrogen-fixing ability of symbiotic bacteria housed in their rood nodules (Bena et al. 2005; Küster 2013). In the light of the agricultural and environmental importance of annual and perennial species of Medicago, many institutions and governments have supported research on several of the Medicago species. Ability to tolerate drought, which is increasingly becoming more common, is the key to creating new cultivars that can provide sustainable fodder and forage (Humphries et al. 2020).
Medicago minima (L.) Bartal. is an ascending herb with branches 5–40 cm long, the vegetative organs, calyx, and fruit covered with dense simple and/or glandular hairs. The seeds are 1–2 per coil, kidney-shaped or oblong-ovoid, 1.5 to 2.5 mm long, and about 1.3 mm wide, isolated by a fruit septum, with yellowish to brownish, smooth seed coat, and a radicle approximately half the length of the seed. The diploid chromosome number is 2n = 16 (Jha and Pal 1992; Small 2011). The ability to withstand the harsh environmental conditions and adaption to various soil types makes M. minima an important potential forage sources in drylands of the world, including the fields and pastures of Iran, where it naturally occurs in northwest, west, and southwest areas (Walsh et al. 2001; Mehregan et al. 2002; Falahati-Anbaran et al. 2006, 2007; Parsamanesh and Sadeghi 2019).
There are very few cultivars of M. minima at this time, but given its numerous agronomical advantages and its economic and ecological importance, the germplasm of this species can be exploited for developing more cultivars. This is best done by first clarifying the genetic diversity structure of the species. Molecular markers have many advantages over morphological or cytological ones, as they are not affected by environmental factors (Werlemark et al. 1999; Nybom et al. 2006). Co-dominant simple sequence repeats (SSRs) have advantages over dominant markers such as random amplified polymorphic DNA (RAPD) or inter simple sequence repeat (ISSR) (Liu et al. 2007; Freeland 2020). SSRs are reliable tools to investigate intra and inter-population genetic diversity (Schlötterer et al. 1997; Belicuas et al. 2007; Xu and Crouch 2008; Silva et al. 2012; Bayat et al. 2021; Emami-Tabatabaei et al. 2021).
This study aimed to examine the genetic variability of the species in Iran in the hope of revealing variations that might be useful for breeding new cultivars.
2 Materials and Methods
2.1 Plant Material
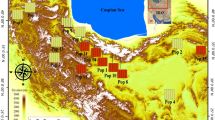
Based on the information in Heyn (1984), and the herbaria of research institute forests and rangelands of Iran, a distribution map for M. minima in Iran was prepared (Fig. 1). Thirteen populations from various localities in Iran were selected for study (note information in Fig. 1 and Table 1). Employing individual plants separated by at least 20 m, leaf samples were taken from 5 to 6 individuals of M. minima in each population. In total, 67 samples were obtained from 13 populations. These were dried on silica gel for molecular studies. Voucher samples are deposited in the Islamic Azad University Herbarium (IAUH) (Table 1).
2.2 DNA Extraction
Total genomic DNA was extracted from silica-gel dried leaf samples using the NucleoSpin® Extract extraction kit (Machery- Nagel, Germany) and CTAB method of Doyle and Doyle (1990). To assess the DNA qualitatively, 1% agarose gel stained with GelRed® Nucleic Acid Gel Stain (San Francisco Bay, CA, USA) was used. Quantitatively, the DNA was tested using NanoDropTM 2000 (Thermo Fisher Scientific, Waltham, MA, USA) at 260 nm.), and samples were stored at − 20 °C.
2.3 Isolation of Microsatellite Loci and Primer Design
100 ng of the extracted genomic DNA was used to generate an Illumina DNA library. After the fragmentation of the DNA, repair of the ends, "a-tailing", and ligation of the TruSeq adapters, the DNA library was amplified with 8 cycles. The library was selected according to the average size of 670 bp. This size corresponded to an average integrate length of 500 bp. The "Library" was sequenced with 300 bp each in the "paired-end" mode in an Illumina-MiSeq run.
The overlapping "paired-end-reads" were first trimmed at the ends to remove residues of adapter sequences. The quality score was set to at least 20. 91.7% of the 56.9 million trimmed sequences had a Q value of 30%. With the help of the “FLASH” tool, the reads were assembled (Magoč and Salzberg 2011). A subset of the sequences was selected for designing primers based on the following criteria: the type and length of the microsatellite and the position within the sequence; as a result, 817,510 loci with any tandem repeat were selected. In this way, 2,410 loci were detected, which contain repetitive elements with the required criteria.
