Abstract
Dracocephalum kotschyi pois’ (Zarrin-Giah) is an endemic endangered medicinal plant in Iran. In this study, 30 inter-simple sequence repeat (ISSR) markers were used to detect genetic relationships among 54 wild individuals from five endemic populations of D. kotschyi. Primers amplified 263 bands, of which 242 (92.1%) were polymorphic. Cluster analysis, population inference, and principal coordinates analysis revealed a broad genetic variation among the populations. The highest intrapopulation diversity was observed within the Arak population. Gst and Nm mean indices of the populations were found to be 0.27 and 1.31, respectively. Analysis of molecular variance showed that the mean genetic diversities within and among the populations were 74% and 26%, respectively. The most similarity was between Isfahan and Alborz populations, whereas the least similarity was between Arak and Tehran. Based on STRUCTURE analysis, samples were divided into four groups. The findings of this work indicate that ISSR analysis could be a suitable technique for genotyping and characterizing D. kotschyi populations. Information on diversity, found in this study, will help to conserve, domesticate and cultivate D. kotschyi for nutraceutical and pharmaceutical uses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dracocephalum kotschyi Boiss, called Zarrin-Giah or Badrandjboie-Dennaie in Persian, is a wild flowering plant of the Lamiaceae family (Golshani et al. 2004). In Iran, this plant is widely distributed in Gorgan, Mazandaran, and Tehran provinces as well as in the Dena mountains. It is used in traditional medicine as an analgesic and anti-inflammatory drug, and its boiled form relieves rheumatic pains and heals wounds. This plant also strengthens the human immune system, and it is used as an additive to improve the taste and aroma of tea and yogurt (Saeidnia et al. 2004). Essential oils bio-produced in this plant include terpenoids such as limonene, α-terpinene, and geranial, which was tested as an analgesic in mice (Golshani et al. 2004). Among the other bioactive compounds in extracts of this herb are phenolic substances such as flavonoids and rosmarinic acid (Saeidnia et al. 2007). The presence of a methoxylated flavone (xanthomicrol), which contributes to the anti-cancer activity, has been reported in D. kotschyi leaves (Jahaniani et al. 2005).
Preserving the genetic diversity of endangered species is one of the primary objectives in survival strategies because conservation of the species depends on the maintenance of sufficient genetic variability (Vicente et al. 2011). However, overharvesting of wild plants, limited distribution areas, and a lack of cultivation and breeding processes prevent species from achieving maturity and reproducing, and consequently results in their mortality and extinction (Noroozisharaf et al. 2011). Information on the diversity among and within populations of a species helps to manage and conserve genetic resources, which could be an important step in preventing the extinction of a species, genetic drift, and development of collections of genetic resources (Rao and Hodgkin 2002). High plant genetic diversity increases the chance to select the appropriate material for breeding. In genetic diversity studies, differences or similarities between species, populations, or individuals are expressed using special statistical methods based on morphological characteristics and genealogical data, or molecular properties (Pank 2006).
Molecular markers are powerful tools to determine the genetic makeup of plants used for comparing DNA sequences and allele compositions among plants. In addition, the markers are frequently used as a quick method for screening germplasm to select crossed parents, for development of genetic linkage maps, and for tagging of genes to control important traits (Ibitoye and Akin-Idowu 2010). Inter-simple sequence repeat (ISSR) is one of the most efficient molecular marker methods in terms of ability to produce abundant polymorphic markers within a short time and limited budget (Reddy et al. 2002). Compared to other techniques, ISSR can discriminate among closely related genotypes and detect polymorphisms without any previous knowledge of the crop’s DNA sequence (Kumar et al. 2006).
To our knowledge, no molecular research has been reported on D. kotschyi for better management of germplasm in Iran. The objectives of this study were (1) to evaluate the genetic diversity within and among D. kotschyi populations using ISSR markers and (2) to provide primary data for future conservation strategies.