Of these sequences, 513 could be used to design primer pairs that met the following criteria: (1) PCR-Product 150–350 bp; (2) Primer size 18–22 bp; (3) TM-Value 58–62 °C. Using these criteria, primers designed by Primer3 software (Kõressaar et al. 2018). The primer pairs were custom synthesized by Qiagen/Operon Technologies (Alame-da, Calif.). Out of the first 96 primer combinations, only 6 polymorphic loci could be detected.
2.4 PCR Amplification and SSR Assay
The 6 SSR primer pairs were tested for PCR amplification to assess the genetic variation in M. minima populations (Table 2). PCR mixture composed of 0.4 mM of each forward and reverse primer, 10 µM deoxyribonucleotides, 50 mM KCl, 10 mM TRIS–HCl (pH 8.3), 1.5 mM MgCl2, 0.01% gelatin, 50–100 ng of DNA, and 1 U Taq DNA (Eujayl et al. 2004). The PCR amplification profile started with 4 min at 94 °C for denaturation, followed by 35 cycles of 30 s at 94 °C, 45 sat the annealing temperature (varied based on the locus), and 60 s at 72 °C, with a final extension for 4 min at 72 °C. Two PCR product pools were made out of Min_A2-Min_A9-Min_C12 and Min_H2-Min_A1b-Min_A7b primer pairs. Next, 2 µL of each pool mixed with 7.75 µL of HiDi formamide (Applied Biosystems, Foster City, CA, USA) and 0.25 µL of ROX-500 internal size standard (Applied Biosystems) in the following were injected to a 3730 × l capillary sequencer (Applied Biosystems-Hitachi).
2.5 Data Analysis
The files obtained were aligned utilizing GeneMarker v2.4.2 (GeneMarker, SoftGenetics, State College, Pennsylvania). A signal intensity of at least 5000 was considered for those scored as present. A binary dataset of 1 as presence and 0 as absence created of combination of two primer sets used for analyses.
For each primer, polymorphic information content (PIC) was calculated according to Nei (1973): PIC = 1−\(1-\sum {p}_{ij}^{2}\) where Pij is the frequency of the jth allele for ith locus across all alleles at a locus.
Genetic variation parameters including the number of observed alleles (Na), the ratio of effective alleles (Ne), the Shannon information index (I), the observed, expected, total expected, and mean expected heterozygosities (HO, He, Ht, and Hs, respectively), unbiased Nei gene diversity (uHe), the fixation index (F), the percentage of polymorphic loci (P%), and gene flow (Nm) were estimated using GenAlEx version 6.5 (Peakall and Smouse 2006). F-statistics, including inbreeding coefficient within individuals (Fis = (Hs-Ho)/Hs); genetic differentiation among populations (Fst); Fit (Ht−Mean Ho)/Ht) (Wright 1978) were utilized to estimate genetic differentiation among populations using GenAlEx version 6.5 (Peakall and Smouse 2006). To examine whether the matrix of genetic distances between M. minima populations correlated with the matrix of geographic distances between locations, the Mantel test (Mantel 1967) in (Tools for population genetic analyses) TFPGA (Miller 1997) was performed for Nei’s unbiased genetic distances matrix and the matrix of geographic distances.
A hierarchical analysis of molecular variance (AMOVA) was carried out using GenAlEx version 6.5 (Peakall and Smouse 2006). A principal coordinate analysis (PcoA) was also carried out. The genetic relationship between populations was assessed by constructing a phylogenetic tree from a genetic distance with the neighbour-joining (NJ) method using POPTREE2 (Takezaki et al. 2010).
The genetic structure of the studied populations was an analysis based on the Bayesian model and using STRUCTURE Ver. 2.3. (Pritchard et al. 2000) was used. Twenty independent replicate ran for 1–7 K. The values sat for the burn-in period, and the value of MCMC (Markov chain Monte Carlo) were 30,000 and 50,000 times, respectively (Porras-Hurtado et al. 2013). The actual number of clusters (K) was determined by identifying the delta K value using an online structure harvester (Earl 2012).