2 Materials and methods
2.1 Plant materials
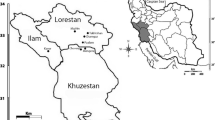
In initial studies, various D. kotschyi habitats were identified and plant samples were collected from five locations in Iran (Fig. 1). According to the meteorological statistics of central Iran, the average annual rainfall for the Isfahan, Markazi, Alborz, Tehran, and Kohgiluyeh and Boyer-Ahmad provinces ranges from 300 to 550 mm and they have semi-arid climatic conditions with cold winters, and hot and dry summers. A total of 54 single plants were harvested (Table 1). The distance between collected plants was at least 100 m. Figure 2 shows D. kotschyi in its natural habitat (Yasouj and Kohgiluyeh and Boyer-Ahmad provinces).
2.2 DNA extraction
DNA was extracted from shadow-dried leaf samples using the Doyle and Doyle (1987) method with little modification. One hundred milligrams of dry leaves was ground and transferred to a 2-ml microfuge tube. One milliliter of extraction buffer including 5% (v/v) 100 mM Tris–HCl (pH 8.0), 4% (v/v) 20 mM EDTA, 28% (v/v) 1.4 M NaCl, 1% (w/v) CTAB, 2% (w/v) PVP-40, and 2% (v/v) β-mercaptoethanol was added. The tube was incubated for 30 min in a water bath at 65 °C and inverted. One milliliter of chloroform:isoamyl alcohol (24:1) was added at 4 °C and inverted for 5 min. The solution was then centrifuged at 10,000 rpm for 15 min and the supernatant transferred to a new tube.
Two hundred microliters of CTAB buffer solution (5 ×) including 5% CTAB and 0.7 M NaCl was added and inverted for 5 min. One milliliter of chloroform:isoamyl alcohol (24:1) was added and inverted for 5 min. The solution was then centrifuged at 10,000 rpm for 15 min and the supernatant transferred to a new tube. Two hundred microliters of cold isopropanol was added and the solution incubated at room temperature for 2 h after inverting. The solution was then centrifuged at 5000 rpm at 4 °C for 15 min to precipitate DNA. The supernatant was discarded. One milliliter of cold washing buffer, including 76% ethanol and 10 mM ammonium acetate, was added and the tube was inverted for 5 min and incubated at room temperature for 30 min. The solution was then centrifuged at 3200 rpm at 4 °C for 10 min. The washing buffer was discarded. The pellet was dried at room temperature for 30 min and dissolved in 100 µl of TE buffer solution at 4 °C overnight. DNA samples were stored at − 20 °C until further analysis.
To determine the quantity and quality of DNA samples, a spectrophotometer at wavelengths of 260 and 280 nm and agarose gel electrophoresis at 1% concentration were used. The samples with a concentration of 50 ng/µl were used for the experiment.
2.3 ISSR-PCR amplification
Thirty primers (Bioneer Company, South Korea) were used to study the genetic diversity (Table 2). The PCR was carried out in a reaction volume of 25 μl containing 3 mmol/l MgCl2, 0.6 mmol/l dNTP, 0.8 mmol/l primers, 50 ng of template DNA, and 1 U of Taq DNA polymerase. The amplification was run under the following conditions: initial denaturation at 94 °C for 5 min, followed by 37 cycles of denaturation at 94 °C for 1 min, primer annealing at 56 °C for 75 s, and elongation at 72 °C for 2 min, ending with a final elongation at 72 °C for 7 min. Amplified products were separated by electrophoresis in 1.50% agarose gel and 1 × TBE buffer and stained with 0.5 µg/ml ethidium bromide.