3 Results
3.1 SSR Loci Polymorphism
The 6 SSR loci produced a mean of 3.16 alleles per locus (N; Table 3); among loci, the effective number of alleles (Ne) did not vary significantly ranged from 1.100 in Min_A7B to 1.541 and 1.532 in Min_A2 and Min_H2, respectively. The latter happened to have the highest value for the Shannon information index (I, 0.395), expected, total, and mean expected heterozygosities (He, 0.258; Ht, 0.672; Hs, 0.258). Additionally, this locus with 0.285 was indicated to have a higher value for unbiased Nei’s gene diversity (uHe) than the rest of the loci. Locus Min_A9 on the other hand was found to have the highest observed heterozygosity (0.069). A notable variation in terms of polymorphic information contents (PIC) was observed among loci in which locus Min_C12 was found to be the most informative (0.996). Other loci, including Min_A9, Min_A2, and Min_H2, also were significantly informative (above 0.50), however, loci Min_A7b (0.478) and Min_A1b (0.517) were moderately informative, respectively, below and close to 0.50. F-statistics revealed invaluable information on loci in this study where the mean of Wright’s fixation index (F) was notably high (0.88) with the lowest value was in Min_A9 (0.698) and the highest in Min_A7b, Min_C12, and Min_A1b (1.00). Similarly, the lowest values for the inbreeding coefficient within individuals (Fis) and Fit (0.599 and 0.758) were observed in Min_A9; other loci had significantly higher, close to 1.00 values.
3.2 Genetic Diversity in M. minima
Among the 13 studied populations of M. minima, the observed mean number of alleles varied from 1 in the Meshkinshahr population to 2.33 in the Marivan population, the former had the value 1 for Ne and the absence of any value for the rest of genetic diversity indices (I, Ho, He, uHe, F, and percentage of polymorphic loci (P%) and the latter also had the highest Ne and I values (1.686 and 0.540, respectively) (Table 4). The population from Garaghaj presented the largest Ho (0.133), whereas He and uHe were the highest in Andimeshk population (0.337 and 0.374, respectively). In the case of Wright’s fixation index, populations with negative F (Garaghaj) had the lowest P% as well (16.67%). However, the increase in F was not consistent with enhancing P%. For example, the population of Rudbar with 50% P had the highest F value (0.841) or Andimeshk population showed 83.33% P while it had the F value of 0.739 (Table 4). Furthermore, the genetic differentiation coefficient (Fst) among the 6 loci was generally high, and Min_A7b and Min_A1b with 0.856 and 0.837 observed to be genetically apart from the rest of the loci, and Min_C12 locus had the most negligible value of Fst (0.250). Following the pattern observed for Fst among loci, gene flow (Nm) was varied where the locus with the lowest Fst (Min_C12) observed to have the highest Nm value (0.750), and Min_A7b with the most elevated genetic differentiation showed the least Nm (0.042) (Table 3).
3.3 Population Genetic Structure of M. minima
The Bayesian-based model method was utilized to investigate the structure of 67 individuals of M. minima. To identify the optimum number of genetic groups of the investigated populations, delta K harvested using the Evanno method was implemented on STRUCTURE HARVESTER website. The K number was determined to be two (Fig. 2A). The membership of each individual in shaping the population structure is presented in Fig. 2B. Each vertical line represents an individual, and those with a probability of 0.75 counted as homogenous and the lower as an admixture. The results indicated the presence of 47 individuals in the “red cluster” and 21 in the “green” without heterogeneity, while only 4 individuals showed genetic admixture. Only three populations, Abdanan, Baghcheh, and Marivan, clustered into the second group showed genetic admixture.
To study correlation between Nei’s unbiased genetic distance and the matrix of geographic distances of 14 populations of M. minima, the Mantel test was utilized to reveal the significant correlation (R = 0.70, P = 0.002) between these two matrixes. The principal coordinate analysis (PCoA) divided the population into two groups, similar to the structure analysis. However, the first group (smaller cluster) harboured geographically closed populations. Still, it did not wholly follow the structure analysis pattern since it contained Marivan, Sarvabad, Abdanan, and Kohdasht. The second group encompassed the rest of the populations (Fig. 3). Furthermore, the neighbour-joining clustering based on genetic distance confirmed the number of genetic groups (2) in structure analysis and PCoA, however in terms of the populations contained, it was only consistent with PCoA result and populations of Marivan, Sarvabad, Abdanan, and Kohdasht grouped similar to NJ result. The remaining 10 populations placed in the second cluster inside the cluster the population grouping mainly indicate significant geographical affinity, for instance, Roudbar and Meshkinshahr, or Poldokhtar and Andimeshk grouped, however populations like Garaghaj from north grouped with Baghcheh from the south (Fig. 4).
To carry out assessments of among-population variation, AMOVA and pairwise Fst analysis were used. AMOVA analysis (Table 5) revealed the distribution of total genetic diversity where the share of among populations (58%) was higher than within-population (42%) from overall genetic diversity. In agreement with Fst, AMOVA showed the presence of a strong genetic differentiation among populations (P, 0.001, PhiPT = 0.617, Table 5). The overall gene flow was minimal (0.155). Moreover, the pairwise Fst test ranged from 0.060 between Marivan and Paveh populations to 0.562 between Meshkinshahr and Kohdasht populations (Table 6). Thus the genetic results roughly parallel the geographical distances between populations.
4 Discussion
4.1 SSR Loci Polymorphism
In this study, the 6 SSR loci produced a mean of 3.16 alleles per locus, which is relatively low, compared to those of 6.57 for the Moroccan M. minima (Zitouna et al. 2014), 3.5 for the Iranian M. polymorpha (Emami-Tabatabaei et al. 2021), and 6.5 for the Iranian M. rigidula complex (Bayat et al. 2021). The mean PIC for the Iranian M. minima was 0.808, compared to 0.66 for Medicago truncatula Gaertner (Eujayl et al. 2004), 0.923 for M. polymorpha (Emami-Tabatabaei et al. 2021), and 0.89 for the M. rigidula complex (Bayat et al. 2021). Dominant markers produce lower PIC contents for Medicago sativa L. (ISSR, Rashidi et al. 2013; AFLP, Keivani et al. 2010). Therefore, these SSR loci proved to be highly informative in investigating the genetic diversity of M. minima populations.
4.2 Genetic Diversity of M. minima
There is only one previous report on the genetic diversity of M. minima in Iran: using 6 SSR markers, an average genetic diversity of 0.13 was reported (Falahati-Anbaran et al. 2006); by contrast, in this study, a significantly higher level of genetic diversity (0.213) was observed. The previous study was based only on 5 samples of M. minima in one location, whereas we included 67 individuals from 13 populations. In the study of various species of Medicago, Zitouna et al. (2014) reported high average values for the majority of genetic diversity parameters in M. minima, including Na (6.571), Ne (4.487), %P (85.71%), Ho (0.330), He (0.671), however, the F value in our study was slightly higher (0.673 vs. 0.536). These differences can be attributed to the difference in sampling (Powell et al. 1996) and the number of molecular markers (Ferrer et al. 2004); additionally, differences in population sizes are another possible reason. Frequently, associations between genetic diversity in wild plant species and geographical distribution, size of the population size, life history parameters, and the breeding system have been found (Hamrick and Godt 1996; Young and Boyle 2000; Ferrer et al. 2004; Li et al. 2018). A higher level of genetic diversity might be expected for species with significant adaptability in harsh environments (Gadissa et al. 2018). However, the average level of genetic diversity of M. minima populations was shown by diversity indices Ho and He (0.076 and 0.225, respectively).
Genetic diversity is highly influenced by life form and breeding system. Outcrossing plant species tend to have higher genetic diversity and a lower differentiation (more homogeneity) among populations (Hamrick and Godt 1996; Young et al. 2002). Inbreeding and outbreeding are present in different species of Medicago, with majority of annual medics being inbreeders (selfers) (Small 2011). The fact that M. minima is a self-pollinating species to a large extent explains the low genetic diversity and significant Fst among populations that were accompanied by low overall gene flow (0.115 < 1). Gene flow greater than 1.0 is considered strong (Slatkin 1985). By comparison, in wild populations of M. sativa, a strongly outcrossing species, expected heterozygosity and PIC content were 0.680 and 0.639, respectively (Qiang et al. 2015). Annual self-pollinating Medicago species have often been found to have a much lower level of expected heterozygosity: Medicago rugosa Desr. (0.0), Medicago scutellata Mill. (0.0), M. truncatula Gaertner. (0.067), Medicago orbicularis (L.) Bartal. (0.067), M. minima (0.13), Medicago rigidula (L.) All. (0.10) (Falahati-Anbaran et al. 2006). Nevertheless, Moroccan annual Medicago species, including those cited above as well as M. murex Willd. and M. polymorpha L. were observed to have high values for expected heterozygosity (Zitouna et al. 2014) similar to M. sativa (Qiang et al. 2015). In two similar studies to ours, Bayat et al. (2021) and Emami-Tabatabaei et al. (2021) reported expected heterozygosities of 0.373 and 0.185, respectively. It appears that various factors affecting the genetic diversity pattern among wild plant populations of Medicago, and perhaps the size of populations is critical: in small populations the chance for mating is significantly higher than larger populations (Moritsuka et al. 2017).