2.4 Data analysis
2.4.1 Polymorphism and marker indices
To provide a matrix of obtained data, 1 was assigned to the presence of a band and 0 was assigned to the absence of a band. Polymorphism information content (PIC) was calculated using polymorphic DNA bands obtained from each primer and using the formula \(PIC = 2f_{i} \left( {1 - f_{i} } \right)\), where PIC is the polymorphic information content of marker i, \(f_{i}\) is the frequency of the amplified allele for a locus, and \(1 - f_{i}\) is the frequency of the null allele (Botstein et al. 1980). Additionally, the marker index (MI), a standard for determining the efficiency of a marker in polymorphic estimation, was calculated according to Powell et al. (1996) using the formula \(MI = EMR*PIC\), where EMR = ɧ * β and ɧ is the mean number of amplification bands in each individual by a particular marker system, and \(\beta = {\raise0.7ex\hbox{${np}$} \!\mathord{\left/ {\vphantom {{np} n}}\right.\kern-0pt} \!\lower0.7ex\hbox{$n$}}\). Resolving power (Rp) was calculated according to Prevost and Wilkinson (1999) using the formula \(R_{P} = \sum I_{b}\), in which Ib = 1 − [2 * (0.5 − p)] and p is the ratio of individuals with a band showing the ability of ISSR markers in distinguishing D. kotschyi populations.
2.4.2 Genetic diversity among and within populations
POPGENE software (version 1.32) was used to analyze the parameters, the observed number of alleles (Na), effective number of alleles (Ne) at each gene location, Shannon’s information index (I) (Shannon and Weaver 1949), and Nei’s gene diversity index (h) (Nei 1978) in both species and population to examine the genetic diversity within populations. To investigate the diversity among populations, the estimation of heterozygosity within populations (Hs), heterozygosity among populations (Dst), total heterozygosity (Ht), coefficients of gene differentiation (Gst), and gene flow (Nm) (Nei 1978) were used. The genetic distance between genotypes and drawing dendrogram were performed based on Nei coefficient (Nei and Li 1979).
The variation among and within the five sampled groups based on ISSR patterns was determined by analysis of molecular variation (AMOVA) using GEN ALEX version 6.3 software (Peakall and Smouse 2006). Genetic similarity based on the Jaccard coefficient was calculated by making a pairwise comparison between all D. kotschyi single plants using the Simqual module of NTSYS-pc version 2.2. These similarity coefficients were used to construct a dendrogram using the unweighted pair group method with arithmetic averages (UPGMA) employing the SAHN algorithm (sequential, agglomerative, hierarchical, and nested clustering) from NTSYS-pc software package (Sokal 1958). In addition, the goodness of fit of the clustering compared to the basic data matrix was also tested by computing the cophenetic correlation coefficient using the normalized Mantel statistics Z test via the COPH and MXCOMP procedures of NTSYS-pc version 2.20e (Rohlf and Sokal 1981). Principal coordinates analysis (PCoA) was carried out on the basis of the pairwise genetic similarity matrix using the Dcenter and Eigen procedures of the NTSYS-pc version 2.2 software, and a two-dimensional model of the distribution of populations was plotted with panels showing the first two principal coordinates obtained from ISSR genotyping (Rohlf 1998).
2.4.3 Inference of population structure
Analysis of the genetic structure of the populations was conducted using STRUCTURE software (version 2.3.3) and the Markov chain-Monte Carlo chain algorithm (MCMC) (Pritchard et al. 2000). In this software, each individual is placed in a subpopulation (K), and the number of clusters is calculated by the possibilities for \(Pr(X | K) K_{r}\) or L(k) in a combined model from K = 1 to K = 10. This allows the comparison of recombination potential between obtained clusters. For calculating each K, 70,000 repeats, followed by 30,000 experiments, were conducted in 10 implementations. Deduction of the number of subpopulations was conducted according to the method described by Evanno et al. (2005) using the logarithmic changes rate of probability \(K \left( {\Delta K} \right)\) to optimal values of K.