4.3 Genetic Differentiation and Population Structure
Annual predominately inbreeding populations sometimes have high levels of differentiation and variation among populations (Culley and Wolfe 2001; Honnay and Jacquemyn 2007; Stöcklin et al. 2009). Indeed, for M. minima the AMOVA test indicated the significant distribution of genetic diversity among populations (58%), while 42% was within populations of M. minima. By comparison, in wild populations of Cicer an ISSR-based study estimated the partitioning of total genetic diversity was 48.90% among the population and 51.10% intrapopulation (Rajesh et al. 2003). In an investigation of M. sativa using SSR, the outcrossing breeding system of M. sativa expectedly led to a distribution of about 97.6% of genetic diversity within the population (Falahati-Anbaran et al. 2007), much higher than self-pollinated annual M. minima.
Gene flow may predominantly affect the genetic differentiation among wild plant populations (Schaal et al. 1998). The overall value of Nm observed in this study (0.115) indicates the occurrence of genetic drift as Nm above 1 facilitates this possibility (Kang and Chung 1997). Also, the PhiPT value of 0.617 confirms the low gene flow among populations. It reflects the high genetic differentiation, similar to the observation of low PhiPT values among the Iranian M. rigidula populations which revealed the absence of or weak genetic differentiation (Bayat et al. 2021). Moreover, F-statistics revealed substantial genetic differentiation among individuals (Fis = 0.87) and among populations (Fst = 0.589, p = 0.001). This seems to reflect significant weak historical gene flow was insufficient to prohibit genetic drift and as a result, diversity among populations increases while decreasing within populations.
Pairwise genetic differentiation coefficient (Fst) had a range of 0.060 to 0.562 (Table 6), indicating a moderate to high population genetic differentiation. The highest genetic differentiation value of 0.562 was between Meshkinshahr, and Kohdasht populations, and the minimum was found between Marivan and Paveh. The physical distance between the first two populations is 953 km and for the second two is 114 km, which was consistent with the generalization that increase in distance can restrict the degree of gene flow between populations (Li et al. 2018). However, northwest populations generally had higher Fst with populations in the southwest.
4.4 Evolution of Two Molecularly Distinctive Geographical Groups in Iran
The structure analysis indicated the presence of two clusters as the optimum genetic group for M. minima populations. Consistently, similar results were produced from both NJ and PCoA analyses. The studied populations appeared to fall into two groups. The groups' composition was the same between NJ and PCoA, whereas the otherwise observed was observed with those two tests and structure analysis. A moderate polarity among population grouping with these tests was observed because populations from northwest and west mainly grouped, while those from the southwest were placed in a group. It seems that the distance between populations played a considerable role in generating such a pattern for populations of M. minima. The Mantel test also indicated the presence of a close link between geographical distance and genetic differentiation between M. minima populations in this study, similar to Wang et al. (2008) report, which indicated the critical role of physical distance in genetic differentiation among populations of Chondrus crispus Stackhouse.
5 Conclusions
This investigation is the first onto examine genetic variation of M. minima populations using SSR markers. Remarkably limited or absent genetic diversity within 13 populations was observed. Nevertheless, substantial genetic differentiation was observed among the populations. This pattern is probably due to the breeding system and physical distance separating the populations. This study is a model for additional examination of M. minima employing larger numbers of populations and SSRs. The data presented are valuable for conservation and breeding projects. The most significant finding was the discovery of two geographically differentiated groups based on SSR markers. These have not yet been carefully analysed for possible corresponding morphological differences, but they suggest very early speciation.
Data Availability
The data that support the findings of this study are available from the corresponding author, IM, upon reasonable request.
References
Bayat M, Assadi M, Small E, Mehregan I (2021) Molecular studies of Iranian populations support the morphology-based taxonomic separation of Medicago rigidula and M. rigiduloides. Phytotaxa 518(4):281–299
Beech E, Rivers M, Oldfield S, Smith P (2017) GlobalTreeSearch: the first complete global database of tree species and country distributions. J Sustain for 36:454–489. https://doi.org/10.1080/10549811.2017.1310049
Belicuas PR, Guimarães CT, Paiva LV, Duarte JM, Maluf WR, Paiva E (2007) Androgenetic haploids and SSR markers as tools for the development of tropical maize hybrids. Euphytica 156:95–102. https://doi.org/10.1007/s10681-007-9356-z
Bena G, Lyet A, Huguet T, Olivieri I (2005) Medicago-Sinorhizobium symbiotic specificity evolution and the geographic expansion of Medicago. J Evol Biol 18:1547–1558
Culley TM, Wolfe AD (2001) Population genetic structure of the cleistogamous plant species Viola pubescens Aiton (Violaceae), as indicated by allozyme and ISSR molecular markers. Heredity 86:545–556. https://doi.org/10.1046/j.1365-2540.2001.00875.x
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Earl DA (2012) Structure harvester: a website and program for visualizing structure output and implementing the Evanno method. Conserv Genet Resour 4:359–361. https://doi.org/10.1007/s12686-011-9548-7
Emami-Tabatabaei SS, Small E, Assadi M, Dehshiri MM, Mehregan I (2021) Genetic variation among Iranian Medicago polymorpha L. populations based on SSR markers. Genet Resour Crop Evol 68:1411–1424. https://doi.org/10.1007/s10722-020-01071-7
Eujayl I, Sledge M, Wang L, May G, Chekhovskiy K, Zwonitzer J, Mian M (2004) Medicago truncatula EST-SSRs reveal cross-species genetic markers for Medicago spp. Theor Appl Genet 108(414–422):422. https://doi.org/10.1007/s00122-003-1450-6
Falahati-Anbaran M, Habashi A, Esfahany M, Mohammadi S, Ghareyazie B (2006) Study of genetic diversity and relationships of diploid and tetraploid annual medics using microsatellite markers. J Sci Tech Agri Nat Resour 10:349–359
Falahati-Anbaran M, Habashi A, Esfahany M, Mohammadi S, Ghareyazie B (2007) Population genetic structure based on SSR markers in alfalfa (Medicago sativa L.) from various regions contiguous to the centres of origin of the species. J Genet 86:59–63. https://doi.org/10.1007/s12041-007-0008-9
Farag MA, Huhman DV, Lei Z, Sumner LW (2007) Metabolic profiling and systematic identification of flavonoids and isoflavonoids in roots and cell suspension cultures of Medicago truncatula using HPLC–UV–ESI–MS and GC–MS. J Phytochem 68:342–354. https://doi.org/10.1016/j.phytochem.2006.10.023
Ferrer MM, Eguiarte LE, Montaña C (2004) Genetic structure and outcrossing rates in Flourensia cernua (Asteraceae) growing at different densities in the South-western Chihuahuan Desert. Ann Bot 94:419–426. https://doi.org/10.1093/aob/mch159
Freeland JR (2020) Molecular ecology, 3rd edn. Wiley-Black- well, London, p 363
Gadissa F, Tesfaye K, Dagne K, Geleta M (2018) Genetic diversity and population structure analyses of Plectranthus edulis (Vatke) Agnew collections from diverse agro-ecologies in Ethiopia using newly developed EST-SSRs marker system. BMC Genet 19:92. https://doi.org/10.1186/s12863-018-0682-z
Hamrick JL, Godt MW (1996) Effects of life history traits on genetic diversity in plant species. Philos Trans R Soc Lond B Biol Sci 351:1291–1298. https://doi.org/10.1098/rstb.1996.0112
Heyn C (1984) Medicago. In: Rechinger KH (ed) Flora Iranica. Akademische Druch-u Verlagsanstalt, Graz, pp 253–271
Honnay O, Jacquemyn H (2007) Susceptibility of common and rare plant species to the genetic consequences of habitat fragmentation. Conserv Biol 21:823–831. https://doi.org/10.1111/j.1523-1739.2006.00646.x
Humphries AW, Ovalle C, Hughes S, del Pozo A, Inostroza L, Barahona V, Yu L, Yerzhanova S, Rowe T, Hill J, Meiirman G, Abayev S, Brummer EC, Peck DM, Toktarbekova S, Kalibayev B, Espinoza S, Ivelic-Saez J, Bingham E, Small E, Kilian B (2020) Characterization and pre-breeding of diverse alfalfa wild relatives originating from drought-stressed environments. Crop Sci 61:69–88