3 Results and discussion
Thirty primers were used to survey the genetic diversity of populations, among which 21 primers showed 100% polymorphism. Primer IS15 showed the lowest polymorphism percentage (50%). In total, 263 bands were obtained, of which 242 were polymorphic (92.1%). The most amplified segment (number 13) was obtained using primers of IS2, IS13, and UBC 810, and the least amplified segment (number 3) was recorded after applying the primer IS19 (Table 2). In this study, ISSR markers showed a high percentage of polymorphism, indicating their effectiveness for investigating genetic diversity. These results were consistent with the results of studies conducted on other medicinal plants, such as Thymus daenensis subsp. Daenensis (Rahimmalek et al. 2009) and Lamiophlomis rotata (Liu et al. 2006).
The mean of the marker index in the populations studied was 2.6, the maximum was 4.93 for IS13, and the minimum was 0.7 for IS19. Resolving power was between 2.04 for IS15 and 14.67 for IS1. The total average value of resolving power was 8.7. Polymorphism information content was calculated using allelic frequency for each primer separately. PIC values ranged from 0.44 (IS17 and IS15) to 0.17 (IS4) (Table 2). Primers with the highest PIC can identify the genetic distance of populations better, and primers with high MI have the highest potential to produce bands (Powell et al. 1996).
MI is a criterion for determining the efficiency of markers for polymorphism estimation. The highest MI in this study was obtained for IS13, suggesting that this primer has produced the highest number of polymorphisms. An important characteristic of a good marker is its capacity to distinguish among different populations (Noroozisharaf et al. 2015).
Prevost and Wilkinson (1999) described the Rp parameter as the measurement of discrimination power for ISSR molecular markers. MI and Rp were calculated to show the ability of ISSRs to discriminate D. kotschyi populations. Results from PIC and MI and Rp means were compatible with the results of former researchers, such as Kumar et al. (2014) with PIC = 0.25, Rp = 4.25, and MI = 3.94 and Hadian et al. (2015) with PIC = 0.29, Rp = 5.71, and MI = 2.30, who used ISSR markers for medicinal plants.
The cophenetic correlation coefficient (r = 0.87) was calculated to assess the efficiency of the UPGMA method in clustering plant populations. If this coefficient is between 0.8 and 0.9, it will be appropriate, and if it is below 0.8 it is weak (Mohammadi and Prasanna 2003). Cluster analysis of five populations, which included 54 individuals, was conducted based on Jaccard’s similarity coefficient and the UPGMA method (Fig. 3). Based on clustering, they were divided into four groups. The first group included three Arak samples, the second group consisted of 47 samples divided into eight subgroups, the third group included two Yasuj samples, and the fourth group included two other samples from Arak. The second group included 47 samples from all provinces, presenting the genetic similarity of these samples. However, in most cases, in each subgroup, the plants of one population have been included. Despite the geographical distance, the genetic affinity and possible relationships among samples from different regions may be justified by the existence of gene flow (Sefc et al. 2000).
Overall, genotypes related to a population were included in the subgroup or similar groups (exception for the Arak population), which could confirm the high correlation between genetic diversity and geographic diversity. Arak samples were separately included in two main groups (groups 1 and 4). In addition, they were included in Group 2 in a subgroup separate from other groups. The inclusion of Arak samples separates from other groups indicated that collected plants from Arak had a higher intra-population variation compared to the other populations. Only the Tehran population was included in a separate subgroup, and it was not included in other subgroups. This could indicate that the Tehran population was purer than the other populations. PCoA was used to obtain a graphical representation of the relationships among the single plants according to the ISSR data, and the 2-D distribution of samples was generated (Fig. 4). In some cases, the distribution of samples was in accordance with its distribution in the branches of the dendrogram obtained from cluster analysis. First and second factors explained 54.36% and 4.32% (total 58.69%) of the total variance.
The mean of observed number allele (Na) at each gene location was between 1.59 and 1.74, with a mean of 1.66. Mean of effective alleles (Ne) at each gene location was between 1.34 and 1.49, with a mean of 1.42. Two indices of Nei’s gene diversity (h) and Shannon’s information (I) are used to assess the genetic diversity within the population in higher plants when the dominant marker is used (Song et al. 2010; Zhang and Dai 2010).