Jha S, Pal A (1992) Seed structure in Medicago L. Flora 187:209–218. https://doi.org/10.1016/S0367-2530(17)32224-7
Judd W, Campbell C, Kellogg E, Stevens P, Donoghue M (2008) Plant systematics: a phylogenetic approach. Sinauer Associates, Massachusetts
Kang SS, Chung MG (1997) Genetic variation and population structure in Korean endemic species: IV. Hemerocallis hakuunensis (Liliaceae). J Plant Res 110:209–217. https://doi.org/10.1007/BF02509309
Keivani M, Ramezanpour S, Soltanloo H, Choukan R, Naghavi M, Ranjbar M (2010) Genetic diversity assessment of alfalfa (Medicago sativa L.) populations using AFLP markers. Aust J Crop Sci 4:491
Kõressaar T, Lepamets M, Kaplinski L, Raime K, Andreson R, Remm M (2018) Primer3_masker: integrating masking of template sequence with primer design software. Bioinformatics 34:1937–1938. https://doi.org/10.1093/bioinformatics/bty036
Küster H (2013) Medicago truncatula. In: Maloy S, Hughes K (eds) Brenner’s encyclopedia of genetics, 2nd edn. Academic Press, pp 335–337
Li X, Li M, Hou L, Zhang Z, Pang X, Li Y (2018) De novo transcriptome assembly and population genetic analyses for an endangered Chinese endemic Acer miaotaiense (Aceraceae). Genes 9:378. https://doi.org/10.3390/genes9080378
Liu ZP, Liu GS, Yang QC (2007) A novel statistical method for assessing SSR variation in autotetraploid alfalfa (Medicago sativa L.). Genet Mol Biol 30(2):385–391
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Mehregan I, Rahiminejad MR, Azizian D (2002) A taxonomic revision of the genus Medicago in Iran. Iran J Bot 9:207–221
Miller MP (1997) Tools for population genetic analysis (TFPGA) 1.3: a Windows program for the analysis of allozyme and molecular population genetic data. Computer software distributed by the author
Moritsuka E et al (2017) Genetic variation and population structure of a threatened timber tree Dalbergia cochinchinensis in Cambodia. Tree Genet 13:115. https://doi.org/10.1007/s11295-017-1199-8
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Nat Acad Sci 70:3321–3323. https://doi.org/10.1073/pnas.70.12.3321
Nybom H, Esselink G, Werlemark G, Leus L, Vosman B (2006) Unique genomic configuration revealed by microsatellite DNA in polyploid dogroses. Rosa Sect Caninae J Evol Biol 19:635–648. https://doi.org/10.1111/j.1420-9101.2005.01010.x
Parsamanesh S, Sadeghi H (2019) The phytoremediation effect of Medicago scutellata (L.) Mill. on soils under Cd–water stress: a good choice for contaminated dry lands. Environ Sci Poll Res 26:29065–29073. https://doi.org/10.1007/s11356-019-05989-z
Peakall R, Smouse PE (2006) Genalex 6: genetic analysis in excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295. https://doi.org/10.1111/j.1471-8286.2005.01155.x
Porras-Hurtado L, Ruiz Y, Santos C, Phillips C, Carracedo Á, Lareu M (2013) An overview of structure: applications, parameter settings, and supporting software. Front Genet 4:98. https://doi.org/10.3389/fgene.2013.00098
Powell W, Morgante M, Andre C, Hanafey M, Vogel J, Tingey S, Rafalski A (1996) The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breed 2:225–238. https://doi.org/10.1007/BF00564200
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Qiang H, Chen Z, Zhang Z, Wang X, Gao H, Wang Z (2015) Molecular diversity and population structure of a worldwide collection of cultivated tetraploid alfalfa (Medicago sativa subsp. sativa L.) germplasm as revealed by microsatellite markers. PLoS ONE. https://doi.org/10.1371/journal.pone.0124592
Rajesh P, Sant V, Gupta V, Muehlbauer F, Ranjekar P (2003) Genetic relationships among annual and perennial wild species of Cicer using inter simple sequence repeat (ISSR) polymorphism. Euphytica 129:15–23. https://doi.org/10.1023/A:1021567821141
Rashidi M, Farshadfar M, Safari H, Shirvani H (2013) Utility of ISSR molecule marker in examine of genetic diversity 17 genotypes of perennial alfalfa (Medicago sativa). J Nov Appl Sci 2:969–973