Shannon’s information index (I) at the population level was between 0.3 and 0.4 (mean of 0.35) and Nei’s gene diversity (h) was between 0.2 and 0.28 (mean of 0.24). These two indices were the lowest for the Alborz population (I = 0.3 and h = 0.2). Indeed, the Arak population showed the highest genetic diversity (I = 0.41 and h = 0.28), and more genetic diversity was observed at the species level (I = 0.46 and h = 0.31) (Table 3). These results are in agreement with the high percentage of genetic diversity at the species level using ISSR markers in plants such as Satureja rechingeri (I = 0.52 and h = 0.35) (Hadian et al. 2015) and Salvia miltiorrhiza (I = 0.19 and h = 0.33) (Song et al. 2010). Also, the percentage of polymorphic gene locations (PPL %) was 59.09% for Tehran and 74.79% for Arak.
Genetic diversity within populations was relatively high (Hs = 0.24), while the diversity among populations was lower (DST = 0.0929) (Table 4). In this study, Dst values were lower than Hs, indicating high diversity within populations. This could be due to the fact that plants in various areas did not have very much gene exchange, while plants of one region had considerable gene exchange leading to high diversity within population and lower diversity among the populations (Wang et al. 2008).
The results of the ISSR analysis showed a high genetic differentiation among the populations at Gst = 0.5. Gst is a statistical measure of the differentiation of gene. This indicates that the genetic diversity among and within populations was equal (Liao et al. 2012).
Nm and Gst are two important indicators to evaluate the genetic structure of a population of one species. Hamrick (1989) showed that the average Gst value was 0.2 for the cross-pollinated plants and 0.5 for the self-pollinated plants. Moreover, an Nm higher than 1 shows that populations are maintaining genetic relationships over time, and an Nm smaller than 1 shows that the populations have become divergent genetically. An Nm of 1.31 indicates that genetic exchange is high among populations of this species (Table 4). In short, the higher the genetic differentiation of the populations, the lower the gene flow (Lowe et al. 2009). These results were supported by Hamrick (1987), who reported high gene flow with the mean of 1.15 tested on 16 cross-pollinated species. Gene flow is a critical ecological process with conservation benefits, which indicate transfer of genes within and among populations (Yang et al. 2007).
Plant population genetic structure is the consequence of the interaction of several factors that create complex genetic structure within populations. Some of these factors include the long history of the evolution of species (i.e. change in distribution, habitat fragmentation, and separation of populations), the pollination system, genetic drift, selection, and gene flow (Reisch et al. 2005). This genetic differentiation among populations may be due to changes in environmental conditions and natural selection or to random processes such as mutation (Bossdorf et al. 2005). Analysis of molecular variance showed that 74% of the genetic diversity was within populations and 26% was among populations. High genetic diversity was observed within populations in S. miltiorrhiza (84.83%) (Song et al. 2010).
The high genetic diversity within D. kotschyi populations may be due to cross-pollination, activity of insects, and pollen transfer among different populations. Genetic variation in cross-pollinated plants is higher than self-pollinated plants and asexual-propagated plants. Transfer of pollen by insects increases the possibility of the creation of new phenotypes and, accordingly, genetic diversity within populations (Shi et al. 2008).
The value of genetic distances among populations was determined by distance matrix based on Nei’s index (Table 5). Nei’s genetic similarity was measured between 0.77 and 0.88. In this evaluation, the highest genetic similarities were observed between Isfahan and Alborz populations (0.886) as well as Tehran and Alborz (0.8751), indicating a stronger gene flow between these populations and a lower level of genetic differentiation between populations. It is possible that these two populations have the same origin. On the other hand, the lowest similarity was observed between Arak and Tehran populations (0.778). The dendrogram created with the ISSR data based on Nei’s index divided the five D. kotschyi populations into two distinct groups (Fig. 5).