Schaal B, Hayworth D, Olsen KM, Rauscher J, Smith W (1998) Phylogeographic studies in plants: problems and prospects. Mol Ecol 7:465–474. https://doi.org/10.1046/j.1365-294x.1998.00318.x
Schlötterer C, Vogl C, Tautz D (1997) Polymorphism and locus-specific effects on polymorphism at microsatellite loci in natural Drosophila melanogaster populations. Genetics 146:309–320. https://doi.org/10.1046/j.1365-294x.1998.00382.x
Silva DC, Dos Santos J, de Souza Barbosa GV, Almeida C (2012) DNA fingerprinting based on simple sequence repeat (SSR) markers in sugarcane clones from the breeding program RIDESA. Afr J Biotechnol, 11:4722–4728. https://doi.org/10.5897/AJB11.2614
Slatkin M (1985) Rare alleles as indicators of gene flow. Evolution 39:53–65. https://doi.org/10.1111/j.1558-5646.1985.tb04079.x
Small E (2011) Alfalfa and relatives: Evolution and classification of Medicago. NRC Research Press, Ottawa
Stöcklin J, Kuss P, Pluess AR (2009) Genetic diversity, phenotypic variation and local adaptation in the alpine landscape: case studies with alpine plant species. Bot Helv 119:125–133. https://doi.org/10.1007/s00035-009-0065-1
Takezaki N, Nei M, Tamura K (2010) POPTREE2: Software for constructing population trees from allele frequency data and computing other population statistics with Windows interface. Mol Biol Evol 27:747–752. https://doi.org/10.1093/molbev/msp312
Walsh MJ, Delaney RH, Groose RW, Krall JM (2001) Performance of annual medic species (Medicago spp.) in Southeastern Wyoming. Agron J 93:1249–1256. https://doi.org/10.2134/agronj2001.1249
Wang X, Zhao F, Hu Z, Critchley AT, Morrell SL, Duan D (2008) Inter-simple sequence repeat (ISSR) analysis of genetic variation of Chondrus crispus populations from North Atlantic. Aquat Bot 88:154–159. https://doi.org/10.1016/j.aquabot.2007.10.001
Werlemark G, Uggla M, Nybom H (1999) Morphological and RAPD markers show a highly skewed distribution in a pair of reciprocal crosses between hemisexual dogrose species Rosa Sect Caninae. Theor Appl Genet 98:557–563. https://doi.org/10.1007/s001220051104
Wright S (1978) The relation of livestock breeding to theories of evolution. J Anim Sci 46:1192–1200. https://doi.org/10.2527/jas1978.4651192x
Xu Y, Crouch JH (2008) Marker-assisted selection in plant breeding: from publications to practice. Crop Sci 48:391–407. https://doi.org/10.2135/cropsci2007.04.0191
Young AG, Boyle TJ (2000) Forest fragmentation. In: Young A, Boshier D, Boyle T (eds) Forest Conservation Genetics: principles practice. CABI Publishing, Wallingford, pp 123–134
Young A, Hill J, Murray B, Peakall R (2002) Breeding system, genetic diversity and clonal structure in the sub-alpine forb Rutidosis leiolepis F. Muell. (Asteraceae). Biol Conserv 106:71–78. https://doi.org/10.1016/S0006-3207(01)00230-0
Zitouna N, Marghali S, Gharbi M, Haddioui A, Trifi-Farah N (2014) Sequence divergence of microsatellites for phylogeographic assessment of Moroccan Medicago species. Genet Mol Res 13:1548–1562. https://doi.org/10.4238/2014.March.12.7
Funding
Authors did not receive any external support for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: IM, ES; Data curation: ZB and IM; Formal analysis: ZB and IM; Investigation: ZB Methodology: IM, ZB, and MA; Project administration: IM; Resources: ZB and IM; Software: ZB and IM; Supervision: IM and MA; Validation: IM, MA, and ES; Visualization: ZB; Writing—original draft: ZB, ES and IM; Writing—review and editing: IM, ES. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable since this study did not involve human participants or material.
Rights and permissions
About this article
Cite this article
Bagheri, Z., Assadi, M., Small, E. et al. Cryptic Molecular-Geographical Divergence within Medicago minima Revealed by SSR Markers. Iran J Sci Technol Trans Sci 46, 49–60 (2022). https://doi.org/10.1007/s40995-021-01236-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-021-01236-8