Inferred population structures by Bayesian clustering analysis for D. kotschyi (54 individuals; 242 loci) performed using STRUCTURE (Fig. 6) Showed that the highest value of \(\Delta K\) was obtained in K = 4. This indicates that the populations consisted of four groups (subpopulations). Based on Fig. 6 and the graph of results, STRUCTURE classified all individuals from the Arak population into two clusters (red and green), which indicates the high level of diversity within this population. This could be due to changes in environmental conditions and natural selection or stochastic processes like mutation. Isfahan has been located in one group, as shown by green color. It is expected that Arak and Isfahan have a common origin because of the genetic exchange via some factors such as transfer of seeds by humans or birds. Alborz and Tehran are in the same group (blue), confirming geographical proximity of these two populations. These results corresponded with the results of cluster analysis. There were relatively high relationships among subpopulations and geographical distributions of the studied population. These inferred subpopulations are in close agreement with the major clusters shown in Figs. 3 and 5.
Bayesian clustering analysis of D. kotschyi (54 individuals; 242 loci) performed using STRUCTURE. The value of Pr (X|K) was optimized at K = 4 for most numerical solutions, with K being the number of subpopulations. The membership of accessions in K = 4 subpopulations was consistent across multiple solutions. Each individual from the predefined populations is represented by a vertical line, which is partitioned into a maximum of K = 4 differently colored segments. Vertical black lines separate individuals of the five predefined populations
As a result, a high correlation was observed between the clustering and the geographical distribution of the studied populations, and the diversity within the population was higher than that among populations. Two important aspects of genetic diversity in the study of plants, especially endangered plants, are the level of diversity and the manner of diversity within and among populations (Shao et al. 2009).
High genetic diversity was observed in D. kotschyi. The level of genetic diversity in a certain species can be affected by various factors including pollination system, pollinators, self-incompatibility, and propagation methods. The pollination system is an important factor in determining genetic diversity. Cross-pollination and pollination by insects are very common in the Lamiaceae family. It has been observed that species with cross-pollination have high genetic diversities (Hajjar et al. 2008).
The high genetic diversity in D. kotschyi could be due to this factor. In this plant, there is a relatively high gene flow among populations, and they have formed a proper genetic relationship with each other. This gene flow can infer the possibility of short-term and long-term adaptation to environmental stress, and it can ensure the survival of these populations to their environment. High gene flow prevents adaptation of populations, the process of speciation, and divergence of populations (Hamrick 1987).
Information on genetic structure and the diversity of plant species is essential for conservation. Despite the presence of genetic diversity in D. kotschyi populations, unfortunately, this herb has not yet been domesticated in Iran and is collected from the wild and threatened with extinction. Seed dormancy is a barrier for bringing D. kotschyi into cultivation (Fattahi et al. 2011). The efforts to domesticate and cultivate this plant should continue.
4 Conclusion
This study showed the analysis of genetic diversity among and within D. kotschyi populations native to Iran. Evaluation of different parameters using ISSR markers showed that they can give clear information about genetic fingerprinting. This marker could show good relationships among populations and specify a good understanding of genetic diversity among and within populations. This was the first effort to assess genetic diversity among native D. kotschyi populations of Iran, and the data generated herein could be effective for germplasm management and parental selection in breeding programs, as well as for the conservation of other endangered Dracocephalum species.
References
Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D (2005) Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144:1–11
Botstein D, White RL, Skolnick M, Davis RW (1980) Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet 32:314
Doyle J, Doyle J (1987) A rapid procedure for DNA purification from small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Fattahi M, Nazeri V, Sefidkon F, Zamani Z, Palazon J (2011) The effect of pre-sowing treatments and light on seed germination of Dracocephalum kotschyi Boiss: an endangered medicinal plant in Iran. Hortic Environ Biotechnol 52:559–566
Golshani S, Karamkhani F, Monsef-Esfehani HR, Abdollahi M (2004) Antinociceptive effects of the essential oil of Dracocephalum kotschyi in the mouse writhing test. J Pharm Pharm Sci 7:76–79
Hadian J, Karami A, Azizi A, Khadivi-Khub A (2015) Ubiquitous genetic diversity among and within wild populations of Satureja rechingeri assessed with ISSR markers. Plant Syst Evol 301:923–930
Hajjar R, Jarvis DI, Gemmill-Herren B (2008) The utility of crop genetic diversity in maintaining ecosystem services. Agric Ecosyst Environ 123:261–270
Hamrick J (1987) Gene flow and distribution of genetic variation in plant populations. In: Differentiation patterns in higher plants, pp 53–67
Hamrick J (1989) Isozymes and the analysis of genetic structure in plant populations. In: Soltis DE, Soltis PS, Dudley TR (eds) Isozymes in plant biology. Springer, Berlin, pp 87–105
Ibitoye D, Akin-Idowu P (2010) Marker-assisted-selection (MAS): a fast track to increase genetic gain in horticultural crop breeding. Afr J Biotechnol 9:8889–8895
Jahaniani F, Ebrahimi SA, Rahbar-Roshandel N, Mahmoudian M (2005) Xanthomicrol is the main cytotoxic component of Dracocephalum kotschyii and a potential anti-cancer agent. Phytochemistry 66:1581–1592
Kumar A, Arya L, Kumar V, Sharma S (2006) Inter simple sequence repeat (ISSR) analysis of cytoplasmic male sterile, male fertile lines and hybrids of pearl millet [Pennisetum glaucum (L.) R. Br.]. Indian J Crop Sci 1:117–119
Kumar A, Mishra P, Singh SC, Sundaresan V (2014) Efficiency of ISSR and RAPD markers in genetic divergence analysis and conservation management of Justicia adhatoda L., a medicinal plant. Plant Syst Evol 300:1409–1420
Liao L, Guo Q-s, Wang Z-y, Liu L, Zhu Z-b (2012) Genetic diversity analysis of Prunella vulgaris in China using ISSR and SRAP markers. Biochem Syst Ecol 45:209–217
Liu J, Wang L, Geng Y, Wang Q, Luo L, Zhong Y (2006) Genetic diversity and population structure of Lamiophlomis rotata (Lamiaceae), an endemic species of Qinghai-Tibet Plateau. Genetica 128:385–394
Lowe A, Harris S, Ashton P (2009) Ecological genetics: design, analysis, and application. Wiley, New York
Mohammadi S, Prasanna B (2003) Analysis of genetic diversity in crop plants-salient statistical tools and considerations. Crop Sci 43:1235–1248
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Nei M, Li W-H (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci 76:5269–5273
Noroozisharaf A, Hamidoghli Y, Zakizadeh H (2011) In vitro seed germination and micropropagation of primrose (Primula heterochroma Stapf.) an endemic endangered Iranian species via shoot tip explants. Hortic Environ Biotechnol 52:298–302
Noroozisharaf A, Hatamzadeh A, Samizadeh Lahiji H, Bakhshi D (2015) Genetic diversity of endangered primrose (Primula heterochroma Stapf.) accessions from Iran revealed by ISSR and IRAP markers. Sci Hortic 190:173–178
Pank F (2006) Breeding of medicinal plants. In: Medicinal plant biotechnology: from basic research to industrial applications, pp 417–449
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Not 6:288–295
Powell W, Morgante M, Andre C, Hanafey M, Vogel J, Tingey S, Rafalski A (1996) The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breed 2:225–238
Prevost A, Wilkinson M (1999) A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor Appl Genet 98:107–112
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Rahimmalek M, Bahreininejad B, Khorami M, Sayed T (2009) Genetic diversity and geographical differentiation of Thymus daenensis, an endangerd medicinal plant, as revealed by Inter Simple Sequence Repeat (ISSR) markers. Biochem Genet 47:831–842
Rao VR, Hodgkin T (2002) Genetic diversity and conservation and utilization of plant genetic resources. Plant Cell Tissue Organ Cult 68:1–19
Reddy MP, Sarla N, Siddiq E (2002) Inter simple sequence repeat (ISSR) polymorphism and its application in plant breeding. Euphytica 128:9–17
Reisch C, Anke A, Röhl M (2005) Molecular variation within and between ten populations of Primula farinosa (Primulaceae) along an altitudinal gradient in the northern Alps. Basic Appl Ecol 6:35–45
Rohlf FJ (1998) On applications of geometric morphometrics to studies of ontogeny and phylogeny. Syst Biol 47:147–158
Rohlf FJ, Sokal RR (1981) Comparing numerical taxonomic studies. Syst Biol 30:459–490
Saeidnia S, Gohari AR, Uchiyama N, Ito M, Honda G, Kiuchi F (2004) Two new monoterpene glycosides and trypanocidal terpenoids from Dracocephalum kotschyi. Chem Pharm Bull 52:1249–1250
Saeidnia S, Gohari AR, Hadjiakhoondi A, Shafiee A (2007) Bioactive compounds of the volatile oil of Dracocephalum kotschyi. Z Naturforsch C J Biosci 62:793–796
Sefc K, Lopes M, Lefort F, Botta R, Roubelakis-Angelakis K, Ibanez J, Pejić I, Wagner H, Glössl J, Steinkellner H (2000) Microsatellite variability in grapevine cultivars from different European regions and evaluation of assignment testing to assess the geographic origin of cultivars. Theor Appl Genet 100:498–505
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Shao J-W, Chen W-L, Peng Y-Q, Zhu G-P, Zhang X-P (2009) Genetic diversity within and among populations of the endangered and endemic species Primula merrilliana in China. Biochem Syst Ecol 37:699–706
Shi W, Yang CF, Chen JM, Guo YH (2008) Genetic variation among wild and cultivated populations of the Chinese medicinal plant Coptis chinensis (Ranunculaceae). Plant Biol 10:485–491
Sokal RR (1958) A statistical method for evaluating systematic relationship. Univ Kans Sci Bull 28:1409–1438
Song Z, Li X, Wang H, Wang J (2010) Genetic diversity and population structure of Salvia miltiorrhiza Bge in China revealed by ISSR and SRAP. Genetica 138:241–249
Vicente MJ, Segura F, Aguado M, Migliaro D, Franco JA, Martínez-Sánchez JJ (2011) Genetic diversity of Astragalus nitidiflorus, a critically endangered endemic of SE Spain, and implications for its conservation. Biochem. Syst. Ecol 39:175–182
Wang Y, Li D, Sui C, Zhao A, Du X (2008) Conservation, characterization, evaluation and utilization of Chinese jujube germplasm resources. In: I International Jujube Symposium, vol. 840, pp 235–240
Yang B, Xiao B, Chen X, Shi C (2007) Assessing the genetic diversity of tobacco germplasm using intersimple sequence repeat and inter-retrotransposon amplification polymorphism markers. Ann Appl Biol 150:393–401
Zhang L-J, Dai S-L (2010) Genetic variation within and among populations of Orychophragmus violaceus (Cruciferae) in China as detected by ISSR analysis. Genet Resour Crop Evol 57:55–64
Acknowledgements
This work was supported by the Grant 9277 by Bu-Ali Sina University to A.A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Additional information
Communicated by Sung-Chur Sim.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maskani Sereshkeh, F., Azizi, A. & Noroozisharaf, A. Structure of genetic diversity among and within populations of the endemic Iranian plant Dracocephalum kotschyi. Hortic. Environ. Biotechnol. 60, 767–777 (2019). https://doi.org/10.1007/s13580-019-00149-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-019-00149-1